Abstract
Background
Pomegranate juice has a number of positive effects on both human and animal subjects.
Material and methods
Four groups were used in this study. i: Control group, ii: H2O2 group, iii: Pomegranate juice (PJ) group and iv: PJ + H2O2 group. Following the sterilization method for pomegranate juice (10%) and H2O2 (6% v/v), Saccharomyces cerevisiae cultures were added and the cultivation incubated at 35°C for 72 hours. Fatty acids and vitamin concentrations were measured using HPLC and GC and the total protein bands profile were determined by SDS-PAGE.
Results
According to our results statistically significant differences have been determined among the study groups in terms of fatty acids and vitamin (p<0,05). Fatty acid synthesis, vitamin control and cell density increased in groups to which PJ was given in comparison with the control group (p<0,05). Pomegranate juice increased vitamins, fatty acids and total protein expression in Saccharomyces cerevisiae in comparison with the control.
Conclusion
Pomegranate juice has a positive effect on fatty acid, vitamin and protein synthesis by Saccharomyces cerevisiae. Accordingly, we believe that it has significantly decreased oxidative damage thereby making a positive impact on yeast development.
Keywords: Pomegranate juice, SDS-PAGE, fatty acid, vitamin
Introduction
Pomegranate juice (PJ) has received a high level of scientific interest in recent years. The effects of PJ on protein, fatty acid and vitamin synthesis in yeasts have been examined; hence we believe that this will contribute meaningfully and pave way for further scientific studies. Pomegranate (Punica granatum) juice and fruit has a high level of polyphenol content and very effective antioxidant capability. As a result, it has attracted the attention of researchers in recent years in relation with human health. Increase in the significance of PJ daily derives from its phenolic compound rich content. It is very important that the anthocyanin amount in its polyphenol content lies within the range of 0.2–1.0g/100g, in addition, it has been stated that the anthocyanin count in this content is between 136 and 23 mg/100 ml (Mena et al. 2012; Rinaldi et al. 2013).
Pomegranate is commonly grown in Turkey, Iran, India, China, Afghanistan, Russia, and America (Rajasekar et al. 2012). Pomegranate is immensely of high nutritional value, owing to its extraordinary nutritional properties. Polyphenols are the vital class of pomegranate phytochemicals, including flavonoids (anthocyanins) and tannins (ellagitannins and gallotannins). Studies have reported that ingestion of pomegranate fruits has nutritional and health benefits, involving reduced oxidatlueive stress, and it has anticancer and antibacterial regulator effects (Santos et al. 2012). Saccharomyces cerevisiae is important yeast that has been used for various studies (Kagan et al. 2005; Comitini et al. 2011). H2O2 is a reactive oxygen species (ROS) in organism, being permanently produced intracellularly as a product of the metabolism in aerobic organisms and otherwise extracellularly during infection in specialist organisms (Lopes et al. 2004; Cipak et al. 2006; Folmer et al. 2008). The consumption of H2O2 by Saccharomyces cerevisiae is to change the synthesis of fatty acid and total protein in plasma membrane (Matias et al. 2007; Folmer et al. 2008). ROS can oxidize nucleic acid, protein, fat and carbohydrates. For example, the oxidative damage to proteins leads to breakdown of amino acid chains decreasing the biologic activity. Under normal physiological situations, oxidative damages are prevented by antioxidant defenses. On the other hand, under abnormal conditions, antioxidant defense system is insufficient and causes oxidative damage in cell. According to a study it has been observed that the consumption of H2O2 at lower dose, caused deadly stress in Saccharomyces cerevisiae and lead to negative effect on the synthesis of essential proteins (Costa et al. 2002; Spiteller 2006; Skoneczna et al. 2007; Folmer et al. 2008; Zupan et al. 2009; Chondrogianni et al. 2012; Yu et al. 2012). In recent years scientists showed that the components of the plant has essential effects in organism. Thus the studies have been confirmed that poliphenols, tannins, flavonoids have antimicrobial effects. Pomegranate fruit is rich in anthocyanin, catechin, and tannins and are the stimulating effect of the oxidative stress (Randhir et al. 2005; Zoreky 2009; Pala and Toklucu 2011). These biological activities have been especially imputed to phenolic compounds, such as anthocyanins and ellagitannins (Mena et al. 2012). In addition the researchers indicated that pomegranate is a strong antioxidant (Mena et al. 2012; Santos et al. 2012; Pala and Toklucu 2011).
In this study we investigated the effect of PJ on cell growth, total protein, fatty acid and vitamin contents in Saccharomyces cerevisiae treated with hydrogen peroxide. We believe that the results we obtained in this study will make an important contribution to current literature.
Material and Methods
Research Groups and Saccharomyces cerevisiae Growth Conditions
In this study, measurements were carried out on culture samples grown for 2, 4, 6 hours and overnight (72 hours). Whereas in other analyses, the overnight developed culture samples were used. In this study, four groups were used; i) control group, ii) H2O2 group, iii) PJ group and iv) PJ+H2O2 group. Saccharomyces cerevisiae growth media used was YEPD in a working volume of 50 mL (2 g yeast extract, 2 g trypton, 2 g glucose). After sterilization, yeast cultures 10 % (v/v) were added to the four group's media and samples were incubated for 72 h at 35°C. At the end of the incubation period, samples were centrifuged (5000 rpm, 4°C for 5 min). The centrifuged pellets were counted and detached for vitamin and fatty acid analysis (Dilsiz et al. 1997; Ozsahin et al. 2009).
Preparation of Extract
Pomegranate fruit (From Alacakaya county of Elazığ city) was crushed in water and the juice was sterilized for further adding of Saccharomyces cerevisiae cultures (10%,v/v).
Application of H2O2 Chemical
H2O2 was supplemented in H2O2 and PJ+H2O2 groups with 6% (v/v) ratio.
Extraction of Fatty Acids and Analyses
Cell pellets were homogenized with Hexaneisopropanol mixture. This solution was centrifuged at 5000 rpm for 5 min at 4°C. The supernatant (containing hexane phase) was used for vitamins A, D, E, K and fatty acid analysis (Ozsahin et al. 2009).
Analysis of Fatty Acid Methyl Esters
Methyl esters were analyzed by gas chromatography (SHIMADZU GC 17 Ver. 3, Japan). For this, a 25 m long Machery-Nagel (Germany) capillary colon with an inner diameter of 0.25 µm and a thickness of 25 micron film was used. During the analysis, the colon temperature was kept at 120–220°C, with an injection temperature of 240°C and detector temperature of 280°C. The colon temperature program was adjusted from 120 to 220°C and the temperature increase was determined to be 5°C minG1 until 200°C and 4°C minG1 from 200–220°C. Temperature was kept at 220°C for 8 min and the total continuance was set as 35 min and nitrogen gas was used as the carrier gas. After this process, the fatty acid methyl esters mixtures of the samples were analyzed
Analysis of Vitamins A, D, E, K and Sterol
A 5 mL of the supernatant was added to tubes with caps (25 mL) and 5% 1ml KOH solution was added. This solution was vortexed and kept at 85°C for 15 min. After that, the tubes were chilled to room temperature and 5 mL of natural water was added and mixed. The Hexane phase was vaporized by nitrogen flow. It was dispersed in 1 ml acetonitril/methanol mixture and then was taken to auto sampler vials and analyzed. The analyses were made by HPLC device. (Shimadzu, Kyoto Japan) (Ozsahin et al. 2009).
Analysis of Total Protein by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
After the protein extraction from cell cultures, protein samples were analyzed by SDS-PAGE. After SDS-PAGE, the gel image was evaluated for total protein bands among the groups studied (Laemmli, 1970).
Analysis of Cell density
Culture samples were collected at 2, 4, 6 hours and overnight (72 hours). The measurement has been carried out using a spectrophotometer at 600nm (OD600) (Lopes et al. 2004).
Statistical Analysis
For statistical analysis, SPSS 20.0 software was used. The comparison between experimental groups and the control was made using One way ANOVA and LSD tests. Statistically significant differences among the groups have been stated as p<0.05 and the statistically nonsignificant differences have been stated as p>0.05. Standard deviations were indicated as mean ±SD. Each assay was repeated 3 times.
Results and Discussion
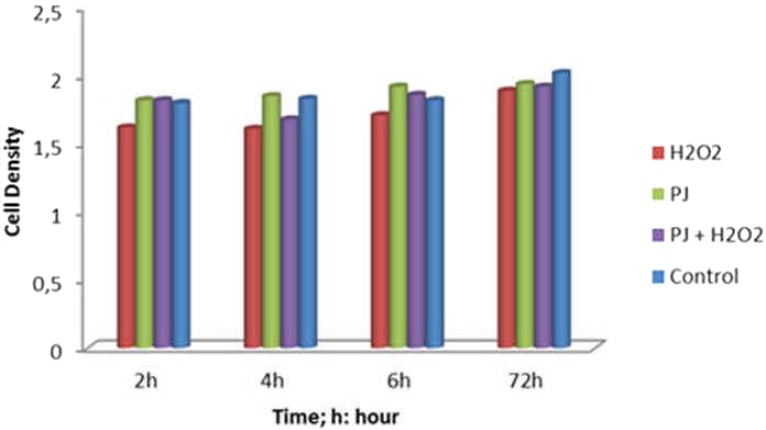
Pomegranate juice added to the yeast culture medium increased cell density in comparison to the control at p<0.05 (Table 1, Figure 1) and vitamins (Table 2) and fatty acid synthesis (Table 3) increased significantly in comparison with the group to which no PJ was added at p<0.05 (Examining the SDS-PAGE protein band profiles, a denser banding is observed in pomegranate added groups in comparison with those to which no PJ was added. In addition, banding has been observed to be denser in the group cultured at 35°C (Figure 2).
Table 1.
Cell density of cultivations at 35°C and PJ addition (10%,v/v).
| 2h | 4h | 6h | 72h | |
| Control | 1,80±0,01a | 1,83±0,03a | 1,82±0,03a | 2,02±0,01a |
| H2O2 | 1,62±0,02b | 1,61±0,01b | 1,71±0,02b | 1,89±0,00b |
| PJ | 1,82±0,01a | 1,85±0,00a | 1,92±0,00c | 1,94±0,05c |
| PJ + H2O2 | 1,82±0,02a | 1,68±0,00c | 1,86±0,00d | 1,92±0,00d |
Means of cell density sharing the same superscript are not significantly different from each other (Anova Post Hoc LSD Test, P<0.05)
Figure 1.
Cell density of Saccharomyces cerevisiae at spectrophotometer OD600
Table 2.
% Fatty acid profiles of cultivations at 35 °C and PJ addition (10%,v/v)
| Control | PJ | H2O2 | PJ + H2O2 | |
| Myristic acid (14:0) | 30,42±0,02a | 29,01±0,00b | 28,01±0,00c | 33,21±0,00d |
| Palmitic acid (16:0) |
218,71±0,01a | 245,01±0,00b | 183,61±0,00c | 269,72±0,00d |
| Palmitoleic acid (16:1) |
38,04±0,00a | 44,18±0,02b | 40,77±0,00c | 50,46±0,00d |
| Stearic acid (18:0) | 114,54±0,47a | 161,17±0,00b | 105,93±0,00c | 143,56±0,58d |
| Monoenoleic acid (18:1) |
306,11±0,11a | 184,41±0,01b | 97,30±0,00c | 172,35±0,31d |
| Dienlinoleic acid (18:2 n6c) |
32,59±0,00a | 67,80±0,58b | 21,75±0,00c | 74,88±0,00d |
| Trienlinoleic acid (18:3) |
99,48±0,00a | 92,01±0,06b | 83,67±0,00c | 105,08±0,08d |
Means of fatty acids amount sharing the same superscript are not significantly different from each other (Anova Post Hoc LSD Test, P<0.05)
Table 3.
% Vitamin profiles of cultivations at 35°C and PJ addition (10%,v/v)
| Control | PJ | H2O2 | PJ + H2O2 | |
| K vit. (1) | 0,34±0,00a | 3,12±0,00b | 0,95±0,02c | 3,52±0,00d |
| 6K vit. (2) | 0,25±0,00a | 0,32±0,00b | 0,18±0,14c | 0,26±0,00a |
| D vit. (2) | 0,58±0,01a | 1,04±0,00b | 1,58±0,00c | 0,96±0,00d |
| α-Tocopherol | 13,11±0,00a | 11,59±0,00b | 15,84±0,01c | 17,16±0,00d |
| γ-Tocopherol | 0,59±0,00a | 0,16±0,00b | 0,09±0,00c | 0,08±0,00d |
| Ergosterol | 9,36±0,00a | 8,15±0,00b | 10,30±0,08c | 10,74±0,01d |
| Cholesterol | 11,84±0,00a | 3,27±0,00b | 28,13±0,00c | 3,68±0,02d |
| Stigmasterol | 54,51±0,00a | 49,44±0,00b | 74,57±0,00c | 55,81±0,00d |
| β-sitosterol | 2,05±0,01a | 23,91±0,01b | 0,93±0,00c | 29,48±0,00d |
Means of vitamin amounts sharing the same superscript are not significantly different from each other (Anova Post Hoc LSD Test, P<0.05)
Figure 2.
SDS-PAGE total protein bands profiles for cultivaions at 35 °C and PJ addition (10%, v/v). Lane 1, Control; lane 2, PJ; lane 3, H2O2; lane 4, PJ+ H2O.
According to our findings, there are a set of alterations in membrane structure in H2O2 groups in comparison with control group. Similar results were obtained by Folmer et al. (2008). Santos et al. (2012) indicated that in groups with added PJ in comparison with the control, cell population survival density was increased. According to Yan et al. (2011), H2O2 has negative effect on β-caroten synthesis in yeast (Saccharomyces cerevisiae) that has preventive effect on oxidative damage. In our results, we observed that in PJ added groups, when compared with control group produced significant increases and so in PJ added groups, in comparison with in added H2O2 group that there are essential increases in cell density (p<0.05) (Table 1). In addition, when cell densities were examined, it has been observed that PJ added groups had higher cell density in comparison with the control (Table 1).
The fatty acid profile from Saccharomyces cerevisiae in this study was: 14:0, 16:0, 16:1, 18:0, 18:1 and 18:2 n-6c, 18:3 with some changes in the composition according to changes in the culture media. We felt that the primary reason in the synthesis of these fatty acids was due to the enzymes that had a role in the synthesis transcribed in Saccharomyces cerevisiae. In various studies, it was discovered that the enzymes, which made fatty acid synthesis in Saccharomyces cerevisiae and other yeasts species were affected by many components in the culture (Ozsahin et al. 2009). In addition, when we looked at the presence of different fatty acid synthesis in comparison with those in H2O2 group, we observed that in PJ added group, the fatty acid synthesis was considerably increased (p<0.05) (Table 2). According to Yan et al. (2011), adding H2O2 can significantly induce the β-carotene production and given H2O2 amount more and more, in culture media measured β-carotene amount declined in parallel with reported that the catalase enzyme activity was decreased. In accordance with Guvenc et al. (2010), different sugar source in nutrition media increased the fatty acid synthesis. Therefore, these circumstances has contributed to the thinking that H2O2 has negative effect on fatty acid synthesis of genes (Kajiwara et al. 1997; Cipak et al. 2006; Ozsahin et al. 2009). In our study, we discovered that vitamin synthesis was made from Saccharomyces cerevisiae: the vitamin rates were α-tocoferol, “γ-tocoferol, β-sitosterol cholesterol, stigmasterol ergesterol, D, and K 1,2. The rates of these vitamins in changing media were identified by computation with statistical methods (Perrone et al. 2008; Ozsahin et al. 2009). Once again, we saw that our results of vitamin contents, in added PJ group was more in comparison with control group (p<0.05) (Table 3).
We saw that in added PJ group; SDS-PAGE total protein band profiles had more intensity than in the H2O2 group (Figure 2). Looking at the effect mechanism of the findings obtained in this study, we believe that significant results have been obtained. Further studies should be carried out and common and effective mechanism of PJ could be tested on different living things. We believe that our study has important contributions to scientific literature.
Conclusion
Pomegranate juice has positive effect on fatty acid, vitamin and protein synthesis by Saccharomyces cerevisiae. Accordingly, we believe that it has significantly decreased oxidative damage thereby making a positive impact on yeast development. In accordance with our results, we believe that PJ might have a similar impact on human health when we consider its effect on yeasts. In addition, further studies with PJ tested on different living things can be carried out and thus PJ consumption can be encouraged in accordance with the obtained results. In line with these conclusions, we believe that people can live a healthy life by consuming PJ regularly.
Acknowledgement
Thanks to Professor Dr. Okkes Yilmaz for his contribution.
References
- 1.Chondrogianni N, Petropoulos I, Grimm S, Georgila K, Catalgol B, Friguet B, Grune T, Gonos ES. Protein damage, repair and proteolysis. Mol Aspects Med. 2014;35:1–71. doi: 10.1016/j.mam.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Cipak A, Hasslacher M, Tehlivets O, Collinson EJ, Zivkovic M. Saccharomyces cerevisiae strain expressing a plant fatty acid desaturase produces polyunsaturated fatty acids and is susceptible to oxidative stress induced by lipid peroxidation. Free Radical Bio Med. 2006;40:897–906. doi: 10.1016/j.freeradbiomed.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Costa VMV, Amorim MA, Quintanilha A, Ferreira PM. Hydrogen peroxide-induced carbonylation of key metabolic enzymes in saccharomyces cerevisiae: the involvement of the oxidative stress response regulators yap1 and skn7. Free Radical Bio Med. 2002;33:1507–1515. doi: 10.1016/s0891-5849(02)01086-9. [DOI] [PubMed] [Google Scholar]
- 5.Dilsiz N, Çelik S, Yılmaz Ö, Digrak M. The effects of selenium, vitamin e and their combination on the composition of fatty acids and proteins in Saccharomyces cerevisiae. Cell Biochem Funct. 1997;15:265–269. doi: 10.1002/(SICI)1099-0844(199712)15:4<265::AID-CBF750>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Folmer V, Pedroso N, Matias AC, Lopes SCDN, Antunes F. H2O2 induces rapid biophysical and permeability changes in the plasma membrane of Saccharomyces cerevisiae. Biochim Biophys Acta. 2008;1778:1141–1147. doi: 10.1016/j.bbamem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Güvenc M, Yılmaz O, Ozsahin AD, Aslan A, Tuzcu M. The growth of Saccharomyces cerevisiae in the different containing grape juices environment affects fatty acid biosynthesis and activities of responsible enzymes. Turkish Journal of Science & Technology. 2010;5:43–51. [Google Scholar]
- 8.Kagan IA, Michel A, Prause A, Scheffler BE, Pace P. Gene transcription profiles of Saccharomyces cerevisiae after treatment with plant protection fungicides that inhibit ergosterol biosynthesis. Pestic Biochem Phys. 2005;82:133–153. [Google Scholar]
- 9.Kajiwara Y, Ogawa K, Takashita H, Omori T, Shimoda M. Intracellular fatty acid formation and alcohol acetyl transferase gene expression in brewing yeast (Saccharomyces cerevisiae) treated with heat shock. Journal of Fermentation and Bioengineering. 1997;84:594–598. [Google Scholar]
- 10.Laemmli UK. leavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Lopes AS, Antunes F, Cyrne L, Marinhoa HS. Decreased cellular permeability to H2O2 protects Saccharomyces cerevisiae cells in stationary phase against oxidative stress. FEBS Letters. 2004;578:152–156. doi: 10.1016/j.febslet.2004.10.090. [DOI] [PubMed] [Google Scholar]
- 12.Matias AC, Pedroso N, Teodoro N, Marinho HS, Antunes F. Down-regulation of fatty acid synthase increases the resistance of Saccharomyces cerevisiae cells to H2O2. Free Radical Bio Med. 2007;43:1458–1465. doi: 10.1016/j.freeradbiomed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Mena P, Vilaplana AG, Marti N, Viguer CG. Pomegranate varietal wines: Phytochemical composition and quality parameters. Food Chem. 2012;133:108–115. [Google Scholar]
- 14.Ozsahin AD, Guvenc M, Yilmaz O, Aslan A, Tuzcu M. The effects of different sugar sources on fatty acid biosynthesis in the Saccharomyces cerevisiae cell culture. J Anim Vet Adv. 2009;8:424–429. [Google Scholar]
- 15.Pala ÇU, Toklucu AK. Effect of UV-C light on anthocyanin content and other quality parameters of pomegranate juice. J Food Compos Anal. 2011;24:790–795. [Google Scholar]
- 16.Perrone GG, Tan SX, Dawes IW. Reactive oxygen species and yeast apoptosis. Biochim Biophys Acta. 2008;1783:1354–1368. doi: 10.1016/j.bbamcr.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Rajasekar D, Akoh CC, Martin,o KG, MacLean DD. Physico-chemical characteristics of juice extracted by blender and mechanical press from pomegranate cultivars grown in Georgia. Food Chem. 2012;133:1383–1393. [Google Scholar]
- 18.Randhir R, Vattem D, Shetty K. Antioxidant enzyme response studies in H2O2-stressed porcine muscle tissue following treatment with oregano phenolic extracts. Process Biochem. 2005;40:2123–2134. [Google Scholar]
- 19.Rinaldi M, Caligiani A, Borgese R, Palla G, Barbanti D. The effect of fruit processing and enzymatic treatments on pomegranate juice composition, antioxidant activity and polyphenols content. Lwt-Food Sci Technol. 2013;53:353–359. [Google Scholar]
- 20.Santos EV, Martinez AO, Munizaga GT, Reyes JE, Won MP. Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innov Food Sci Emerg. 2012;13:13–22. [Google Scholar]
- 21.Skoneczna A, Micialkiewicz A, Skoneczny M. Saccharomyces cerevisiae Hsp31p, a stress response protein conferring protection against reactive oxygen species. Free Radical Bio Med. 2007;42:1409–1420. doi: 10.1016/j.freeradbiomed.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 22.Spiteller G. Peroxyl radicals: Inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radical Bio Med. 2006;41:362–387. doi: 10.1016/j.freeradbiomed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Yan Gl, Liang HY, Wang ZQ, Yang XF, Liu D. Important role of catalase in the production of β-carotene by recombinant Saccharomyces cerevisiae under H2O2 Stress. Curr Microbiol. 2011;62:1056–1061. doi: 10.1007/s00284-010-9826-8. [DOI] [PubMed] [Google Scholar]
- 24.Yu S, Zhang Xen, Chen G, Liu W. Compromised cellular responses to DNA damage accelerate chronological aging by incurring cell wall fragility in Saccharomyces cerevisiae. Mol Biol Rep. 2012;39:3573–3583. doi: 10.1007/s11033-011-1131-5. [DOI] [PubMed] [Google Scholar]
- 25.Zoreky NSA. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int J Food Microbiol. 2009;134:244–248. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Zupan J, Mavri J, Raspor P. Quantitative cell wall protein profiling of invasive and non-invasive Saccharomyces cerevisiae strains. J Microbiol Meth. 2009;79:260–265. doi: 10.1016/j.mimet.2009.09.003. [DOI] [PubMed] [Google Scholar]