Abstract
Background
Currently, there has been extensive research interest for inorganic nanocrystals such as calcium phosphate, iron oxide, silicone, carbon nanotube and layered double hydroxide as a drug delivery system especially in cancer therapy. However, toxicological screening of such particles is paramount importance before use as delivery carrier. In this study we examine the biocompatibility of CaCO3 nanocrystal on NIH 3T3 cell line.
Material and Methods
Transmission and field emission scanning electron microscopy (TEM and FESEM) were used for the characterisation of CaCO3 nanocrystals. Cytotoxicity and genotoxic effect of calcium carbonate nanocrystals in cultured mouse embryonic fibroblast NIH 3T3 cell line using various bioassays including MTT, and Neutral red/Trypan blue double-staining assays. LDH, BrdU and reactive oxygen species were used for toxicity analysis. Cellular morphology was examined by scanning electron microscopy (SEM) and confocal fluorescence microscope.
Results
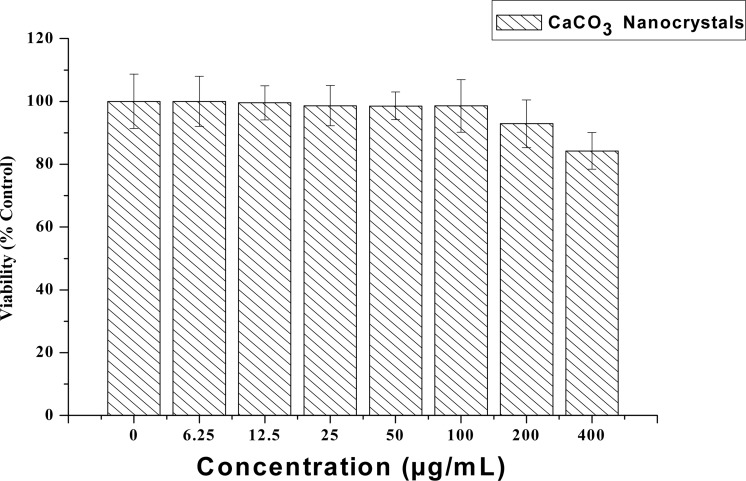
The outcome of the analyses revealed a clear rod-shaped aragonite polymorph of calcium carbonate nanocrystal. The analysed cytotoxic and genotoxicity of CaCO3 nanocrystal on NIH 3T3 cells using different bioassays revealed no significance differences as compared to control. A slight decrease in cell viability was noticed when the cells were exposed to higher concentrations of 200 to 400 µg/ml, while increase in ROS generation and LDH released at 200 and 400 µg/ml was observed.
Conclusions
The study has shown that CaCO3 nanocrystal is biocompatible and non toxic to NIH 3T3 fibroblast cells. The analysed results offer a promising potential of CaCO3 nanocrystal for the development of intracellular drugs, genes and other macromolecule delivery systems.
Keywords: Biocompatibility, Calcium carbonate; nanocrystals, drugs and Cockle shells
Introduction
Nanoparticles consist of larger classes of inorganic nanomaterials, different methods and techniques were employed for the synthesis of different classes of nanomaterials (Rao et al., 2012). There has been a considerable exertion for nanotechnology to selectively produce nanoparticles with a wide range of shapes, size distribution and functional surface modifications (Hernández-Ortiz et al., 2012). Therefore, development of nanoparticles for drug delivery systems, medical imaging and other biomedical relevance are currently undergoing a dramatic expansion (Bengt et al., 2010). However, as the range of nanoparticle types and applications increases in the aforementioned areas, it is important to study the potential toxicities of these novel materials and the properties driving such toxic responses must also be understood (Bengt et al., 2010).
Many organic materials were investigated for their ability to serve as carriers in drug delivery, but these organic materials (such as liposome, polymers and micelles) still create problems, such as swelling, low chemical stability, susceptibility to microbial attack and inadequate control over the drug release rate (Ying-Jie, 2008). These problems led many researchers to investigate inorganic materials hoping to overcome these problems as several of these inorganic materials are non-toxic, biocompatible, hydrophilic and chemically stable (Wang, 2010). Researchers had demonstrated that inorganic materials such as calcium phosphate, iron oxide, silicone and colloidal gold particles. Layered hydroxide (LDH) and carbon nanotubes showed potential for the development of drug delivery systems, especially in controlled release, gene therapy, and can be engineered into various forms, such as porous inorganic particles, which have the ability to control drug loading and releasing rate with no change in porosity or swelling under different pH conditions (Wang, 2010).
Calcium carbonate is a novel inorganic material with a feasible prospect in biomedical applications, and also acting as a carrier for control delivery systems due to its biodegradability, biocompatibility and porous nature (Vijaya et al., 2013). CaCO3 has tunable physicochemical properties, surface chemistry (shape and size) with an easy method of production at a large scale (Rodríguez et al., 2013). Another advantage of CaCO3 is pH sensitivity; as the pH decreases the solubility increases exponentially (Ming-Jium et al., 2011). These unique properties of calcium carbonate make it a potential and useful carrier for intracellular drug delivery systems.
The current study is designed to specifically synthesize aragonite polymorphs of calcium carbonate nanocrystals derived from cockle shells (Anadara granosa) in which the primary composition is approximately 95–99% calcium carbonate (Kamba et al., 2013). To the best of our knowledge, no report or research has been done using the same material in this regard. Herein, the research focus on the use of calcium carbonate as the main sources of calcium and carbonate ions, which is in contrast to the most of the reported literature where the researchers described the use of chemical reactions such as co-precipitation or double-decomposition methods whereby salts of calcium and carbonate ions such as reactions between CaCl2 and Na2CO3, CaCl2 and NH4CO3 or Ca(NO2)3 and Na2CO3, act as the precursor for the synthesis of calcium carbonate nanocrystals (Babou-Kammoe et al.,2012). An assessment of biocompatibility of the synthesized calcium carbonate nanoparticles is necessary before they can be used as a delivery agent. One of the criteria for this assessment is that the particles should be non-toxic or less toxic when attached to cells, which is the first step in the cellular interaction and leads to the next cellular response.
Materials and Methods
Synthesis and Characterization of Calcium Carbonate Nanocrystals
Synthesis of calcium carbonate nanocrystals was carried out in oil-in-water (O/W) microemulsions using a high-pressure homogeniser (HPH) as described by Kamba et al. (2013). In this technique, the particle sizes were reduced after leaving the homogenising gap by cavitations, particle collisions, and shear forces.
Characterisation of Calcium Carbonate Nanocrystals
Particle size and morphology were characterised by transmission electron microscopy (TEM, Hitachi H-7100) and field emission scanning electron microscopy (FESEM, JOEL 7600F) with energy-dispersive X-ray spectroscopy (EDX) as described previously (Kamba et al., 2013).
Cell Culture
Mouse embryonic fibroblast NIH 3T3 cell-line cells were cultured in 75-ml flasks in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL) supplemented with 10% foetal bovine serum (FBS), containing antibiotics (100 µg/ml each of penicillin and streptomycin) at 37°C in a humidified atmosphere containing 5% CO2.
Evaluation of Cells Viability
The NIH 3T3 fibroblast cell lines were seeded in 96-well plates for 24 hrs; the cells were treated with different concentrations of the nanocrystal suspension (0 to 400 µg/ml) for 24, 48 and 72 hrs. After the exposure was completed, the media was removed and replaced with a fresh media containing 20 µl of MTT reagents (Sigma Aldrich) at 37°C for 4 hrs. Then, this culture medium was removed, and dimethylsulfoxide (DMSO) was added to 96-well plates. Optical density of the solution was measured at 570 nm with a microplate reader to release the coloured product into the solution.
Genotoxicity (BrdU) Assay
Cellular genotoxicity was analysed based on the incorporation of BrdU (5-bromo-2-deoxyuridine) into the synthesized DNA during cell proliferation. NIH 3T3 cells were seeded in 96-well plates at a density of 1×105 cells per well and incubated for 24 hrs, the cells were washed with PBS and treated with CaCO3 nanocrystals suspension, control was treated with 0.1% DMSO, then the cells was subjected to a 5-bromo-2-deoxyuridine labelling assay according to the manufacturer's protocols (Roche Diagnostics GmbH, Mannheim, Germany). The absorbance was measured at 550 nm using a microplate reader and the data is presented as mean ± S.D.
Neutral Red/Trypan Blue Double-staining Assay
Cells were seeded into 6-well plate at a seeding density of 2×104 and incubated for 24 hours at 37°C, it was supplemented with 5% CO2. The cells were treated as previously described in MTT above. The treated cells were washed with PBS three times and trypsinised. Fifty microlitres of a neutral red/trypan blue mixture was added to the cell suspension in a 2 ml eppendorf tube and incubated at room temperature for 5 min. The dead and viable cells were microscopically counted using a haemocytometer chamber (Invitrogen, Ltd., Renfrew, UK).
Cell Membrane Integrity Analysis (LDH release)
The cells were seeded in 96-well plate and incubated for 24 hrs. The medium was removed and replaced with CaCO3 nanocrystals at concentrations of 0 to 400 µg/mL and incubated for 24, 48 and 72 hrs. Lysis buffer (2 µl) was added to the positive control wells, and the plates were centrifuged at 1500 rpm for 10 min at 37°C. Membrane integrity assay reagent (50 µL) was added to each well and re-incubated for 10 min at 37°C in the dark. Stop reagent (30 µl of 1N HCl) was added to each well. The sample fluorescence was measured at 560 nm (excitation) and 590 nm (emission) using a microplate reader. The percentage of LDH released was calculated with respect to the positive control where the lysed cells were assumed to have 100% lactate dehydrogenase (LDH) release.
Generation of Reactive Oxygen Species (ROS)
ROS generation is an indication of cellular oxidative stress. When 2, 7-dichlorofluorescein diacetate (DCFH-DA) passively enter the cells, it will react with ROS and be oxidized to dichlorofluorescein (DCF). Briefly, ROS generation was measured by culturing cells to >80% confluence, and then the cells was seeded into 24-well plates and treated with different concentrations of nanocrystals for 24, 48 and 72 hrs. Cells were washed twice with PBS and then incubated with 10 mM DCFH-DA for 30 min at 37°C. After the DCFH-DA incubation, then washed with PBS again and lysed with alkaline solution, 100 µl of the supernatant was transferred to 96-well plates and the fluorescence of DCF was measured using a microplate ELISA reader (Bio-Rad) at 520 nm.
Morphological Observation
Fluorescence microscope observation is one of the reliable methods for determining and quantifying viable and dead cells using different cellular stains.
Acridine Orange (AO) and AO/Propidium Iodide Double Staining
The cells were seeded in 6-well plates for 1 day and CaCO3 nanocrystals were administered for a period of 24 hrs. The medium containing treatment was removed, and the cells were washed twice with PBS. The NIH 3T3 cells were double stained with Acridine orange dye (1 µg/ml)/ propidium iodide (0.5 µg/mL) for 5 minutes, and the stained cells were examined using confocal fluorescence microscopy.
Effect of calcium carbonate nanocrystals on NIH 3T3 fibroblast cells by Scanning Electron Microscopy Examination
NIH 3T3 mouse fibroblasts were placed in 24-well plates at a seeding density of 2×105 cells/mL, after ∼ 80% of cells confluence, suspension of calcium carbonate nanocrystals was treated with UV-light before the administration of suspension to the cells, treated cells were incubated in CO2 at 37°C for 24 hours. Cells were washed twice with PBS (pH 7.4) immediately after the incubation period and centrifuge for 10 min at 3000 rpm. The pellets were fixed in 4% (v/v) glutaraldehyde in 0.1 M coccadylate buffer (pH 7.4) for 4 hours at 4°C. The fixed cells were washed in three changes of sodium coccadylate buffer for 10 minutes each, postfixed in 1% osmium tetraoxide at 4°C. The specimens were then washed in three changes of sodium coccadylate buffer (pH 7.4) for 10 minutes each, dehydrated in ascending grades of acetone (35%, 50%, 75%, 95% and 100%), and brought to critical point drying by the critical point drier (CPD 030, Bal-TEC, Switzerland) for 30 minutes. The cells were affixed to a metal SEM stub and sputter coated in gold by using a SEM coating unit (E5100 Polaron, UK). The coated specimens were viewed under scanning electron microscopy (JOEL 64000, Japan) at accelerating voltage of 15–25 KV.
Statistical Analysis
All statistical analyses were performed using Minitab Statistical Software (Minitab Inc., State College, PA, USA) and Origin 8. Treatment effects were determined using one-way analysis of variance followed by Tukey's post-hoc analysis. A value of P < 0.05 was considered significant unless indicated otherwise.
Results and Discussion
Investigation of nanoparticle cytotoxicity is of paramount importance in various biological fields, including drug discovery, toxicology and ecotoxicology. In vitro cell viability (cytotoxicity) assays are used to avoid any unnecessary animal testing, waste of longer time for animal observation after treatment and higher costs materials compared to In vitro assays, which is faster and cheaper to conduct. Selection of the best assay format for meeting a particular need and understanding the endpoint of each assay; what is measured and how the measurement correlates with cell viability are the central key for any analysis.
Field Emission Scanning Electron Microscope and Transmission Electron Microscope Characterisation
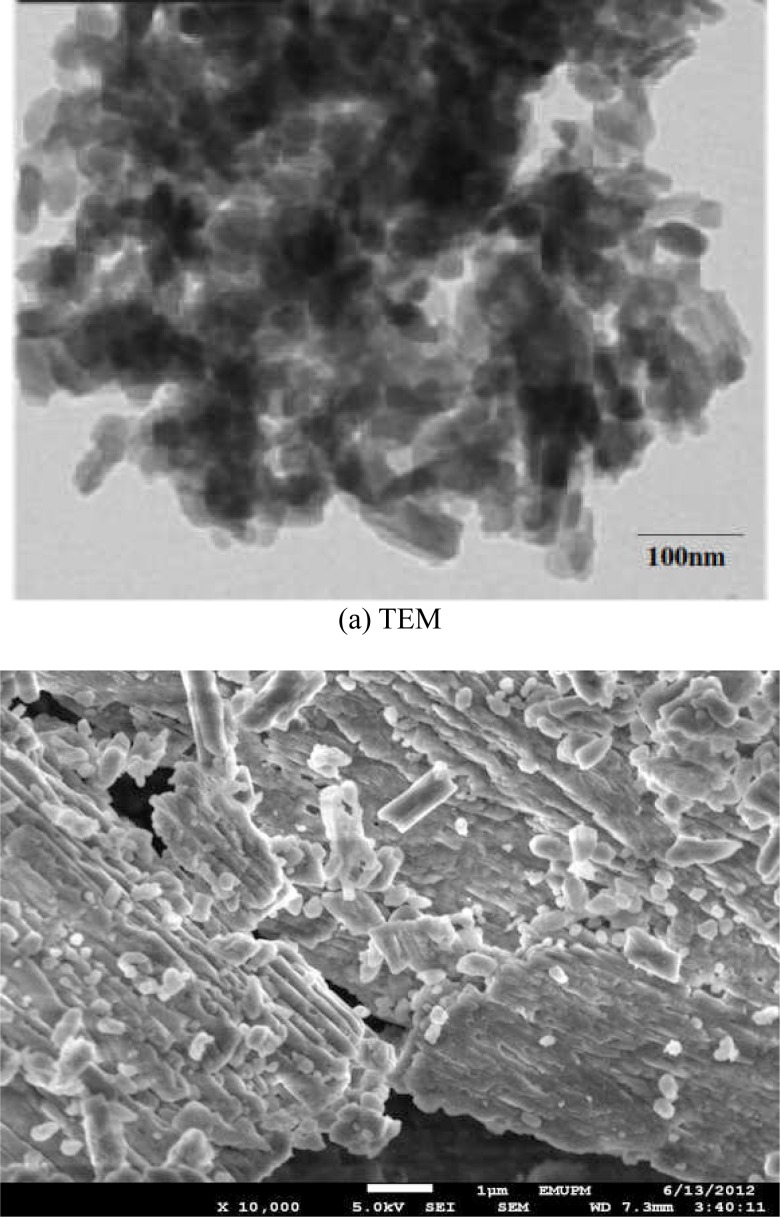
Figures (1a and 1b) demonstrate micrographs of transmission electron microscope (TEM) and field emission scanning electron microscope (FESEM) for the synthesized calcium carbonate nanocrystals, respectively. The images illustrate a uniform particle size distribution with rod-shaped morphology; the synthesized nanoparticles showed individual particle average size of 40 to 60 nm. As shown by the TEM image in Figure (1a), all the particles are within 100 nm, also particle surfaces characterized by FESEM in figure (1b) displayed a rod-shaped particles being the main target of our synthesis.
Figure 1.
(a and b) is a micrograph images for transmission electron microscope (TEM) and Field Emission scanning electron microscope (FESEM).
In Vitro Cellular Toxicity of CaCO3 nanocrystals against NIH 3T3 Mouse Fibroblasts
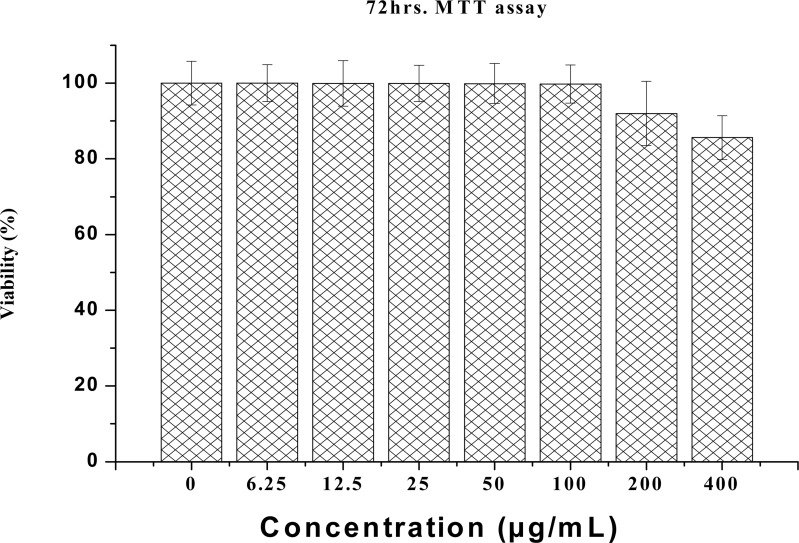
MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) is a water-soluble tetrazolium salt, and the assay is based on the principle that mitochondrial dehydrogenase of intact cells may converts the soluble yellow MTT tetrazolium salt into an insoluble purple formazan by cleaving the tetrazolium ring; the formazan product formed is impermeable to cell membranes and therefore only accumulates in healthy cells. Based on the analysis, calcium carbonate nanocrystals show no apparent toxicity to NIH 3T3 cells, as shown by the MTT results in Figure 2. The results revealed that none of the concentrations (0 to 400 µg/ml) used during the study were toxic to the NIH 3T3 fibroblasts cells, and even at the higher concentrations of 200 and 400 µg/mL, the percentages of viable cells were 92% and 85%, respectively. The remaining concentrations of calcium carbonate nanocrystals showed no significant difference from the control group (100% viability). Therefore, the result indicates that calcium carbonate nanocrystals did not interfere with the mitochondrial metabolism of the NIH 3T3 fibroblast cells.
Figure 2.
NIH 3T3 cell viability (MTT) in response to different concentrations of calcium carbonate nanocrystals.
Modified Double-Staining Neutral Red/Trypan Blue Exclusion Assay
The effect of the calcium carbonate nanocrystals on cell proliferation was analysed by a modified double-staining neutral red/trypan blue method. The results from cells counting demonstrated that the treated cells (10×104 cells/mL) showed no significant differences (p>0.05) compared to untreated cells (10.1 ×104), which indicates that most of the cells were viable; however, reduced number of cells was observed in the treated cells with 200 to 400 µg/mL compared to control (untreated) as shown in Figure 3. Therefore, the modified neutral red/trypan blue double-staining method is consistent with our MTT result in Figure 2. Moreover, live cells possess intact cell membranes, which exclude certain dyes, such as trypan blue, propidium and ethidium bromide, whereas dead cells do not; however, trypan blue stains only dead cells, whereas neutral red (NR) assay is based on the ability of viable cells to incorporate and bind neutral red dye into the lysosomes. NR uptake by cells occurs through a process requiring energy and is sensitive to substances that interfere with cell membrane and lysosomal permeability, as well as the process of energy-dependent endocytosis (Repetto and Zurita, 2008) causing cytotoxicity through the lysosomal activity.
Figure 3.
Neutral Red/Trypan Blue Exclusion Assay for cells viability.
The analysis revealed low levels of toxicity at concentrations of 200 and 400 µg/ml, where the cells exhibited survival rates of 92% and 84–85%, respectively. The decrease in cell viability may be due to the overburden of cells with nanocrystals. However, the cells observed with double staining (i.e. single cells stained with blue and red dye) is a clear indication that the cells were affected or damaged during the preparation or procedure and was not killed by the toxic effect of the nanocrystals.
BrdU Test (Genotoxicity Assays)
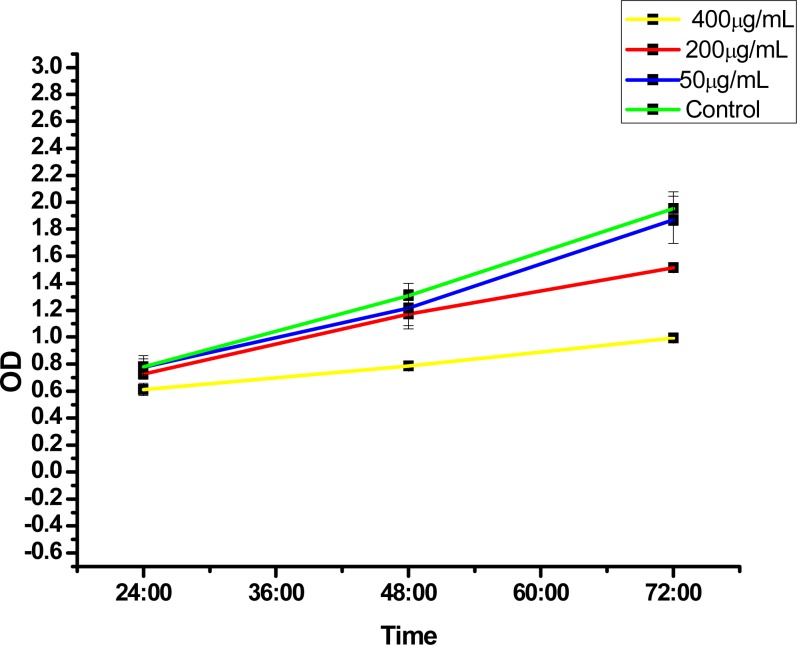
Figure 4 shows the effect of CaCO3 nanocrystals on the NIH 3T3 cell line. The analysis suggests a proliferation ability of the cells within the period of 24, 48 and 72 hours of treatment compared to the control group. A slight decrease was observed at 200 and 400 µg/mL, with the highest inhibition rate at 400 µg/mL. Furthermore, Figure 4 reveals the percentage of BrdU incorporated in NIH 3T3 cells and, therefore, the decrease in cells proliferation at 200 and 400 µg/mL indicates minor DNA damage. The principle of BrdU analysis was based on the ability of proliferating cells to incorporate BrdU reagent into their DNA as they incorporate thymidine during the replication process (DNA synthesis) and as such when DNA is damaged the BrdU cannot be incorporated into the damaged DNA chain during the replication process which indicate a genotoxic effect (Hernández-Ortiz, 2012).
Figure 4.
BrdU assays (Genotoxicity test), NIH 3T3 fibroblast.
The current research displays the percentage of BrdU incorporation in NIH 3T3 cells after exposure to calcium carbonate nanocrystals, which indicate that CaCO3 nanocrystals are cytocompatible due to the higher percentage of incorporated BrdU with lesser toxicity to the cells and does not cause irrepairable DNA damage such as strand breaks and alkali-labile sites (Hernández-Ortiz, 2012).
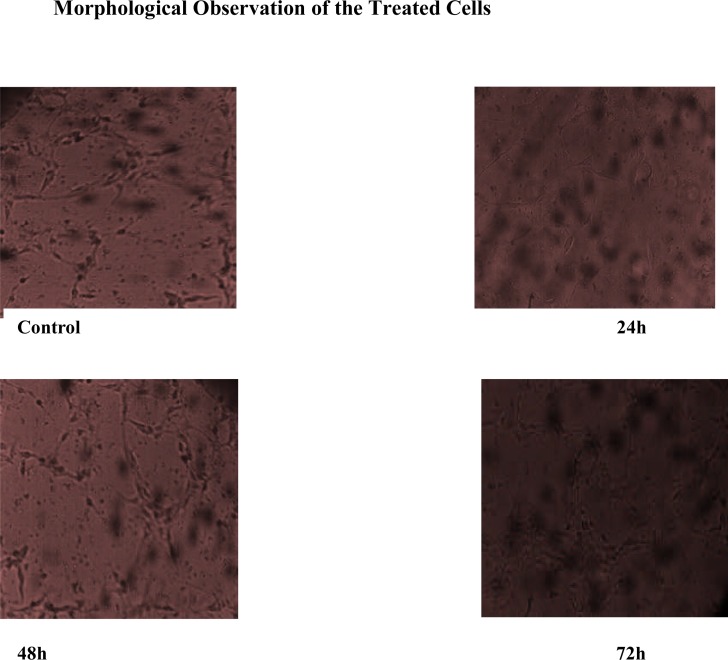
Live cells are very sensitive to their environment, the ability of cells to attach to a material's surface and proliferate is an indication that the particular material and environment are nontoxic and contain no toxic substances because the presence of toxic substances on or within the surface material may lead to cell detachment from the surface or eventually cell death (Nazir et al., 2012).
The images in Figure 5 indicate direct contact of NIH fibroblast cells with nanocrystals. The cell showed good attachments with no significant difference compared to control; almost all the cells in the treatment group proliferated. The interaction and adherence of cells to calcium carbonate nanocrystals surface demonstrated a good cytocompatibility of the material, which could be a primary gateway for drug delivery and other biomedical applications. This result was similar to the reported research on calcium phosphate nanoparticles (Liang et al., 2010).
Figure 5.
Inverted microscope images of NIH fibroblast cells after treatment with calcium carbonate nanoparticles at 24, 48 and 72 hrs.
Acridine Orange (AO)/Propidium Iodide (PI) Double-Staining (Cellular Morphological Analysis)
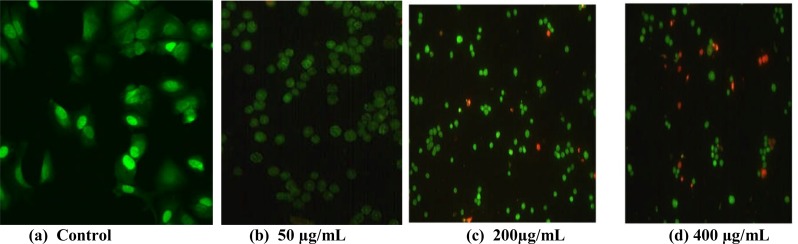
Dual-fluorescence viability assays, using Acridine orange (AO) and propidium iodide (PI), is an effective method for accurate measurement of cell viability. Acridine orange (AO) is a lipid soluble dye permeable to both live and dead cells and stains all nucleated cells green, while propidium iodide (PI) enters dead cells or cells with compromised membranes and stains dead nucleated cells red. Figure 6a illustrates a control group that was not treated, while in Figure 6b the cells were treated and double stained with both AO and PI. The images in control cells (Figure 6a) and the treated cells (Figure 6b) show no significant differences since the cells were stained green indicating active and viable cells and no indication of dead nucleated cells (red) by the propidium iodide. Moreover, Figure 6 (c and d) show treated cells stained with AO/PI, showing some dead cells that are noticeably red and the effect was observed to be concentration dependent. The results reflect the viability of most cells because PI does not enter cells with intact cell membranes. Therefore, the florescence observation confirms our previous results based on the MTT, LDH and reactive oxygen species, thus calcium carbonate nanocrystals could be a potential drug delivery carrier due to its biocompatibility.
Figure 6.
Fluorescence images of NIH 3T3 fibroblast cells stained with Acridine orange AO (a) Control Propidium iodide (b, c and d) AO/ propidium iodide double staining.
Lactate Dehydrogenase (LDH)
Elevated lactate dehydrogenase (LDH) activity in the cell supernatant is a popular parameter for assessing the in vitro cytotoxicity potential of a compound, extract or formulation. LDH is released from damaged cells, and oxidises lactate to pyruvate (Mbeh et al., 2012).
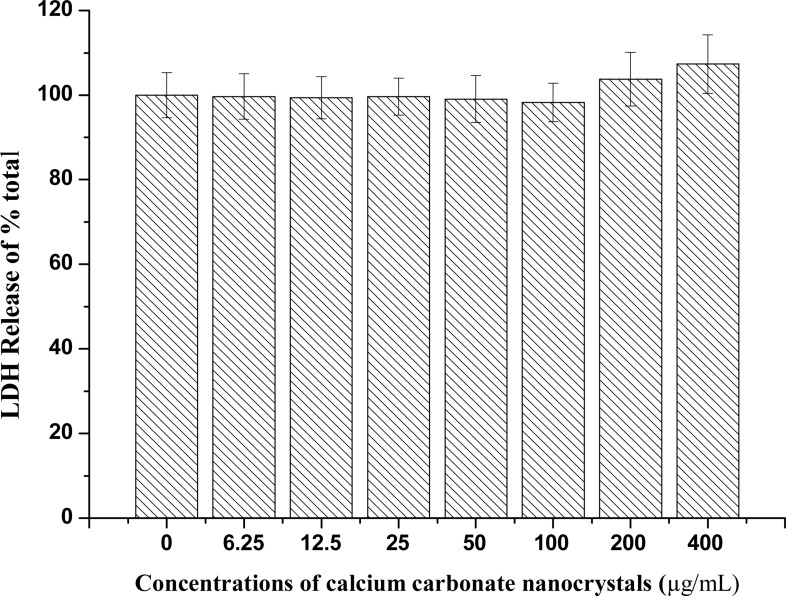
Membrane integrity was analysed by measuring LDH released by lysed cells, which is proportional to the number of cells damaged or lysed (Mbeh et al., 2012). The exposure of NIH 3T3 fibroblast cells to calcium carbonate nanocrystals resulted in minimal LDH leakage from the cell membrane, which was observed when calcium carbonate nanocrystals (200 and 400 µg/mL) were administered for 72 hours as indicated in Figure 7. The cytosolic enzymes can leak into the extracellular fluids only when the cell membrane integrity is lost (Choi et al., 2009). Therefore, LDH results indicate no toxicity observed due to the effect of CaCO3 nanocrystals at certain times and concentrations.
Figure 7.
NIH 3T3 LDH release from different concentrations of calcium carbonate nanocrystals
Reactive Oxygen Species
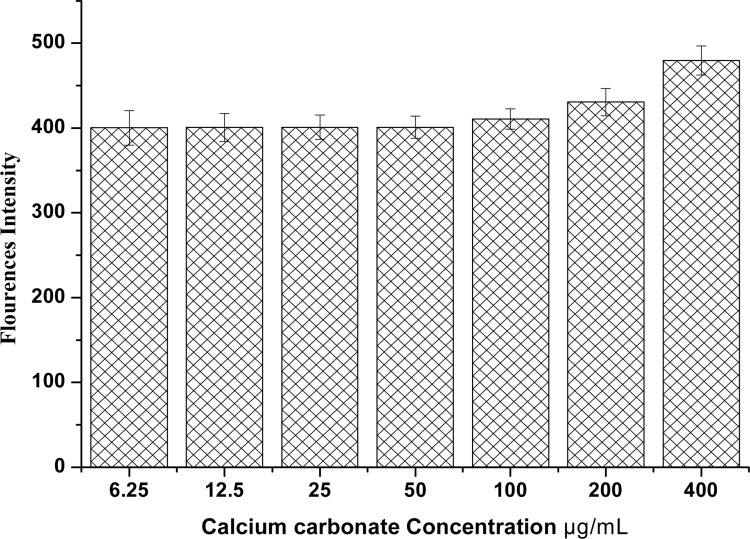
The production of reactive oxygen species by cells is a natural consequence of normal cell metabolism. At low physiological levels, ROS act as a second messenger in signalling pathways and the regulation of healthy cells (Yaran et al., 2012). However, when excessive generation of ROS overcomes the antioxidant protective ability of the cells, this may lead to unfavourable biological effects including oxidative damage to cellular macromolecules, inhibition of protein function, triggering of the apoptosis pathway and, eventually, cell death (Li et al., 2012). Evaluation of reactive oxygen species in NIH 3T3 cells after exposure to CaCO3 nanocrystals (0 to 400 µg/mL) showed no increase in ROS generation until 200 and 400 µg/mL where a slight increase in ROS generation was shown at 72 hours as indicated in Figure 8. However, increase in fluorescence intensity at higher concentrations is a indication that calcium carbonate nanoparticles may generate more ROS as the concentration and time increases, which eventually results in cell damage.
Figure 8.
NIH 3T3 ROS generation on different concentrations of calcium carbonate nanocrystals
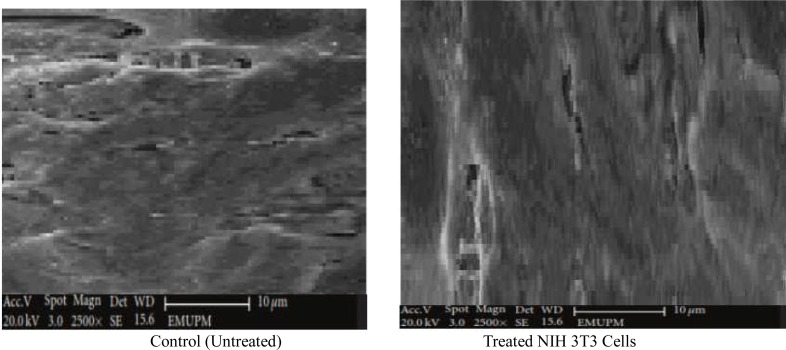
Morphological Evaluation of NIH 3T3 Fibroblast Cells by Scanning Electron Microscope (SEM)
Ultrastructural characteristic of NIH 3T3 cells cultured on CaCO3 nanocrystals were analysed by scanning electron microscope (Figure 9). The SEM micrographs of analysed cells signify that the cultured cells adhered to the surface; well-attached and proliferating cells were progressively growing on the surface of calcium carbonate nanocrystals. Densely packed elongated cell colonisation covered the surfaces of nanocrystal materials. Thus, the study provides more evidences on the biocompatibility of CaCO3 nanocrystal on NIH mouse fibroblast showing distinctive characteristics of a biomaterial for regeneration medicine.
Figure 9.
SEM micrographs of surface ultrastructural characteristics of NIH 3T3 cultured on calcium carbonate nanocrystals after 4 day of treatment.
Conclusions
In summary, a simple, cost-effective and environmental synthesis of biogenic calcium carbonate nanocrystal from cockle shells was shown to be cytocompatible to NIH 3T3 mouse fibroblast cells. Based on the presented evidences, calcium carbonate nanocrystals may be a good biomaterial and a potential for drug carrier systems and other biomedical applications due to its biocompatibility with no observed apparent toxicity on NIH 3T3 fibroblast cells, even at a high concentration of 400 µg/mL, according to the calculated parameters based on the cell viability, membrane integrity, oxidative stress and BrdU assays.
References
- 1.Babou-Kammoe R, Hamoudi S, Larachi F, Belkacemi K. Synthesis of calcium carbonate nanoparticles by controlled precipation of saturated carbonated and calcium Nitrate aqueous solution. The Canadian journal of chemical engineering. 2012;9:26–33. [Google Scholar]
- 3.Beng F, Alfonso E, Garcia B. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Advanced Drug Delivery Reviews. 2010;62:362–374. doi: 10.1016/j.addr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Choi, Soo-Jin, Oh Jae-Min, Choy Jin-Ho. Toxicological effects of inorganic nanocrystals on human lung cancer A549 cells. Journal of Inorganic Biochemistry. 2009;103:463–471. doi: 10.1016/j.jinorgbio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Frank A D, Schechtman A. Staining by Neutral Red and Trypan Blue in Sequence for Assaying Vital and Nonvital Cultured Cells. Biotechnic & Histochemistry. 1973;48(3):135–136. doi: 10.3109/10520297309116602. Informa Healthcare. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Ortiz M, Laura SA, Genoveva H, Rodolfo B, Catalina C, Victor M C, Alicia I M. Biocompatibility of crystalline opal nanoparticles. BioMedical Engineering OnLine. 2012;2(1):78. doi: 10.1186/1475-925X-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamba AS, Ismail M, Ibrahim TAT, Zakaria ZAB. Synthesis and characterisation of calcium carbonate aragonite nanocrystals from cockle shell powder (Anadara granosa) J Nanomater. 2013;398357 1-398357:9. [Google Scholar]
- 8.Li Yan, Chen Zhongwen, Ning Gu. In vitro biological effects of magnetic nanocrystals Chinese Science Bulletin. Special topic. Multifunctional Nanocarriers for Biomedical Applications. 2012 11434-012-5295-8. [Google Scholar]
- 9.Chen Liang, Mccrate Joseph M, Lee James C-M, Li Hao. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanocrystals with osteoblast cells. Journal of Nanotechnology. 2010;22 doi: 10.1088/0957-4484/22/10/105708. 105 708 (10pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mbeh DA, Franç R, Merhi Y, Zhang X,F, Veres T, Sacher E, Yahia L. In vitro biocompatibility assessment of functionalized magnetite nanocrystals: Biological and cytotoxicological effects. J Biomed Mater Res Part A. 2012;100A:1637–1646. doi: 10.1002/jbm.a.34096. [DOI] [PubMed] [Google Scholar]
- 11.Ming-Jium S, Chia-Yen H, Ling-Yi H, Hsuan-Ying C, Fei-Hong H, Ping-Shan L. Reversal of doxorubicin-resistance by multifunctional nanoparticles in MCF-7/ADR cells. Journal of Controlled Release. 2011;152(3):418–442. doi: 10.1016/j.jconrel.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Nazir N M, Dasmawati MMD, Azman S M, Nor S O, Radzali O. Biocompatibility of in House β-tricalciumphosphate ceramics with normal human Osteoblast Cell. Journal of Engineering Science and Technology. 2012;7(2):169–176. [Google Scholar]
- 13.Raju LC, Narasimhulu KV, Gopal NO, Rao JL, Reddy BCV. Electron paramagnetic resonance, optical and infrared spectral studies on marine mussels Arca burneisi shells. Journal of Molecular structure. 2002;608:201–211. [Google Scholar]
- 14.Rao CN, Ramakrishna Matte HS, Voggu R, Govindaraj A. Recent progress in the synthesis of inorganic nanoparticles. Dalton Trans. 2012;41(17):5089–5120. doi: 10.1039/c2dt12266a. [DOI] [PubMed] [Google Scholar]
- 15.Repetto G, Del Zurita J L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nature Protocol. 2008;3(7):1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez R, Anna T, Jose M, Delgado-Lopez D, Colangelo M P, Miguel A, Dura n-Olivencia, Gomez-Morales Jaime, Iafisco Michele. PH-Responsive Delivery of Doxorubicin from Citrate-Apatite Nanocrystals with Tailored Carbonate Content. Langmuir. 2013;29:8213–8221. doi: 10.1021/la4008334. [DOI] [PubMed] [Google Scholar]
- 17.Vijaya R, Diane R, Khalda F, Temesgen S, Shaik J. Bio-based calcium carbonate nanoparticles for drug delivery Applications. International Journal of Biomedical Nanoscience and Nanotechnology. 2013 In press. [Google Scholar]
- 18.Wang Y, Xin XM, Chen C, Gunawan P, Xu R. Fast precipitation of Calcium carbonate and their transformation to hollow hydroxyappatite nanospheres. Journal of Colloid and Interface Science. 2010;352:393–400. doi: 10.1016/j.jcis.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 19.Yaran Z, Ping M, Yao W, Du Juan, Zhou Qi, Zhu Zhihong, Yang Xu, Yuan Junlin. Biocompatibility of Porous Spherical Calcium Carbonate Microparticles on Hela Cells. World Journal of Nano Science and Engineering. 2012;2:25–31. [Google Scholar]
- 20.Ying-Jie Biomaterials Fabrication and Processing Handbook: Pages 217–234; Inorganic Nanostructures for Drug Delivery. International Journal of Nanomedicine. 2008;3(2):133–149. [Google Scholar]