Abstract
Background
Studies have suggested an increasing practice of concurrent herb-drug consumption. One of the major clinical risks of such concomitant herb-drug use is pharmacokinetic herb-drug interaction (HDI). This is brought about by the ability of phytochemicals to inhibit or induce the activity of metabolic enzymes. The aim of this study was to investigate the potential of the crude aqueous extracts of three popular medicinal herbs used in South Africa to inhibit major cytochrome P450 (CYP) enzymes.
Materials and Methods
The extracts of Bowiea volubilis, Spirostachys africana and Tulbaghia violacea were incubated with human liver microsomes (HLM) to monitor the phenacetin O-deethylation, diclofenac 4′-hydroxylation, S-mephenytoin 4′-hydroxylation and testosterone 6β-hydroxylation as respective probe reactions for CYP1A2, CYP2C9, CYP2C19 and CYP3A4. The inhibitory activity, where observed, was profiled against the extract concentration.
Results
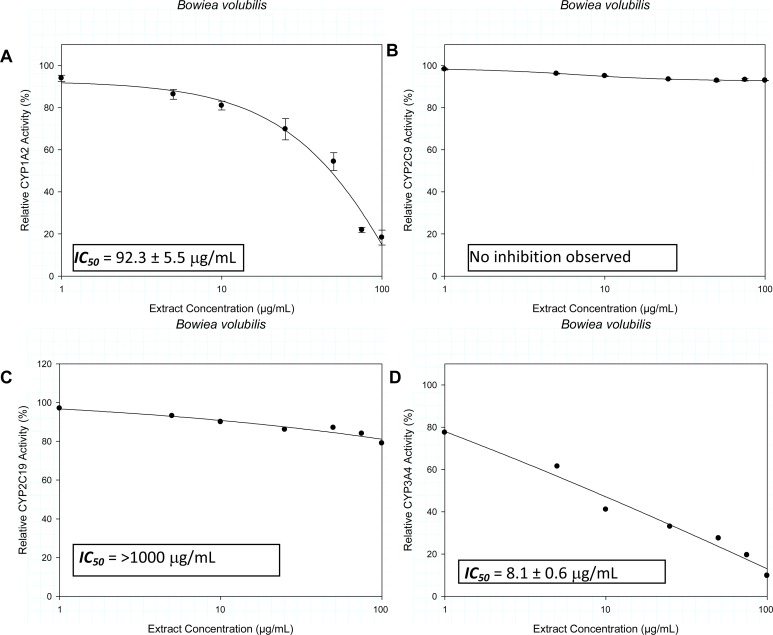
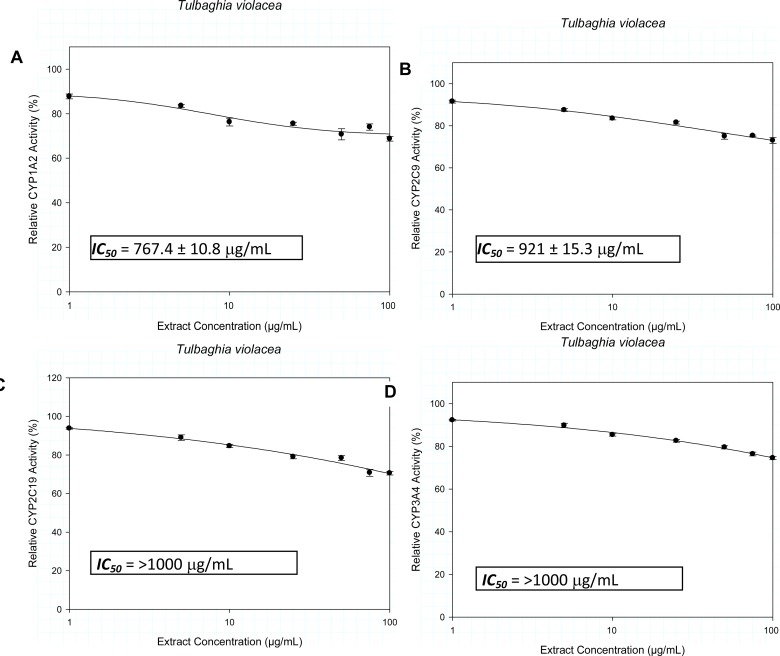
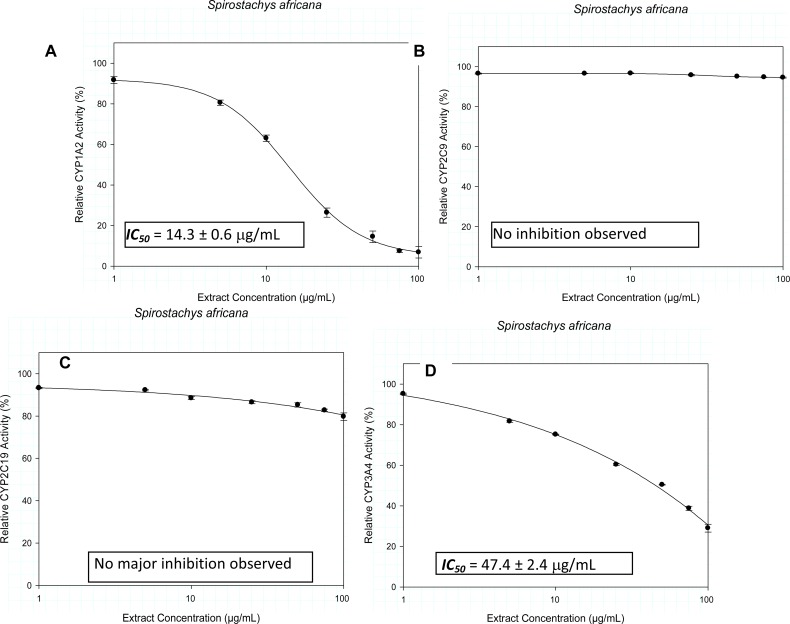
Extracts of Bowiea volubilis inhibited the metabolic activity of CYP1A2 and CYP3A4 with IC50 values of 92.3 ± 5.5 µg/mL and 8.1 ± 0.6 µg/mL respectively. Similar observation with Spirostachys africana showed inhibitory activity against CYP1A2 and CYP3A4 with respective IC50 values of 14.3 ± 0.6 µg/mL and 47.4 ± 2.4 µg/mL. Tulbaghia violacea demonstrated relatively weak inhibitory activity against CYP1A2 (767.4 ± 10.8 µg/mL) and CYP2C9 (921 ± 15.3 µg/mL).
Conclusion
The results suggest the potential for HDI between the herbs and the substrates of the affected enzymes, if sufficient in vivo concentration is attained.
Keywords: Cytochrome P450, drug metabolism, enzyme inhibition, herb-drug interaction, liver microsomes
Introduction
The concomitant use of medicinal herbs and orthodox medicine has been well reported and documented (Canter and Ernst, 2004; Delgoda et al., 2010). This is despite the lack of sufficient scientific information on the efficacy and toxicology on most of the herbal products. One of the clinically important consequences of the concomitant use of herbs and prescription drugs is the risk of pharmacokinetic herb-drug interaction (HDI). The ability of phytochemicals to inhibit or induce drug metabolizing enzymes especially the cytochrome P450 (CYP) enzyme family is known to be the key mechanism for pharmacokinetic HDI (Fasinu et al., 2012). While a number of plant products such as garlic and St John's wort, that are popular in developed countries have been well studied for their effects on CYP and transport proteins, there is dearth of information on the potential of most African herbal products for HDI (Fugh-Berman, 200; Nowack, 2008). In our earlier studies, extracts of Sutherlandia frutescens and Hypoxis hemerocallidea showed strong inhibitory activity against CYP and transport proteins (Fasinu et al., 2013a; 2013b). In this current study, three important herbs - Bowiea volubilis, Spirostachys africana and Tulbaghia violaceae - widely consumed in South Africa for medicinal purposes were identified and investigated for potential HDI.
Bowiea volubilis is known as ‘climbing onion’, and locally as ugibisisila (isiZulu), umgaqana (IsiXhosa) or Knolklimop (Afrikaans). It is widely sought after by indigenous South Africans for numerous medical conditions including dermatological disorders, sore eyes, urinary complications, infertility, facilitation and induction of abortion (Steenkamp, 2003). Some studies have claimed that its extracts demonstrated moderate in vitro antibacterial and antifungal activity (Buwa et al., 2006; Van Vuuren and Naidoo, 2010).
Spirostachys africana is widely distributed in the vegetations of Southern Africa. A number of phytochemicals including stachenone, diosphenol and diterpenoid derivatives have been isolated and identified as active constituents (Duri et al., 1992). The latex is traditionally used in South Africa and Zimbabwe as purgative, to stimulate emesis, and manage infective diarrhoea and dysentery (Gelfand et al., 1995); as anti-malarial in Tanzania and Mozambique and as topical anti-infective in South Africa (Munkombwe et al., 1997; 1998). It is used in South Africa, especially among the IsiXhosa-speaking people for the topical treatment of infantile cradle cap known locally as ishimca (Beach et al., 2010). In vitro studies have shown anti-microbial activity of isolated compounds against Escherichia coli, Salmonella typhi, Shigella dysentery, Staphylococcus aureus and Vibrio cholera (Mathabe et al., 2006; 2008). A clinical study of the anti-malarial preparation from S. africana conducted in Mozambique reported significant reduction in parasitaemia (Jurg et al., 1991).
Tulbaghia violacea is traditionally used for the treatment of respiratory disorders including tuberculosis, asthma; gastrointestinal ailments, oesophageal cancer, fungal infection, fever and colds (Van den Heever et al., 2008). The close association between HIV/AIDS and tuberculosis has made it popular among people living with HIV/AIDS in South Africa. It contains a C-S lyase, cysteine and tetrathiaoctane derivatives (Kubeca et al., 2002). It inhibits angiotensin converting enzyme (ACE) supporting its use in hypertension (Duncan et al., 1999). Its extracts inhibited the growth of cancer cells and induced apoptosis in an in vitro study (Bungu et al., 2006). Other studies have suggested its anthelmintic and antifungal activity especially against Candida albicans, a common cause of infection in HIV/AIDS patients (McGaw et al., 2000; Motsei et al., 2003; Thamburan et al., 2006).
Concomitant use of herbs and orthodox medicine has been reported to be common among South African patients (Bepe et al., 2011). The popularity of Bowiea volubilis, spirostachys Africana and Tulbaghia violacea makes them likely candidates for such concurrent use. While a number of CYP isozymes participate in drug metabolism, CYP1A2, CYP2C9, 2C19 and 3A4 are responsible for the CYP-dependent metabolism of over 90% of marketed drugs. CYP1A2 has been shown to be exclusively expressed in the liver constituting about 13–15% of the total hepatic CYP content (Shimada et al., 1994; Martignoni et al., 2006). It is the primary enzyme responsible for the phase I metabolism of a number of drugs including tacrine, theophylline, and clozapine. CYP1A2-catalyzed O-deethylation to phenacetion to form the active acetaminophen is the most widely used in vitro and in vivo probe reaction for evaluating the activity of CYP1A2 (Faber et al., 2005).
CYP2C (mainly 9 and 19) accounts for about 20% of the total human hepatic CYP (Gerbal-Chaloin et al., 2001; Martignoni et al., 2006), and the second most abundant CYP after 3A. CYP2C9 is the most abundantly expressed CYP2C isozyme (Rettie and Jones, 2005). One of the most well established in vitro CYP2C9-specific probe substrate is diclofenac (Konecný et al., 2007; Tai et al., 2008) while S-mephenytoin has been established, and used extensively as an in vitro probe substrate for CYP2C19 metabolic activity.
CYP3A4/5 is expressed in the liver and in extra-hepatic tissues and accounts for about 80% of intestinal CYP where it is responsible for pre-systemic drug metabolism (Paine et al., 2006). The CYP3A4 enzyme is the most important drug-metabolizing CYP accounting for about 40% of the total hepatic CYP (although the levels may vary 40-fold among individuals), and 50% of all CYP-mediated drug metabolism (Rendic, 2002; Ferguson and Tyndale, 2011; Singh et al., 2011). The inhibition of both intestinal and liver CYP3A4 has been shown to contribute to drug-drug interaction (Galetin et al., 2007). Testosterone hydroxylation is one of the very CYP3A4-specific reactions and is thus used as CYP3A4 probe in an enzyme mix.
The aim of the current study was to investigate the inhibitory effects of the crude extracts of Bowiea volubilis, Spirostachys africana and Tulbaghia violacea on CYP1A2, CYP2C9, CYP2C19 and CYP3A4 using pooled human liver microsomes (HLM) expressing these CYP isozymes. The specimens were to be sourced from traditional health practitioners (THP) and prepared in reflection of traditional use.
Materials and Methods
Plant materials
Fresh samples of the specimens (bulbs of Bowiea volubilis and Tulbaghia violacea; and stem bark of Spirostachys africana) were obtained from two South African THPs after a mutually acceptable material transfer agreement (MTA) was signed in accordance with the University's and the country's regulations on indigenous knowledge. The samples together with their representative flowering portions were identified by Kwaleta Sibuyile and Viola Kalitz of the Stellenbosch University botanical garden where prepared voucher specimens were deposited with voucher identification numbers PSF#12, PSF#13 and PSF#14 respectively. Information on the mode of use, dosage and specific indications were obtained and documented through semi-structured interview. Ethical approval was obtained from the University of Stellenbosch Health Research Ethics Committee.
Chemical compounds
The chemical compounds employed in the study were obtained as indicated: 4′-hydroxymephenytoin, 6β-hydroxytestosterone, 4′ hydroxydiclofenac, diclofenac acetaminophen, NADPH and testosterone from Sigma-Aldrich (Pty) Ltd (St. Louis, USA); dimethylsulfoxide (DMSO), phenacetin, magnesium chloride hexahydrate, potassium dihydrogen phosphate (KH2PO4), di-potassium hydrogen phosphate (K2HPO4), acetonitrile, formic acid and methanol from Merck Chemicals (Pty) Ltd (Darmstadt, Germany). All other chemicals and reagents used were of analytical grade.
Assay enzymes
Pooled mixed gender HLM prepared from 50 individual donors with total CYP and cytochrome b5 content of 290 pmol/mg protein and 790 pmol/mg proteins respectively was obtained from Gentest BD Biosciences (Woburn, MA, USA), stored at −80°C and thawed according to supplier instructions before use. The catalytic activities of the constituent CYP enzymes were provided by the manufacturer.
Extraction of Plant Material
The medicinal samples were air-dried and ground. This was followed by aqueous extraction (50mg/mL) in a round bottom flask. After the initial stirring for 2 hours, the mixture was allowed to extract for 24 hours, decanted, and centrifuged (20,000 rpm, 5min). The supernatant was filtered (0.45µL; Whatman International LTD, Maidstone, England) and freeze-dried. The dried extracts were stored at −20°C in air-tight containers, and were reconstituted in water just before use.
Incubation in HLM
Thawed HLM were diluted with potassium phosphate buffer (50mM; pH = 7.4) and incubated in 96-well plate format. Graded concentrations of the herbal extracts were prepared in methanol such that the addition of 1µL to 200µL incubation mixture yielded a final extract concentration of 0.39, 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50 and 100µg/mL respectively. Appropriate CYP substrate (table 1) was added and the mixture pre-incubated at 37°C for 10 minutes using an IS89 96-well plate incubator (Wesbart, Leimuiden, The Netherlands). Metabolic reactions were initiated by adding NADPH (1mM) and magnesium chloride (5mM) solution, and terminated after 20 minutes through the addition of formic acid (10 µL; 50%). All incubations were performed in duplicate. Control incubations contained CYP-specific inhibitor, and incubations without inhibitors (Table 1). The final methanol concentration in the incubations was 0.5% (v/v). Probe substrate concentrations used were less or equal to published Km values. Other conditions for microsomal incubation are summarized in Table 1.
Table 1.
Probe substrates, microsomal protein concentrations and incubation time
| Enzyme | Probe substrate | Microsome concentration (mg protein/mL) |
Probe substrate concen-tration (µM) |
Literature Km value (µM) |
CYP-specific inhibitors |
| CYP1A2 | Phenacetin | 0.2 | 10 | 9.01, 142, 313, 544 |
Furafylline |
| CYP2C9 | Diclofenac | 0.1 | 5 | 3.45, 9.06 | Sulfaphenazole |
| CYP2C19 | S-mephenytoin | 0.5 | 30 | 517, 428, 319 | Ticlopidine |
| CYP3A4/5 | Testosterone | 0.2 | 30 | 50–6010, 5111 | Ketoconazole |
Quantitative analysis of metabolites
LC-MS methods were developed to analyze CYP substrates/metabolites. Total separation and elution of the analytes were achieved within 10 minutes run time. Reproducibility of the quantitative analysis was assessed through repeat injections at different times. Intra-day and inter-day variations were insignificant (less than 5%). LC/MS conditions are summarized in Table 2. Solid-phase extraction using OASIS HLB 96-well elution plate (Waters, Milford, USA) was performed on the samples prior to LC-MS analysis. This was performed by sequential washing with 1 mL each of water and water-methanol (95/5; v/v) followed by two elutions with 1 mL methanol. The elutes were dried employing the 96-well Micro-DS96 evaporator (Porvair Sciences Ltd., Shepperton, UK) at 37°C and reconstituted in 100 µL of 10% acetonitrile containing 0.1% formic acid for LC-MS analysis.
Table 2.
Summary of the LC/MS analytical conditions for the quantitative determination of the metabolites
| Conditions | Acetaminophen | 4′-hydroxy-diclofenac | 4′-hydroxy-mephenytoin | 6β-hydroxy-testosterone | ||||||
| Mobile phase (plus 0.1% formic acid) |
A: Water B: Acetonitrile |
|||||||||
| Gradient of mobile phase: | Time range |
A (%) |
B (%) |
Time range |
A (%) |
B (%) |
Time range |
A (%) |
B (%) |
Isocratic 70% A and 30% B |
| 0.0–1.0 | 65 | 35 | 0.0–1.0 | 50 | 50 | 0.0 – 0.5 | 85 | 15 | ||
| 1.0–1.1 | 1.0 | 99 | 1.0–2.0 | 5 | 95 | 0.5 – 2.0 | 55 | 15 | ||
| 1.1–5.5 | 1.0 | 99 | 2.0–8.0 | 5 | 95 | 2.0 – 4.0 | 5 | 95 | ||
| 5.5–5.6 | 65 | 35 | 8.0–8.1 | 50 | 50 | 4.0 – 4.2 | 5 | 95 | ||
| 5.6–10 | 65 | 35 | 8.1–11. | 50 | 50 | 4.2 – 8.0 | 85 | 15 | ||
| 8.0 | 85 | 15 | ||||||||
| Injection volume (µL) | 2.5 | 2 | ||||||||
| Flow speed (µL / min) | 300 | 60 | ||||||||
| Column type | Luna Phenyl-Hexyl 3 µm, inner dimensions 50 × 1 mm (Phenomenex, Torrance, USA) | |||||||||
| Column temperature | 40°C | 30°C | ||||||||
| Nebulizing temperature | 375°C | |||||||||
| Mode | Positive | Negative | Positive | |||||||
| Quantifier transition (m/z) | 180.2 / 110.1 | 293.88/249.00 | 169.4/102.3 | 289.2/96.8 | ||||||
| Qualifier transition(s) (m/z) | 180.2 / 138.1 | No qualifier with a suitable intensity | ||||||||
| Dwell time (msec) | 400 | 300 | 200 | |||||||
| Retention time (min) | 3.35 | 6.65 | 6.65 | 3.2 | ||||||
| Total run time (min) | 10 | 11 | 8 | 5 | ||||||
| Lower limit of detection | 0.1 µM | 0.1 µM | 0.1 µM | 0.05 µM | ||||||
| Internal standard | Thiacetazone (Retention time-3.4 min; Quantifier transitions - 293.88/249.00) | |||||||||
Enzyme activity was monitored through CYP-specific metabolite production. Relative activity (100%) was defined in terms of metabolite production in the absence of inhibitor. The nonlinear-regression was generated by profiling the extract concentration against observed enzyme activity. The kinetic parameters were determined from the generated plot using the SigmaPlot® Enzyme Kinetic software. For each regression, the R2 > 0.9 and p < 0.05 were taken as the minimum level of significance. The enzyme inhibition parameter (IC50) was calculated based on kinetic equation for sigmoid curves (Equation 1) where x = concentration; y = relative enzyme activity; and s = slope factor
Results
The inhibitory activity of the crude extracts on the CYP isozymes, profiled against the extract concentration is presented in Figures 1–3. The IC50 values and their estimated extrapolative significance are presented in Table 3. Extracts of Bowiea volubilis inhibited the metabolic activity of CYP1A2 and CYP3A4 with IC50 values of 92.3 ± 5.5 µg/mL and 8.1 ± 0.6 µg/mL respectively (Figure 1). No significant effect was observed on the activity of CYP2C9 and CYP2C19. Similarly, Spirostachys africana showed inhibitory activity against CYP1A2 and 3A4 with respective IC50 values of 14.3 ± 0.6 µg/mL and 47.4 ± 2.4 µg/mL (Figure 2). Tulbaghia violacea only demonstrated a relatively mild inhibitory activity against CYP1A2 (767.4 ± 10.8 µg/mL) and CYP2C9 (921 ± 15.3 µg/mL) (Figure 3).
Figure 1.
The influence of the crude extracts of Bowiea volubilis on the metabolic activity of A) CYP1A2, B) CYP2C9, C) CYP2C19 and D) CYP3A4.
Figure 3.
The influence of the crude extracts of Tulbaghia violacea on the metabolic activity of A) CYP1A2, B) CYP2C9, C) CYP2C19 and D) CYP3A4.
Table 3.
Summary of the inhibitory effects of the medicinal herbs on CYP1A2, CYP2C9, CYP2C19 and CYP3A4
| Bowiea volubilis | Spirostachys africana | Tulbaghia violacea | |
| CYP1A2 [IC50 (µg/mL)] | 92.3 ± 5.5 | 14.3 ± 0.6 | 767.4 ± 10.8 |
| CYP2C9 [IC50 (µg/mL)] | - | - | 921 ± 15.3 |
| CYP2C19 [IC50 (µg/mL)] | >1000 | - | >1000 |
| CYP3A4 [IC50 (µg/mL)] | 8.1 ± 0.6 | 47.4 ± 2.4 | >1000 |
| Extraction yielda (%w/w) | 15.6 | 18.1 | 16.4 |
| Usual (single) dose (mg)b | 10,000 | 10,000 | 10,000 |
| Estimated extract per dose (mg)c | 1560 | 1810 | 1640 |
| Putative GIT conc (µg/mL)d | 6240 | 7240 | 6560 |
This is laboratory extraction yield using water
Usual traditional doses vary widely. On the average, 10–50g of the herbal materials is extracted and the decoctions are taken over time (3–6 times). Thus each consumption represent an extract of >1g
This is based on the assumption that similar yield is obtained in the traditional process of decoction or extraction
The estimation of putative GIT concentration is based on the average volume of GIT fluid (250mL)
(-) indicates the absence of inhibition
Figure 2.
The influence of the crude extracts of Spirostachys africana on the metabolic activity of A) CYP1A2, B) CYP2C9, C) CYP2C19 and D) CYP3A4.
Discussion
This study has demonstrated the potential of Bowiea volubilis, Spirostachys africana and Tulbaghia violacea, three of the most commonly consumed medicinal herbs in South Africa, to inhibit metabolic activity of CYP1A2, CYP2C9, CYP2C19 and CYP3A4. A summary of the results presented in Table 3 suggests a high likelihood of in vivo inhibitory effects. No study has reported the influence of any of the herbs on metabolic enzymes.
In traditional practice, all selected herbs are taken as aqueous extracts, as gathered from the THPs. Thus, an aqueous extraction was used in this study to reflect the consumption and what consumers are exposed to during medical use of the plants. While single-dose decoctions are common as dosage regimes, higher amounts of herbal products are often decocted and taken several days in the course of treatment. Thus, it is difficult to determine uniform doses of traditional medications. The estimates provided however, were determined based on the information gathered from the THPs and the determined weight of sampled doses.
Based on the approximated intestinal fluid volume of 250 mL and the laboratory extraction yields, putative achievable concentrations of the herbal extracts in the GIT were determined as shown in Table 3. The extent and efficiency of extraction of the herbs in the intestine may vary from laboratory extraction due to several factors: intestinal fluid composition, GIT transit time, disease state and dosage form. While the IC50 values vary, a cursory look at Table 3 shows that the putative and achievable GIT concentration of the herbal extract is 20–500 times the observed IC50 values. Such high concentrations in the GIT portend a high potential to inhibit the pre-systemic CYP-dependent metabolism of drugs, thus altering the expected pharmacokinetic profiles. This is important because of the rich expression of CYP in the intestines (Ufer et al., 2008).
Each of the herbs investigated in this study inhibited at least one metabolic enzyme. Information on the systemic bio-availability of the herbal extracts is not available. However, the long history of the folkloric use of these medicinal herbs for systemic disorders, and the high putative GIT concentrations of the extracts, are indications of their potentially high systemic bioavailability. Systemic availability of phyto-constituents increases the potential for enzyme inhibition and HDI. The traditional indications of the medicinal herbs cut across a wide variety of disease conditions. This makes them candidates for potential HDI of variety of prescription drugs.
There are a few limitations to the current study. The known variation of the phytochemical composition of medicinal plants due to place and time of harvest limits the generalization of the findings of this study (Ozkan et al., 2010). Although, the process of sourcing and extraction of the herbs was considered representative of the popular practice in South Africa, it should be noted that several herbal products are now available in capsules and other dosage forms and may be obtained commercially from non-THPS. The findings in this study are based on crude extracts only. The determination of the exact compounds exerting inhibitory activity on the drug metabolism was beyond the scope of this study.
The use of HLM is well accepted to assess the potential of new chemical entities and drug candidates for drug interactions. This technology has also been widely used to assess the HDI potentials. The results from such studies provide an indication for clinically significant interactions. Results from in vitro metabolic studies have been extrapolated for in vivo correlation with some degree of predictability (Umehara and Camenisch, 2012). However, there are still challenges in quantitative extrapolation of in vitro data to humans. At best, the results from in vitro assessment of HDI can be considered qualitative only.
Conclusion
Extracts of Bowiea volubilis inhibited the metabolic activity of CYP1A2 and CYP3A4; Spirostachys africana showed inhibitory activity against CYP1A2 and CYP3A4; while the extracts of Tulbaghia violacea inhibited the metabolic activity of CYP1A2 and CYP2C9. The results suggest the potential for HDI between the herbs and the substrates of the affected enzymes, if sufficient in vivo concentration is achieved.
Acknowledgement
Ms Nomsisi Stefans and Nombuso Keme for providing the herbal materials; Dr Heinart Seifart who helped with the bioanalysis; HOPE Kapstadt-Stiftung (HOPE Cape Town), and the Stellenbosch University Rural Medical Education Partnership Initiative (SURMEPI) for providing funds for this study.
References
- 1.Beach RA, Gantsho N, Flesche J, Scott C, Khumalo NP. Possible drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an infant from ingestion of Spirostachys africana complicated by measles co-infection. S Afr J Child Health. 2010;4(4):112–113. [Google Scholar]
- 2.Bepe N, Madanhi N, Mudzviti T, Gavi S, Maponga COC, Morse GD. The impact of herbal remedies on adverse effects and quality of life in HIV-infected individuals on antiretroviral therapy. J Infect Dev., Ctries. 2011;5(1):48. doi: 10.3855/jidc.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bort R, Macé K, Boobis A, Gómez-Lechón MJ, Pfeifer A, Castell J. Hepatic metabolism of diclofenac: Role of human CYP in the minor oxidative pathways. Biochem Pharmacol. 1999;58:787–796. doi: 10.1016/s0006-2952(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 4.Brosen K, Skjelbo E, Rasmussen BB, Poulsen HE, Loft S. Fluvoxamine is a potent inhibitor of cytochrome P4501A2. Biochem Pharmacol., 1993;45:1211–1214. doi: 10.1016/0006-2952(93)90272-x. [DOI] [PubMed] [Google Scholar]
- 5.Bungu L, Frost CL, Brauns SOC, van de Venter M. Tulbaghia violacea inhibits growth and induces apoptosis in cancer cells in vitro. Afr J Biotechnol. 2006;5(20):1936–1943. [Google Scholar]
- 6.Buwa LV, van Staden J. Antibacterial and antifungal activity of traditional medicinal plants used against venereal diseases in South Africa. J Ethnopharmacol. 2006;103:139–142. doi: 10.1016/j.jep.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Canter PH, Ernst E. Herbal supplement use by persons aged over 50 years in Britain. Drugs & Aging. 2004;21(9):597–605. doi: 10.2165/00002512-200421090-00004. [DOI] [PubMed] [Google Scholar]
- 8.Coller JK, Somogyi AA, Bochner F. Comparison of (S)-mephenytoin and proguanil oxidation in vitro: contribution of several CYP isoforms. Br J Clin Pharmacol. 1999;48:158–167. doi: 10.1046/j.1365-2125.1999.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgoda R, Younger N, Barrett C, Braithwaite J, Davis D. The prevalence of herbs use in conjunction with conventional medicines in Jamaica. Complement Ther Med. 2010;18(1):13–20. doi: 10.1016/j.ctim.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Draper AJ, Madan A, Smith K, Parkinson A. Development of a non-high pressure liquid chromatography assay to determine testosterone hydroxylase (CYP3A) activity in human liver microsomes. Drug Metab Dispos. 1998;26:299–304. [PubMed] [Google Scholar]
- 11.Duncan AOC, Jäger AK, van Staden J. Screening of Zulu medicinal plants for angiotensin converting enzyme (ACE) inhibitors. J Ethnopharmacol. 1999;68:63–70. doi: 10.1016/s0378-8741(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 12.Duri ZJ, Hughes NA, Munkombwe NM. Diterpenoids from Spirostachys africana. Phytochemistry. 1992;31(2):699–702. [Google Scholar]
- 13.Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125–134. doi: 10.1111/j.1742-7843.2005.pto_973160.x. [DOI] [PubMed] [Google Scholar]
- 14.Fasinu PS, Bouic PJ, Rosenkranz B. An overview of the evidence and mechanisms of herb-drug interactions. Front Pharmacol. 2012;2012:3. doi: 10.3389/fphar.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasinu PS, Gutmann H, Schiller H, Bouic PJ, Rosenkranz B. The potential of Hypoxis hemerocallidea for herb-drug interaction. Pharm Biol. 2013;51(12):1499–1507. doi: 10.3109/13880209.2013.796393. [DOI] [PubMed] [Google Scholar]
- 16.Fasinu PS, Gutmann H, Schiller H, James AD, Bouic PJ, Rosenkranz B. The Potential of Sutherlandia frutescens for Herb-Drug Interaction. Drug Metab Dispos. 2013;41(2):488–497. doi: 10.1124/dmd.112.049593. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol Sci. 2011;32:708–714. doi: 10.1016/j.tips.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 19.Galetin A, Hinton LK, Burt H, Obach RS, Houston JB. Maximal inhibition of intestinal first-pass metabolism as a pragmatic indicator of intestinal contribution to the drug-drug interactions for CYP3A4 cleared drugs. Curr Drug Metab. 2007;8(7):685–693. doi: 10.2174/138920007782109805. [DOI] [PubMed] [Google Scholar]
- 20.Gelfand M, Mavi S, Drummond RB, Ndemera B. The traditional medical practitioner in Zimbabwe. Gweru, Zimbabwe: Mambo Press; 1985. [Google Scholar]
- 21.Gerbal-Chaloin S, Pascussi JM, Pichard-Garcia L, Daujat M, Waechter F, Fabre JM, Carrère N, Maurel P. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab Dispos. 2001;29(3):242–251. [PubMed] [Google Scholar]
- 22.Jurg A, Tomás T, Pividal J. Antimalarial activity of some plant remedies in use in Marracuene, southern Mozambique. J Ethnopharmacol. 1991;33(1–2):79–83. doi: 10.1016/0378-8741(91)90165-a. [DOI] [PubMed] [Google Scholar]
- 23.Kenworthy KE, Clarke SE, Andrews J, Houston JB. Multisite kinetic models for CYP3A4: simultaneous activation and inhibition of diazepam and testosterone metabolism. Drug Metab Dispos. 2001;29:1644–1651. [PubMed] [Google Scholar]
- 24.Konecný J, Jurica J, Tomandl J, Glatz Z. Study of recombinant cytochrome P450 2C9 activity with diclofenac by MEKC. Electrophoresis. 2007;28(8):1229–1234. doi: 10.1002/elps.200600560. [DOI] [PubMed] [Google Scholar]
- 25.Kubeca R, Velisek J, Musah RA. The amino acid precursors and odor formation in society garlic (Tulbaghia violacea Harv.) Phytochemistry. 2002;60:21–25. doi: 10.1016/s0031-9422(02)00065-1. [DOI] [PubMed] [Google Scholar]
- 26.Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2(6):875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 27.Mathabe MOC, Hussein AA, Nikolova RV, Basson AE, Meyer JJ, Lall N. Antibacterial activities and cytotoxicity of terpenoids isolated from Spirostachys africana. J Ethnopharmacol. 2008;116(1):194–197. doi: 10.1016/j.jep.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Mathabe MOC, Nikolova RV, Lall N, Nyazema NZ. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. J Ethnopharmacol. 2006;105(1–2):286–293. doi: 10.1016/j.jep.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 29.McGaw LJ, Jager AK, Van Staden J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J Ethnopharmacol. 2000;72:247–263. doi: 10.1016/s0378-8741(00)00269-5. [DOI] [PubMed] [Google Scholar]
- 30.Motsei ML, Lindsey KL, Van Staden J, Jager AK. Screening of traditionally used South African plants for antifungal activity against Candida albicans. J Ethnopharmacol. 2003;86:235–241. doi: 10.1016/s0378-8741(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 31.Munkombwe NM, Hughes NA, Duri ZJ. Acid metabolites from Spirostachys africana. Phytochemistry. 1998;47:1653–1655. [Google Scholar]
- 32.Munkombwe NM, Matswabi T, Hughes NA. Diosphenols from Spirostachys africana. Phytochemistry. 1997;45:1217–1220. [Google Scholar]
- 33.Nowack R. Review Article: Cytochrome P450 enzyme, and transport protein mediated herb-drug interactions in renal transplant patients: Grapefruit juice, St John's Wort-and beyond! Review Article. Nephrology. 2008;13(4):337–347. doi: 10.1111/j.1440-1797.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- 34.Ozkan G, Baydar H, Erbas S. The influence of harvest time on essential oil composition, phenolic constituents and antioxidant properties of Turkish oregano (Origanum onites L.) J Sci Food Agric. 2010;90(2):205–209. doi: 10.1002/jsfa.3788. [DOI] [PubMed] [Google Scholar]
- 35.Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DOC. The human intestinal cytochrome P450 ‘pie’. Drug Metab Dispos. 2006;34:880–886. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34:83–448. doi: 10.1081/dmr-120001392. [DOI] [PubMed] [Google Scholar]
- 37.Rettie AE, Jones JP. Clinical and toxicological relevance of CYP2C9: drug-drug interactions and pharmacogenetics. Annu Rev Pharmacol Toxicol. 2005;45:477–494. doi: 10.1146/annurev.pharmtox.45.120403.095821. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues AD, Surber BW, Yao Y, Wong SL, Roberts EM. [O-ethyl 14C]phenacetin O-deethylase activity in human liver microsomes. Drug Metab Dispos. 1997;25:1097–1100. [PubMed] [Google Scholar]
- 39.Schmider J, Greenblatt DJ, von Moltke LL, Harmatz JS, Duan SX, Karsov D, Shader RI. Characterization of six in vitro reactions mediated by human cytochrome P450: application to the testing of cytochrome P450-directed antibodies. Pharmacology. 1996;52:125–134. doi: 10.1159/000139376. [DOI] [PubMed] [Google Scholar]
- 40.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 41.Singh D, Kashyap A, Pandey RV, Saini KS. Novel advances in cytochrome P450 research. Drug Discov Today. 2011;16:793–799. doi: 10.1016/j.drudis.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Steenkamp V. Traditional herbal remedies used by South African women for gynaecological complaints. J Ethnopharmacol. 2003;86:97–108. doi: 10.1016/s0378-8741(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 43.Tai G, Dickmann LJ, Matovic N, DeVoss JJ, Gillam EM, Rettie AE. Re-engineering of CYP2C9 to probe acid-base substrate selectivity. Drug Metab Dispos. 2008;36(10):1992–1997. doi: 10.1124/dmd.108.022186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tassaneeyakul W, Birkett DJ, Veronese ME, McManus ME, Tukey RH, Quattrochi LOC, Gelboin HV, Miners JO. Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J Pharmacol Exp Ther. 1993;265:401–407. [PubMed] [Google Scholar]
- 45.Thamburan S, Klaasen J, Mabusela WT, Cannon JF, Folk W, Johnson Q. Tulbaghia alliacea Phytotherapy: A Potential Anti-infective Remedy for Candidiasis. Phytother Res. 2006;20:844–850. doi: 10.1002/ptr.1945. [DOI] [PubMed] [Google Scholar]
- 46.Transon C, Lecoeur S, Leemann T, Beaune P, Dayer P. Interindividual variability in catalytic activity and immunoreactivity of three major human liver cytochrome P450 isozymes. Eur J Clin Pharmacol. 2006;51:79–85. doi: 10.1007/s002280050164. [DOI] [PubMed] [Google Scholar]
- 47.Ufer M, Dilger K, Leschhorn L, Daufresne L, Mosyagin I, Rosenstiel P, Haesler R, Kuehbacher T, Nikolaus S, Schreiber S, Cascorbi I. Influence of CYP3A4, CYP3A5, and ABCB1 genotype and expression on budesonide pharmacokinetics: a possible role of intestinal CYP3A4 expression. Clin Pharmacol Ther. 2008;84(1):43–46. doi: 10.1038/sj.clpt.6100505. [DOI] [PubMed] [Google Scholar]
- 48.van den Heever E, Allemann J, Pretorius J. Influence of nitrogen fertilizers on yield and antifungal bioactivity of Tulbaghia violacea L. Hum Exp Toxicol. 2008;27(11):851–857. doi: 10.1177/0960327108099529. [DOI] [PubMed] [Google Scholar]
- 49.van Vuuren SF, Naidoo D. An antimicrobial investigation of plants used traditionally in southern Africa to treat sexually transmitted infections. J Ethnopharmacol. 2010;130:552–558. doi: 10.1016/j.jep.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 50.Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Human cytochromes P450 mediating phenacetin O-deethylation in vitro: validation of high affinity component as an index of CYP1A2 activity. J Pharm Sci. 1998a;87:1502–1507. doi: 10.1021/js980255z. [DOI] [PubMed] [Google Scholar]
- 51.Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Relative quantities of catalytically active CYP 2C9 and 2C19 in human liver microsomes: application of the relative activity factor approach. J Pharm Sci. 1998b;87:845–853. doi: 10.1021/js970435t. [DOI] [PubMed] [Google Scholar]