Summary
Background
WHO recommends that Xpert MTB/RIF replaces smear microscopy for initial diagnosis of suspected HIV-associated tuberculosis or multidrug-resistant pulmonary tuberculosis, but no data exist for its use in children. We aimed to assess the accuracy of the test for the diagnosis of pulmonary tuberculosis in children in an area with high tuberculosis and HIV prevalences.
Methods
In this prospective, descriptive study, we enrolled children aged 15 years or younger who had been admitted to one of two hospitals in Cape Town, South Africa, with suspected pulmonary tuberculosis between Feb 19, 2009, and Nov 30, 2010. We compared the diagnostic accuracy of MTB/RIF and concentrated, fluorescent acid-fast smear with a reference standard of liquid culture from two sequential induced sputum specimens (primary analysis).
Results
452 children (median age 19·4 months, IQR 11·1–46·2) had at least one induced sputum specimen; 108 children (24%) had HIV infection. 27 children (6%) had a positive smear result, 70 (16%) had a positive culture result, and 58 (13%) had a positive MTB/RIF test result. With mycobacterial culture as the reference standard, MTB/RIF tests when done on two induced sputum samples detected twice as many cases (75·9%, 95% CI 64·5–87·2) as did smear microscopy (37·9%, 25·1–50·8), detecting all of 22 smear-positive cases and 22 of 36 (61·1%, 44·4–77·8) smear-negative cases. For smear-negative cases, the incremental increase in sensitivity from testing a second specimen was 27·8% for MTB/RIF, compared with 13·8% for culture. The specificity of MTB/RIF was 98·8% (97·6–99·9). MTB/RIF results were available in median 1 day (IQR 0–4) compared with median 12 days (9–17) for culture (p<0·0001).
Interpretation
MTB/RIF testing of two induced sputum specimens is warranted as the first-line diagnostic test for children with suspected pulmonary tuberculosis.
Funding
National Institutes of Health, the National Health Laboratory Service Research Trust, the Medical Research Council of South Africa, and Wellcome Trust.
Introduction
Diagnosis of pulmonary tuberculosis in children has relied predominantly on clinical, radiological, and tuberculin skin-test findings.1 However, clinical diagnosis has low specificity, radiological interpretation is subject to interobserver variability, and the tuberculin skin test is a marker of exposure, not disease.1–3 Microbiological confirmation with identification of drug resistance is increasingly important in the context of an emerging drug-resistant tuberculosis epidemic. Furthermore, confirmation is useful in children with HIV, in whom pill burden, drug interactions, and adherence issues make treatment of HIV and tuberculosis difficult. Use of repeated induced sputum specimens in children is simple, well tolerated, and effective for microbiological confirmation of pulmonary tuberculosis, even in infants.4,5 One induced sputum specimen provides a similar microbiological yield to three gastric lavage specimens in children admitted to hospital with pulmonary tuberculosis.4
Smear microscopy is typically negative in children with culture-confirmed tuberculosis, even when optimised fluorescence microscopy is used on concentrated specimens. 1,6 Mycobacterial culture of induced sputum specimens is therefore needed, but culture can take weeks and is consequently unavailable to inform clinical decisions on initial treatment. A rapid diagnosis of tuberculosis in children is desirable because delayed diagnosis is associated with poor outcome.7
An urgent need therefore exists for a rapid, sensitive, and specific test for tuberculosis and for identification of drug-resistant disease in children. The performance of Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA), an integrated sample processing and nucleic acid amplification test for detection of Mycobacterium tuberculosis and resistance to rifampicin, was assessed in a large multicentre study in adults with suspected tuberculosis.8 One MTB/RIF test accurately detected the presence of tuberculosis in 98·2% of smear-positive and 72·5% of smear-negative tuberculosis cases. Rifampicin resistance was detected with a sensitivity of 99·1% and specificity of 100%. The test results were available within 100 min of testing, much less time than for results from culture.
WHO have endorsed the use of MTB/RIF as the initial diagnostic test in people suspected of having drug-resistant or HIV-associated tuberculosis,9 but no data are available on its accuracy in children. We prospectively assessed use of MTB/RIF compared with culture of repeated induced sputum specimens for the diagnosis of pulmonary tuberculosis in children.
Methods
Participants
We did a prospective study in the general paediatric wards of Red Cross War Memorial Children’s Hospital and Somerset Hospital, Cape Town, South Africa. Children aged 15 years or younger were eligible for enrolment if they had been admitted to hospital between Feb 19, 2009, and Nov 30, 2010, with pulmonary tuberculosis suspected on the basis of having a cough for more than 14 days and one of the following: a household contact infected with tuberculosis within the previous 3 months, loss of weight or failure to gain weight in the previous 3 months, a positive skin test to purified protein derivative (PPD; 2TU, PPD RT23, Staten Serum Institute, Denmark, Copenhagen), or a chest radiograph suggestive of pulmonary tuberculosis. A positive skin test was defined as 5 mm or more of transverse induration in children with HIV infection or 10 mm or more in children without HIV infection. Children were excluded if they had received more than 72 h of tuberculosis treatment or prophylaxis during their hospital admission, if they were not resident in Cape Town and could not be followed up, if informed consent was not obtainable, or if an induced sputum specimen could not be obtained.
Consecutive children meeting the entry criteria were enrolled, except when patients were discharged before recruitment could be completed. Written, informed consent for enrolment in the study was obtained from a parent or legal guardian. The Research Ethics Committee of the University of Cape Town approved the study.
Procedures
A history and physical examination were done at enrolment. Routine clinical investigations included chest radiography, tuberculin skin test, and HIV testing in children whose HIV status was not known (HIV rapid test in all children, followed by a confirmatory PCR for children younger than 18 months or HIV ELISA for children aged 18 months or older). Children with HIV infection were classified according to WHO clinical staging10 from stage 1 to stage 4. We recorded CD4 cell count and HIV viral load for children with HIV; children were classified according to the US Centers for Disease Control and Prevention immunological classification11 as category 1 to category 3 (no immune deficiency to severe immune deficiency). We did CD4 cell counts with the panleucogating method and viral load testing with Abbott RealTime HIV-1 assay (Abbott, Des Plaines, IL, USA). Two reviewers, masked to microbiological and other results, reported all chest radiographs according to a standardised format.
Children were followed up for the duration of their stay in hospital. The decision to start tuberculosis treatment was at the discretion of the medical doctor caring for the child. Follow-up ambulatory visits were done at 3 months for all children to assign a diagnostic category by assessment of response to treatment or recovery in the absence of tuberculosis treatment. Response to treatment or recovery was assessed at follow-up visits by recording symptoms, signs, and weight gain, and by repeating chest radiograph at the completion of treatment. A study paediatrician who had access to all laboratory results made this assessment.
Sputum induction was done after a 2–3 h fast in a dedicated sputum induction room by a trained research nurse, as previously described.4 A second induced sputum specimen was obtained, whenever possible, the following day or a minimum of 4 h after the first specimen. Baseline arterial pulse oximetry was done in all children; monitoring was done throughout the sputum induction procedure and for 30 min thereafter.
Sputum specimens were processed within 2 h of collection in an accredited routine diagnostic microbiology laboratory by trained technicians who used standardised protocols. This laboratory also participated in the previously reported multicountry study of MTB/RIF.8 After decontamination with N-acetyl-L-cysteine and sodium hydroxide (1·0% final concentration), centrifuged sputum deposits were resuspended in 1·5 mL of phosphate buffer. A drop of sediment was used for fluorescent acid-fast smear microscopy. For MTB/RIF testing, 1·4 mL of MTB/RIF sample reagent was added to 0·7 mL of the resuspended sputum pellet and subsequently processed as previously reported.8 Automated liquid culture (mycobacterial growth indicator, BACTEC MGIT, Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) was done with 0·5mL of the resuspended pellet. Cultures were incubated for 6 weeks if negative. Positive cultures were identified by acid-fast staining followed by MTBDRplus testing (Hain Lifesciences, Hehren, Germany)12 to confirm the presence of M tuberculosis and to test for resistance to rifampicin and isoniazid. When clinically indicated, hospital staff collected specimens from additional disease sites—eg, in children with suspected extrapulmonary and pulmonary tuberculosis. Because culture results were not available at the time of MTB/RIF testing, staff doing and recording MTB/RIF tests were unaware of culture results.
If MTB/RIF or MTBDRplus tests identified the presence of rifampicin resistance, the corresponding cultured isolate also underwent testing for phenotypic resistance to rifampicin and isoniazid by automated liquid MGIT culture.
On the basis of clinical and microbiological investigations children were classified as having definite tuberculosis (induced sputum culture positive for M tuberculosis), not tuberculosis (negative tuberculosis cultures and documented resolution of symptoms and signs at 3 month follow-up visit in children who did not receive treatment), or possible tuberculosis (all other children). Children with possible tuberculosis therefore included children not receiving treatment, who had no documented recovery at follow-up, and all children placed on treatment in the absence of microbiological confirmation. An automatic computed algorithm made the assignment to diagnostic category.
Statistical analysis
The primary reference standard was a positive culture for M tuberculosis from an induced sputum specimen. We analysed patients separately with interpretable results from at least one induced sputum specimen and those with results from two induced sputum specimens. For the primary analysis of the specificity of MTB/RIF we included only those children with two interpretable MTB/RIF and culture results, because one negative culture result is likely to miss a substantial proportion of culture-confirmed cases.4 We excluded children with negative induced sputum cultures who had a positive culture from another (extrapulmonary) site from the analysis of sensitivity and specificity, because of the difficulty in interpretation of MTB/RIF findings in this group. The sensitivity, specificity, and predictive values of the assays with 95% CIs were established. Data were analysed with Stata (version 10) and EpiInfo (version 6). Simple descriptive statistics were used to characterise the study population, normally distributed continuous data were summarised by mean and 95% CI, and non-normally distributed continuous data by median and IQR. Categorical data were summarised as proportions with 95% CIs. Statistical tests included two-sample test of proportions, ×2 test, and Wilcoxon rank-sum test. All statistical tests were two-sided at with an α value of 0·05.
Results
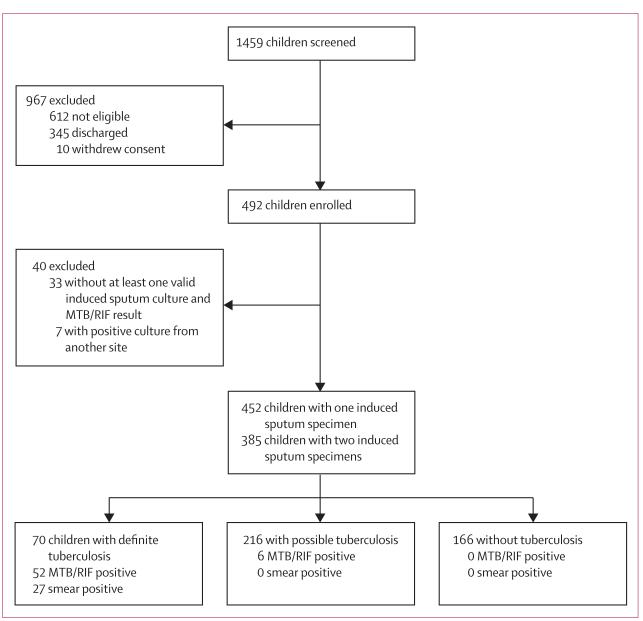
We screened 1459 children, 492 of whom were enrolled (figure). 452 children had MTB/RIF and culture test results from at least one induced sputum specimen and 385 had test results from two specimens. 70 (16%) of 452 children were classified as having definite tuberculosis, 216 (48%) as having possible tuberculosis, and 166 (37%) as not having tuberculosis (table 1). Most children with HIV had moderate or severe immune suppression, and many children had nutritional impairment (table 1).
Figure. Study profile.
Table 1. Baseline characteristics.
| All (n=452) | Definite (n=70) | Possible (n=216) | Not tuberculosis (n=166) | |
|---|---|---|---|---|
| Median (IQR) age (months) | 19·4 (11·1 to 46·2) | 23·7 (15·2 to 59·5) | 17·6 (10·6 to 40·6) | 18·3 (10·9 to 39·9) |
| Sex (male) | 250 (55%) | 39 (56%) | 116 (54%) | 95 (57%) |
| HIV infection | 108 (24%) | 17 (24%) | 55 (26%) | 36 (22%) |
| WHO clinical staging | ||||
| Stage 1 | 15 (14%) | 2 (13%) | 7 (47%) | 6 (40%) |
| Stage 2 | 43 (40%) | 7 (16%) | 20 (47%) | 16 (37%) |
| Stage 3 | 27 (25%) | 4 (15%) | 13 (48%) | 10 (37%) |
| Stage 4 | 23 (21%) | 4 (17%) | 15 (65%) | 4 (17%) |
| HIV CDC immune suppression | ||||
| None | 11 (10%) | 2 (18%) | 7 (64%) | 2 (18%) |
| Moderate | 34 (31%) | 3 (9%) | 16 (47%) | 15 (44%) |
| Severe | 54 (50%) | 9 (17%) | 28 (52%) | 17 (32%) |
| Unknown | 9 (8%) | 3 (33%) | 4 (44%) | 2 (22%) |
| History of tuberculosis | 51 (11%) | 7 (10%) | 23 (11%) | 21 (13%) |
| Radiological changes suggestive of tuberculosis | 274 (64%) | 44 (68%) | 139 (68%) | 91 (57%) |
| Started on tuberculosis treatment | 216 (48%) | 69 (99%) | 147 (68%) | 0 |
| Median (IQR) height for age Z score | −1·50 (−2·5 to −0·5) | −1·58 (−2·78 to −0·68) | −1·69 (−2·7 to −0·71) | −1·28 (−2·1 to −0·2) |
| Median (IQR) weight for age Z score | −1·5 (−2·3 to −0·6) | −1·77 (−2·86 to −0·89) | −1·52 (−2·37 to −0·65) | −1·24 (−2·16 to −0·43) |
| Median (IQR) weight for height Z score | −0·56 (−1·6 to 0·4) | −0·93 (−2·29 to −0·28) | −0·39 (−1·53 to 0·53) | −0·39 (−1·24 to 0·32) |
| Malnutrition (weight for age Z score <−2) | 155 (34·3%) | 31 (44·3%) | 76 (35·2%) | 48 (28·9%) |
| TST positive/TST result known (%) | ||||
| All children | 128/372 (34%) | 39/57 (68%) | 78/176 (44%) | 11/139 (8%) |
| HIV-infected | 13/85 (15%) | 3/11 (27%) | 10/44 (23%) | 0/30 |
| HIV-uninfected | 115/287 (40%) | 36/46 (78%) | 68/132 (52%) | 11/109 (10%) |
Data are n (%) unless otherwise stated. CDC=US Centers for Disease Control and Prevention. TST=tuberculin skin test.
At the time of enrolment, 34 children had previously been given tuberculosis treatment for a median 1 day—no children received treatment for more than 3 days. Almost all children with definite tuberculosis and most children with possible tuberculosis were given tuberculosis treatment on enrolment (table 1), and only one child—who had possible tuberculosis—did not improve on follow-up.
We recorded at least one positive MTB/RIF test in 74·3% of children with definite tuberculosis (table 2), 2·8% of children with possible tuberculosis, and no children who did not have tuberculosis. MTB/RIF detected all 27 smear-positive definite cases and 25 of 43 smear-negative definite cases (table 2).
Table 2. Accuracy of MTB/RIF and smear for case detection with liquid culture as the reference standard.
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Sensitivity (95% CI) for smear-positive definite tuberculosis | Sensitivity (95% CI) for smear-negative definite tuberculosis | |
|---|---|---|---|---|---|---|
| All children with complete results from at least one induced sputum specimen (n=452) | ||||||
| MTB/RIF | ||||||
| All | 52/70, 74·3% (63·8–84·8) | 376/382, 98·4% (97·2–99·7) | 89·7% | 95·4% | 27/27, 100% (87·2−100) | 25/43, 58·1% (42·8–73·5) |
| HIV-infected | 17/17, 100% (80·5–100) | 91/91, 100% (96·0–100) | 100% | 100% | 10/10, 100% (69·2–100) | 7/7, 100% (59·0–100) |
| HIV-uninfected | 35/53, 66·0% (52·9–79·2) | 285/291, 97·9% (96·3–99·6) | 85·4% | 94·1% | 17/17, 100% (80·5–100) | 18/36, 50% (32·8–67·2) |
| Smear microscopy | 27/70, 38·6% (26·9–50·3) | 382/382, 100% (99·0–100) | 100% | 89·9% | .. | .. |
| Children with complete results from two induced sputum specimens (n=385) * | ||||||
| MTB/RIF (first induced sputum specimen) | 34/58, 58·7% (45·6 –71·1) | 325/327, 99·4% (98·5–100) | 94·4% | 93·1% | 22/22, 100% (84·6–100) | 12/36, 33·3% (17·2–49·5) |
| MTB/RIF (both induced sputum specimens) | ||||||
| All | 44/58, 75·9% (64·5–87·2) | 323/327, 98·8% (97·6–99·9) | 91·7% | 95·8% | 22/22, 100% (84·6–100) | 22/36, 61·1% (44·4–77·8) |
| HIV-infected | 14/14, 100% (76·8–100) | 80/80, 100% (95·5–100) | 100% | 100% | 9/9, 100% (66·4–100) | 5/5, 100% (47·8–100) |
| HIV-uninfected | 30/44, 68·2% (53·9–82·5) | 241/245, 98·4% (96·8–99.9) | 88·2% | 94·5% | 13/13, 100% (75·3–100) | 17/31, 54·8% (36·3–73·4) |
| Smear microscopy (two smears) | ||||||
| All | 22/58, 37·9% (25·1–50·8) | 327/327, 100% (98·9–100) | 100% | 90·1% | ||
Data are number correct/number tested, % (95% CI).
Sensitivity and specificity calculated with results from both induced sputum cultures.
When including only the 385 children with both culture and MTB/RIF results from two induced sputum specimens, two MTB/RIF tests detected twice as many definite cases as did smear microscopy, including all smear-positive cases but only 61·1% of smear-negative cases (table 2). The first MTB/RIF test detected all smear-positive cases but only a third of smear-negative cases; the second test increased the sensitivity of MTB/RIF for smear negative tuberculosis by 27·8%. By comparison with MTB/RIF tests, a second test increased the sensitivity of culture by 13·8% (eight of 58 cases).
The proportion of children diagnosed with definite tuberculosis was much the same in HIV-infected (17 of 108, 15·7%) and uninfected children (53 of 344, 15·4%). The sensitivity of MTB/RIF was higher in children with HIV than in children who did not have HIV (p=0·042; table 2). We recorded more smear-positive tuberculosis cases in children with HIV than in children without HIV, although the difference was not significant (p=0·072; table 2). The specificity of MTB/RIF in children with two induced sputum culture results available was 98·8%, with only four of 327 cases not detected (table 2). Two additional children had a positive MTB/RIF test with only one negative induced sputum culture result available. All of these six children were classified as having possible tuberculosis and had a documented response to treatment at 3 month follow-up, and probably had tuberculosis. None of the 166 children in the not tuberculosis group had a positive MTB/RIF result (specificity 100%, 95% CI 97·8–100). The specificity of smear microscopy in this group was also 100%.
When results for rifampicin susceptibility testing were interpretable from both line probe assay and MTB/RIF, MTB/RIF correctly identified all 70 rifampicin-susceptible-cases and two rifampicin-resistant cases on a per-sample analysis (table 3). However, we recorded one case of rifampicin-resistant tuberculosis and four cases of rifampicin-sensitive tuberculosis (by line-probe assay and confirmatory culture-based testing), which were reported as indeterminate by MTB/RIF. Whenever rifampicin resistance was identified by line-probe assay, resistance was confirmed by phenotypic susceptibility testing.
Table 3. Concordance between MTB/RIF and culture-based drug susceptibility testing for identification of resistance to rifampicin (per-sample analysis).
| LPA/DST resistant | LPA/DST sensitive | LPA/DST inconclusive | |
|---|---|---|---|
| MTB/RIF resistant | 2 | 0 | 0 |
| MTB/RIF sensitive | 0 | 70 | 1 |
| MTB/RIF indeterminate | 1 | 4 | 0 |
LPA/DST=culture-based drug susceptibility testing (line probe assay).
Fewer MTB/RIF tests were recorded as failures or invalid (one of 867, 0·001%) than cultures were recorded as contaminated (19 of 867, 2·2%; p<0·0001). MTB/RIF provided faster results, within a median of 1 day (IQR 0–4) compared with 12 days (IQR 9–17) for culture.
Discussion
Two MTB/RIF tests on induced sputum specimens detected three-quarters of culture-confirmed tuberculosis in young children admitted to hospital with suspected pulmonary tuberculosis, with very high specificity. MTB/RIF detected all smear-positive cases, but had a lower sensitivity in smear-negative cases, with two tests detecting about three-fifths of cases. The yield of MTB/RIF was twice that of smear microscopy. This test is widely anticipated to replace smear microscopy in resource-poor settings where HIV co-infection or drug-resistant tuberculosis are common,9 and our results suggest that its use is a major improvement over use of smear microscopy. Although time to detection was not a primary outcome for this study, MTB/RIF results were available within 1 day, which was substantially faster than for culture results.
The sensitivity for smear-negative disease was lower than that previously reported in adults with suspected tuberculosis of 85% for two MTB/RIF tests.8 The incremental increase in sensitivity of a second MTB/RIF test for smear-negative tuberculosis was substantial. WHO recommends one MTB/RIF test for adults with suspected tuberculosis,9 but our findings suggest that a second test should be recommended in children who have a negative first test. The benefit of improved sensitivity of a second test would need to be balanced against the increased costs associated with repeat testing.
Although MTB/RIF was more sensitive than smear microscopy, almost a quarter of children with culture-confirmed tuberculosis were negative on MTB/RIF testing; this proportion was even higher in smear-negative, culture-positive children. Although a positive MTB/RIF test is helpful, a negative test should therefore be interpreted in the context of the child’s clinical and radiological findings. Culture remains an important diagnostic method.
Most children were treated for tuberculosis on the basis of clinical rather than microbiological findings. In view of the fact that an ideal test for tuberculosis in children is likely to have better sensitivity than culture, culture might be an imperfect reference standard. The absence of a highly sensitive reference standard makes assessment of this and other, potentially more sensitive, future technologies challenging. The development of better diagnostic methods for childhood tuberculosis remains a major priority.13
In this study, MTB/RIF detected all cases of definite tuberculosis in children with HIV. However, because the number of children with HIV and culture-proven disease was small, further study is needed to confirm whether sensitivity is increased in these patients.
The specificity of MTB/RIF was high with only six children having a positive MTB/RIF test and at least one negative induced sputum culture. Interpretation of the results for these six children is difficult, because of the poor sensitivity of culture for the diagnosis of childhood tuberculosis.1 However, these children probably had pulmonary tuberculosis because they all had a good clinical response to treatment at follow-up visits. The true specificity of MTB/RIF might be even higher, because none of the 166 children in whom tuberculosis was excluded had a positive MTB/RIF test.
Sputum induction was not regarded as feasible for diagnosis in young children until about 6 years ago. Several studies have now shown the efficacy and safety of sputum induction in infants and young children, particularly in those who have been admitted to hospital.5,14,15 However, data on the use of sputum induction in primary care facilities, where the major burden of childhood tuberculosis occurs, are scarce (panel). If MTB/RIF testing is to be implemented at or close to the point of care, then the capacity for sputum induction in children at health facilities will need to be scaled up. More widespread use of sputum induction in children poses operational challenges, including training of staff, operator time, and the need for precautions to prevent transmission. For ambulatory children, two induced sputum procedures necessitate a second visit or an extended stay at a health facility. In our study, MTB/RIF testing was done at a large diagnostic laboratory in an academic centre; however, the test is robust and can be easily and competently done at microscopy centres.22
MTB/RIF is designed to detect not only the presence of tuberculosis, but also RIF resistance. This study was primarily aimed at assessment of the use of MTB/RIF for tuberculosis detection. Because few rifampicin-resistant cases were detected in this study, we are unable to draw conclusions about the ability of MTB/RIF to detect resistance. Further limitations of this study include the few children with HIV infection and culture-confirmed disease, and the need to split sputum sediment between culture and MTB/RIF testing. MTB/RIF is designed for use directly on sputum specimens, rather than on sputum pellet. However a study in adults showed equivalent performance for both specimen types.8 For this study we chose to split the sputum pellet for tests to obtain a direct comparison of culture and MTB/RIF on the same specimen. In view of the paucibacillary nature of childhood tuberculosis, had we processed one specimen by MTB/RIF and the other by culture, we would have had many discrepant results, which would have been difficult to interpret. Because induced sputum specimens are typically very low volume, and M tuberculosis bacilli are probably lost during the specimen decontamination process, direct testing of induced sputum specimens with MTB/RIF (when the whole specimen volume would be used) might have improved sensitivity compared with testing of the pellet.
This study enrolled children who were admitted to a secondary or tertiary care facility, who could have had more severe disease than those seen in primary care settings. However, these hospitals are major referral centres for children across the Greater Cape Town region. This urban area has one of the highest rates of tuberculosis in South Africa, but has good access to health services.23 The performance of MTB/RIF might differ in children with less severe illness or in other settings where the spectrum of illness is not the same because of differing access to health care; further studies are needed. Further work is also needed to assess MTB/RIF with other, easily obtained specimen types, such as urine and stool.
MTB/RIF is a reliable test for rapid diagnosis of tuberculosis in children when used on induced sputum specimens. Testing two specimens substantially increases diagnostic yield for smear-negative tuberculosis. To maximise the effect of this technology and benefit child health, increased capacity for sputum induction in children at health-care facilities is needed.
Panel: Research in context.
Systematic review
We searched PubMed for studies about the Xpert MTB/RIF test published in English up to June 14, 2011, with the search terms “Xpert” or “MTB/RIF” and “tuberculosis”. We did not identify any systematic reviews. We identified eight studies that assessed the use of MTB/RIF for detection of tuberculosis in respiratory specimens, all of which were in adult patients.8,14,16-21
Interpretation
Most studies, apart from a large multicentre study8 and a multicentre demonstration study,14 were small, with many including testing of archived samples. The sensitivity of MTB/RIF for detection of smear-positive tuberculosis varied between 95% and 100%, with most studies reporting sensitivity of 99–100%. Sensitivity for smear-negative tuberculosis varied substantially, between 47% and 77%. In studies with adequate numbers of rifampicin-resistant samples, sensitivity for detection of rifampicin resistance varied between 94% and 100%, and specificity varied between 98% and 100%. Our study adds to these findings by documenting the accuracy of MTB/RIF on induced sputum specimens for the detection of culture-confirmed tuberculosis in children, a previously unreported population. We showed that the sensitivity of MTB/RIF for smear-negative tuberculosis is lower in children that it is in adults, but is twice as sensitive compared with smear microscopy in children. We also showed that the incremental benefit in testing a second induced sputum specimen is substantial, suggesting that, in children, a second specimen should be tested to optimise sensitivity.
Acknowledgments
This study was funded by the National Institutes of Health, USA (1R01HD058971-01), the National Health Laboratory Service Research Trust, the Medical Research Council of South Africa and the Wellcome Trust (085251/B/08/Z). We thank the National Health Laboratory Service diagnostic microbiology at Groote Schuur Hospital, the children who participated in the study, and the children’s carers.
Footnotes
Conflicts of interest: MPN has received funding from the Foundation for Innovative New Diagnostics (FIND, Geneva, Switzerland) to assess the performance and effect of MTB/RIF. CCB is employed by FIND. FIND is a non-profit organisation that collaborates with industry partners, including Cepheid (the manufacturers of the Xpert MTB/RIF test), on the development, assessment, and demonstration of new diagnostic tests. All other authors declare that they have no conflicts of interest.
References
- 1.Zar HJ, Connell TG, Nicol M. Diagnosis of pulmonary tuberculosis in children: new advances. Expert Rev Anti Infect Ther. 2010;8:277–88. doi: 10.1586/eri.10.9. [DOI] [PubMed] [Google Scholar]
- 2.Hatherill M, Hanslo M, Hawkridge T, et al. Structured approaches for the screening and diagnosis of childhood tuberculosis in a high prevalence region of South Africa. Bull World Health Organ. 2010;88:312–20. doi: 10.2471/BLT.09.062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis. 2002;6:1038–45. [PubMed] [Google Scholar]
- 4.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005;365:130–34. doi: 10.1016/S0140-6736(05)17702-2. [DOI] [PubMed] [Google Scholar]
- 5.Zar HJ, Tannenbaum E, Apolles P, Roux P, Hanslo D, Hussey G. Sputum induction for the diagnosis of pulmonary tuberculosis in infants and young children in an urban setting in South Africa. Arch Dis Child. 2000;82:305–08. doi: 10.1136/adc.82.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marais BJ, Pai M. Recent advances in the diagnosis of childhood tuberculosis. Arch Dis Child. 2007;92:446–52. doi: 10.1136/adc.2006.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viani RM, Lopez G, Chacon-Cruz E, Hubbard P, Spector SA. Poor outcome is associated with delayed tuberculosis diagnosis in HIV-infected children in Baja California, Mexico. Int J Tuberc Lung Dis. 2008;12:411–16. [PubMed] [Google Scholar]
- 8.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO [accessed April 26, 2011];Tuberculosis diagnostics: Automated DNA test. http://www.who.int/tb/features_archive/xpert_factsheet.pdf.
- 10.WHO [accessed July 5, 2011];WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2006 http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf.
- 11.Caldwell MB, Oxtoby MJ, Simonds RJ, Lindegren ML, Rogers MF. Vol. 43. MMWR; Centers for Disease Control and Prevention; 1994. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age; pp. 1–10. [Google Scholar]
- 12.Barnard M, Albert H, Coetzee G, O’Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177:787–92. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 13.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shata AM, Coulter JB, Parry CM, Ching’ani G, Broadhead RL, Hart CA. Sputum induction for the diagnosis of tuberculosis. Arch Dis Child. 1996;74:535–37. doi: 10.1136/adc.74.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiersma HE, Van Aalderen WM, Hoekstra MO. Sputum induction for the diagnosis of pulmonary tuberculosis. Arch Dis Child. 2000;83:276. doi: 10.1136/adc.83.3.276g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armand S, Vanhuls P, Delcroix G, Courcol R, Lemaitre N. Comparison of the Xpert MTB/RIF test with an IS6110-TaqMan real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol. 2011;49:1772–76. doi: 10.1128/JCM.02157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrich SO, Venter A, Kayigire XA, Dawson R, Donald PR, Diacon AH. Xpert MTB/RIF and Genotype MTBDRplus for patient selection for a tuberculosis clinical trial (JCM00138-11) J Clin Microbiol. 2011 doi: 10.1128/JCM.00138-11. Published online June 8. DOI:10.1128/JCM.00138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–37. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marlowe EM, Novak-Weekley SM, Cumpio J, et al. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49:1621–23. doi: 10.1128/JCM.02214-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moure R, Munoz L, Torres M, Santin M, Martin R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol. 2011;49:1137–39. doi: 10.1128/JCM.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theron G, Peter J, van Zyl-Smit R, et al. Evaluation of the Xpert(R) MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201101-0056OC. Published online April 14. DOI:10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 22.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day C, Gray A. Health and related indicators. In: Ijumba P, Padarath A, editors. South African Health Report 2006. Health Systems Trust; Durban: 2006. pp. 369–506. [Google Scholar]