Abstract
AIM: To evaluate the accuracy of red cell distribution width (RDW) to platelet ratio (RPR) to predict in-hospital mortality in acute pancreatitis (AP).
METHODS: Between January 2010 and June 2012, 102 patients with AP were recruited to the study. In this retrospective cohort study, for all subjects, demographic data on hospital admission, AP etiology, co-morbid diseases, organ failure assessment, laboratory parameters and length of hospital stay were examined. Additionally, we used a non-invasive prediction method in addition to the RPR to evaluate the disease severity. Multivariate logistic regression analyses were used to evaluate the impact of RPR on hospital admission to predict mortality.
RESULTS: The male-female ratio (59/43) was 1.37 with a median age of 56.5 years (17-89 years). In both univariate and multivariate analyses, RDW and RPR were presented as independent and significant variables on admission to predict mortality. The RPR obtained on hospital admission was persistently higher among non-survivors than among survivors (P < 0.0001). The median RPR was 0.000087 in the non-survivor group and 0.000058 in the survivor group. RPR with a cutoff value of 0.000067 presented an area under the curve of 0.783 (95%CI: 0.688-0.878) in receiver operating characteristic curves and could predict the mortality of approximately 80% of the patients.
CONCLUSION: We identified RPR as a valuable, novel laboratory test to predict mortality in AP.
Keywords: Acute pancreatitis, Red cell distribution width, Red cell distribution width to platelet ratio
Core tip: Although the majority of patients with acute pancreatitis have a mild course of the disease, severe forms require more attention because of their high morbidity and mortality. Several single- and multi-parameter scoring systems, including laboratory parameters, physiological and radiological assessments, have been described to evaluate disease severity. Sometimes, it is not clinically practicable to use these scoring systems for evaluation; therefore, an easy to use and accurate method is needed to predict mortality.
INTRODUCTION
Acute pancreatitis (AP) is an acute inflammatory disease, and is one of the most frequent gastrointestinal causes of hospital admission. The incidence of AP is 150-420 cases per million in the United Kingdom, and 330-430 cases per million in the United States[1]. The majority of patients have mild, self-limited disease; however approximately 20% of patients have a severe form[2]. AP has three phases. The first phase is characterized by enzymatic activation and cellular injury that leads to early symptoms. In the second phase, pro-inflammatory and anti-inflammatory mediators play a role in a systemic inflammatory response and an intrapancreatic inflammatory reaction occurs. The third phase involves complications of AP[1]. The early assessment of disease severity to estimate the complications and even organ failure is fundamental: approximately 23% of the deaths attributable to AP occur in the first 3 d, and 53% within the first week [3].
Several single- and multi-parameter predictors have been described to evaluate the severity of the disease. Some scoring systems, such as Ranson, Glasgow and APACHE II, provide valuable clues to evaluate the severity and mortality of AP. In several studies, certain biological markers, such as elevated C-reactive protein, elevated creatinine, high blood glucose and hemoconcentration on admission, have been used to predict mortality[4].
Complete blood count is a laboratory test frequently used in clinical practice and comprises white blood cell, red blood cell and platelet counts, and their morphological indices, such as the red cell distribution width (RDW). RDW measures the size variability of erythrocytes. It is widely used to differentiate the etiology of anemia; thus, in previous studies, the RDW was shown to be a useful marker for celiac disease, colon cancer and acute coronary syndromes[5,6]. In addition, hemostatic disorders ranging from hypercoagulopathy to disseminated intavascular coagulation appear in the acute phase of AP and are related to disease severity[7]. In a recent study to predict hepatic fibrosis stages in patients with chronic Hepatitis B, Chen et al[8] used the RDW to platelet ratio (RPR). Using these two parameters, which are easily calculated, the necessity for liver biopsy for these patients will be reduced. The RPR ratio reflects inflammation severity; therefore, we aimed to evaluate the severity of patients with AP using RPR on hospital admission.
MATERIALS AND METHODS
A total of 102 patients with AP were recruited in the study between January 2010 and June 2012. AP was diagnosed with typical physical examination findings associated with a plasma amylase level greater that 500 IU/L, and radiological verification of the disease by ultrasonography and/or abdominal tomography. In this retrospective study, for all subjects, demographic data on hospital admission, etiology of pancreatitis, organ failure, co-morbid diseases, length of hospital stay, and the following laboratory measures such as serum amylase, C-reactive protein, complete blood count, serum electrolytes, serum enzymes associated with cholestasis, serum hepatic and renal function tests, bilirubin levels, fasting blood glucose, lactate dehydrogenase, capillary gas analysis (partial pressure of oxygen, pH, and base excess), were analyzed from each patient records. Also we calculate the RPR using the following index. RPR = RDW (%)/platelet (109/L)[8].
Statistical analysis
Data were presented as median values (interquartile range), and Kolmogorov-Smirnov and Shapiro-Wilk tests were used to assess the normality. The prognostic factors that influenced mortality in univariate analyses were determined using the Mann-Whitney U and χ2 tests. Factors identified as significant in univariate analyses were included in multivariate logistic regression analysis. Receiver operating curve analyses were used to evaluate the mortality predictive performances and sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) measures of each significant variable, with different cutoff values.
All statistical procedures were performed with SPSS 15.0 (SPSS Inc, Chicago, Illinois). A P value < 0.05 was considered to statistically significant.
RESULTS
In this retrospective cohort study, 59 female and 43 male patients were included, with a median age of 56.5 years (17-89 years). AP etiology was biliary stones in 75 and alcohol in 37 of the patients. Local complications observed during follow-up were; 15 pseudocysts, four pancreatic abscesses and six necrotizing pancreatitis. Thirteen patients died after a median of 6 d of hospitalization (1-88 d). The demographic and clinical features of the patients are summarized in Table 1.
Table 1.
Summary of the demographic and clinical characteristics of the patients
| Non-survivors (n = 13) | Survivors (n = 89) | Univariate P | Multivariate P | |
| Age | 52 (41-72) | 77 (73-82) | 0.001 | NS |
| Sex (M/F) | 4/9 | 39/50 | NS | |
| BUN (mg/dL) | 20.56 (12.29-48.6) | 12.66 (10.28-17.76) | 0.001 | NS |
| RDW1 | 15.6% (14%-21%) | 13.3% (12.5%-14.3%) | 0.000 | 0.001 |
| RDW/PLT ratio1 | 0.000 | 0.000 | 0.000 | 0.001 |
| Hematocrit | 45.5 (37.2-49.5) | 41.7 (37.9-45.2) | NS | |
| Platelet count (thousand per μL) | 193 (173-212) | 236 (197-298) | 0.037 | NS |
| Amylase (U/L) | 1223 (820-2860) | 1114 (614-1663) | NS | |
| WBC count (thousand per μL) | 14.4 (12.9-16.0) | 11.4 (8.2-14.3) | 0.018 | NS |
| Albumin (g/L) | 29 (22-31) | 35 (30-38) | 0.001 | NS |
| Total bilirubin (mg/dL) | 2 (1.5-2.7) | 2 (0.90-3.60) | NS | |
| Lactate dehydrogenase (U/L) | 274 (185-331) | 301 (195-481) | NS | |
| Alanine aminotransferase (U/L) | 77 (29-108) | 118 (30-291) | NS | |
| Aspartate aminotransferase (U/L) | 121 (38-186) | 122 (41-253) | NS | |
| Calcium (mg/dL) | 7.84 (7.39-8.49) | 8.75 (8.32-9.13) | 0.001 | NS |
| Serum glucose (mg/dL) | 149 (128-200) | 138 (112-172) | NS | |
| Hospital stay | 3 (2-19) | 6 (3-9) | NS |
Indicates statistical significance. Data are presented as median value (interquartile range). BUN: Blood urea nitrogen; WBC: White blood cell; RDW: Red cell distribution width; PLT: Platelet count; NS: Non significant.
Age, WBC count, platelet count, calcium level, BUN level, RDW, RPR and albumin level were the significant variables in the univariate analysis that influenced the survival rates of AP on admission. These significant variables in univariate analyses were entered into multivariate analyses. In multivariate analyses, the RDW and RPR were predicted as independent and significant variables on admission to predict mortality (Table 1). The RPR obtained on hospital admission was persistently higher among non-survivors than among survivors (P < 0.0001). Median RPR was 0.000087 in the non-survivor group and 0.000058 in the survivor group. We further analyzed the PPV (26.67%, 95%CI: 14.6-41.9) and NPV (96.39%, 95%CI: 89.7-99.2) of high RPR. An RPR with a cutoff value of 0.000067 presented an area under the curve of 0.783 (95%CI: 0.688-0.878) in Receiver operating characteristic curves (Figure 1) and predicted mortality in approximately 80% of the patients (sensitivity, 80.00%, 95%CI: 51.91-95.43; specificity, 70.08%, 95%CI: 61.50-78.97; +LR: 2.74; -LR: 0.28).
Figure 1.
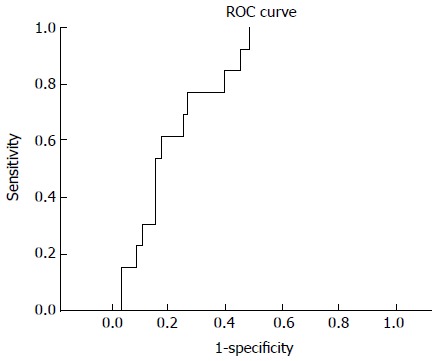
Receiver operating characteristic curve analysis of red cell distribution width to platelet ratio levels. ROC: Receiver operating characteristic.
DISCUSSION
Although a majority of patients with acute pancreatitis have a mild course of the disease, severe forms require more attention because of its high morbidity and mortality. The rate of mortality in severe acute pancreatitis may be as high as 25%[4]. Thus, early diagnosis and management is fundamental to maximize organ support and to prevent irreversible organ dysfunction.
There are several simple predictors to evaluate the severity of the disease. Several scoring systems, such as Ranson, Glasgow and APACHE II, are useful in clinical practice. These scoring systems are expected to provide valuable clues in the assessment of severity. However, a meta-analysis of 110 studies demonstrated that the Ranson score proved insufficient data to predict the severe form of disease with lower positive and negative predictive values[9]. In addition, it has been argued that SIRS on admission to assess the severity of the disease is more accurate than the APACHE II scoring system[10]. In the present study, the performance of the RPR was compared with the Ranson criteria, and found to be more sensitive than this severity score.
To recognize organ dysfunction earlier, some biological markers could be used. In a large observational cohort study, serial BUN measurements of AP patients on admission, and at 24 and 48 h were described as the most predictive marker of in-hospital mortality compared with the serial hemoglobin/hematocrit measurements. The authors also noticed that the extent of BUN increase was a single accurate prognostic marker that was highly associated with the risk of mortality[11]. In another study, hemoconcentration caused by fluid loss in a third space was mentioned as a single reliable severity predictor of AP. Patients presented with a hematocrit level below 44%-47% on admission and patients not responding to resuscitation within 24 h have are at risk of developing necrotizing pancreatitis; therefore, patients with hemoconcentration should be monitored in an intensive care unit with exclusive attention on aggressive fluid resuscitation. Hemoconcentration usually correlates with pancreatic necrosis, and absence of hemoconcentration suggests a benign course of the disease[12,13]. In addition, many other serum markers have been studied to predict the severity of the disease, such as C-reactive protein, urinary trypsinogen activation peptide, procalcitonin, polymorphonuclear elastase, interleukins-1, 6, 8 and 10, antithrombin III (AT III) and platelet activating factor[14]. Each of these markers have some limitations in clinical use. For example, C-reactive protein and procalcitonin are non-specific and require a longer time for the results. A test for urinary trypsinogen activating peptide, the specific marker of protease activation, is not currently available[11].
Inflammatory mediators have a pivotal role in the pathogenesis of AP, and also influence hemostasis, leading to coagulation abnormalities. These range from intravascular thrombosis to disseminated intravascular coagulation. Platelet activating factor is an inflammatory mediator and plays a role in the early stages, where it activates platelets, neutrophils, mast cells and amplifies the production of other cytokines. Another important factor in the coagulation cascade, known as tissue factor, induces coagulopathy and leads to reduced platelet count, fibrinogen and AT III levels[7]. In a study of 27 patients with acute pancreatitis, platelet counts, prothrombin levels were decreased[15,16]. In another study, plasma prekallikrein, AT III and platelet count were reduced during the first week after admission[14].
The RDW, an index of variability of erythrocyte size, has been reported as a predictor of mortality in some conditions, such as cardiac disease, strokes, infections and peripheral artery disease[17]. Hu et al[17] evaluated the RDW in various liver diseases. The RDW was increased in patients and was positively correlated with bilirubin, creatinine levels, prothrombin time, and negatively correlated with platelet count and albumin level. The half life of red blood cells is higher than bilirubin and albumin; therefore, the RDW represented a more stable index. Proinflammatory cytokines of sepsis affect the survival of erythrocytes in circulation, damage the membranes, suppress maturation and lead to larger and newer reticulocytes to enter circulation and increase the RDW. In addition, high oxidative stress can also reduce erythrocyte survival and increase the release of large premature erythrocytes into the circulation. Sadaka et al[18] demonstrated that an RDW on the first day of septic shock was very strongly associated with mortality and morbidity.
As mentioned above, the RDW was increased in some pathological conditions, including acute pancreatitis. Some of the inflammatory cytokines that play a role in the etiology of pancreatitis affect hemostasis and lead to coagulation abnormalities. In a recent study, Chen et al[8] evaluated the RPR to predict hepatic fibrosis stages in patients with chronic hepatitis B. Using these two indices, they stated that with such a simple and non-invasive method, the necessity for liver biopsy would be reduced because they found that this ratio provided the greatest value of liver fibrosis. In a study from our clinic, Şenol et al[19] demonstrated that an increased RDW level was an independent predictor of mortality in AP patients. Based on this study, we asked whether RPR could be useful to assess the mortality of patients with AP. We then evaluated these parameters for our patients on hospital admission.
Early recognition of disease severity and early treatment interventions are very important to reduce the rates of morbidity and mortality. Severe AP needs urgent management, admission to an intensive care unit, optimization of oxygen delivery and maintenance of tissue perfusion. Improved outcome is associated with early restoration of blood volume circulation[20]; therefore, careful monitoring of patients with AP improves survival. Consistent with the correlations mentioned above, we found that the RPR could predict mortality in patients with AP. The CBC is a simple and inexpensive laboratory test that is used routinely in clinical practice and these two indices will help us to assess the mortality rate of this disease.
In our study, we found that if RPR is used in clinical practice with the accompanying assessments, it could be a useful and important marker for predicting the mortality of patients with acute pancreatitis.
COMMENTS
Background
Acute pancreatitis, one of the most frequent gastrointestinal causes of hospital admission, requires early diagnosis and management to maximize organ support and to prevent irreversible organ dysfunction. To evaluate the severity of the disease, several scoring systems and simple predictors can be used.
Research frontiers
Red cell distribution width (RDW) and platelet count are the two parameters in a complete blood count, and using these indices, the RDW to platelet ratio (PRP) can be calculated easily. In a recent study, this ratio was shown to reflect the severity of inflammation in patients with chronic hepatitis B; therefore, authors aimed to evaluate the severity of patients with acute pancreatitis using the RPR on hospital admission.
Innovations and breakthroughs
There are several clinical, laboratory, radiological factors and scoring systems that have been used to evaluate the severity of acute pancreatitis. However, it is sometimes not clinically practicable to use these scoring systems for evaluation. Therefore, an easy and useful method is needed to predict severity. RPR was investigated a useful marker for this purpose.
Applications
The authors found that RPR could predict the mortality in patients with acute pancreatitis. If used in clinical practice with the accompanying assessments, this could be useful as an important marker for predicting the mortality of patients with acute pancreatitis.
Terminology
RPR, an easily calculated index, can predict the mortality of patients with acute pancreatitis.
Peer review
The authors examined the accuracy of RPR for the prediction of in-hospital mortality in acute pancreatitis. The performance of RPR was compared with the Ranson criteria for predicting the mortality rate and found to be more sensitive than this severity score.
Footnotes
Supported by Ankara Numune Education and Research Hospital
P- Reviewer: Braden B, Tandon RK S- Editor: Gou SX L- Editor: Stewart G E- Editor: Wang CH
References
- 1.Stevenson K, Carter CR. Acute pancreatitis. Surgery. 2013;31:295–303. [Google Scholar]
- 2.Al Mofleh IA. Severe acute pancreatitis: pathogenetic aspects and prognostic factors. World J Gastroenterol. 2008;14:675–684. doi: 10.3748/wjg.14.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isenmann R, Rau B, Beger HG. Early severe acute pancreatitis: characteristics of a new subgroup. Pancreas. 2001;22:274–278. doi: 10.1097/00006676-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Pitchumoni CS, Patel NM, Shah P. Factors influencing mortality in acute pancreatitis: can we alter them? J Clin Gastroenterol. 2005;39:798–814. doi: 10.1097/01.mcg.0000177257.87939.00. [DOI] [PubMed] [Google Scholar]
- 5.Lippi G, Filippozzi L, Montagnana M, Salvagno GL, Franchini M, Guidi GC, Targher G. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med. 2009;47:353–357. doi: 10.1515/cclm.2009.066. [DOI] [PubMed] [Google Scholar]
- 6.Spell DW, Jones DV, Harper WF, David Bessman J. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prev. 2004;28:37–42. doi: 10.1016/j.cdp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Kakafika A, Papadopoulos V, Mimidis K, Mikhailidis DP. Coagulation, platelets, and acute pancreatitis. Pancreas. 2007;34:15–20. doi: 10.1097/01.mpa.0000240617.66215.d2. [DOI] [PubMed] [Google Scholar]
- 8.Chen B, Ye B, Zhang J, Ying L, Chen Y. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One. 2013;8:e68780. doi: 10.1371/journal.pone.0068780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bernardinis M, Violi V, Roncoroni L, Boselli AS, Giunta A, Peracchia A. Discriminant power and information content of Ranson’s prognostic signs in acute pancreatitis: a meta-analytic study. Crit Care Med. 1999;27:2272–2283. doi: 10.1097/00003246-199910000-00035. [DOI] [PubMed] [Google Scholar]
- 10.Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Mortele KJ, Banks PA. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7:1247–1251. doi: 10.1016/j.cgh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Wu BU, Johannes RS, Sun X, Conwell DL, Banks PA. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology. 2009;137:129–135. doi: 10.1053/j.gastro.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 12.Baillargeon JD, Orav J, Ramagopal V, Tenner SM, Banks PA. Hemoconcentration as an early risk factor for necrotizing pancreatitis. Am J Gastroenterol. 1998;93:2130–2134. doi: 10.1111/j.1572-0241.1998.00608.x. [DOI] [PubMed] [Google Scholar]
- 13.Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas. 2000;20:367–372. doi: 10.1097/00006676-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lee WS, Huang JF, Chuang WL. Outcome assessment in acute pancreatitis patients. Kaohsiung J Med Sci. 2013;29:469–477. doi: 10.1016/j.kjms.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Lasson A, Ohlsson K. Consumptive coagulopathy, fibrinolysis and protease-antiprotease interactions during acute human pancreatitis. Thromb Res. 1986;41:167–183. doi: 10.1016/0049-3848(86)90227-6. [DOI] [PubMed] [Google Scholar]
- 16.Aasen AO, Kierulf P, Ruud TE, Godal HC, Aune S. Studies on pathological plasma proteolysis in patients with acute pancreatitis. A preliminary report. Acta Chir Scand Suppl. 1982;509:83–87. [PubMed] [Google Scholar]
- 17.Hu Z, Sun Y, Wang Q, Han Z, Huang Y, Liu X, Ding C, Hu C, Qin Q, Deng A. Red blood cell distribution width is a potential prognostic index for liver disease. Clin Chem Lab Med. 2013;51:1403–1408. doi: 10.1515/cclm-2012-0704. [DOI] [PubMed] [Google Scholar]
- 18.Sadaka F, O’Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med. 2013;28:307–313. doi: 10.1177/0885066612452838. [DOI] [PubMed] [Google Scholar]
- 19.Şenol K, Saylam B, Kocaay F, Tez M. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am J Emerg Med. 2013;31:687–689. doi: 10.1016/j.ajem.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, Clain JE, Pearson RK, Levy MJ, Sarr MG. Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology. 2009;9:770–776. doi: 10.1159/000210022. [DOI] [PubMed] [Google Scholar]


