Abstract
Background
The preference for a fairer skin-tone has become a common trend among both men and women around the world. In this study, seaweeds Sargassum polycystum and Padina tenuis were investigated for their in vitro and in vivo potentials in working as skin whitening agents. Seaweed has been used as a revolutionary skin repairing agent in both traditional and modern preparations. The high antioxidant content is one of the prime reasons for its potent action. It has been employed in traditional Chinese and Japanese medicine. For centuries, most medical practitioners in the Asian cultures have known seaweed as an organic source of vitamins, minerals, fatty acids like omega-3 and omega-6 and antioxidants. The present objective of the study was to evaluate the potent dermal protective effect of the two seaweeds Sargassum polycystum and Padina tenuis on human cell lines and guinea pigs.
Material and Methods
Seaweeds were extracted with ethanol and further fractionated with hexane, ethyl acetate and water. The extracts were tested for mushroom tyrosinase inhibitory activity, cytotoxicity in human epidermal melanocyte (HEM), and Chang cells. Extracts with potent melanocytotoxicity were formulated into cosmetic cream and tested on guinea pigs in dermal irritation tests and de-pigmentation assessments.
Results
Both Sargassum polycystum and Padina tenuis seaweeds showed significant inhibitory effect on mushroom tyrosinase in the concentration tested. SPEt showed most potent cytotoxicity on HEM (IC50 of 36µg/ml), followed by SPHF (65µg/ml), and PTHF (78.5µg/ml). SPHF and SPEt reduced melanin content in skin of guinea pigs when assessed histologically.
Conclusion
SPEt, SPHF and PTHF were able to inhibit HEM proliferation in vitro, with SPHF being most potent and did not cause any dermal irritation in guinea pigs. The results obtained indicate that SPHF is a promising pharmacological or cosmetic agent.
Keywords: Hyper-pigmentation, Melanogenesis, Padina tenuis, Sargassum polycystum, Tyrosinase, Whitening effect
Introduction
The human skin covers the external surface of the body, is the largest organ accounting for about 16% of total body weight. Human skin has a wide spectrum of colors, ranging from shades of aphotic amber to white. The color of the skin is largely determined by the amount, size and type of melanin produced by melanocytes, the subsequent transfer of the pigments to keratinocytes and their eventual distribution and deposition in the skin (Miyamura et al., 2007). Nowadays, an alternative for fairer derma tone has become one of the accepted trends for both men and women across the globe, especially the Asian continent. Rapid increase in sales and varieties of whitening cosmetic products in the market is noticeable nowadays for this reason.
Whitening cosmetic products may improve skin lightness and dullness by interfering with the skin pigmentation cascade, such as the melanin biosynthesis pathway. A pivotal and rate-limiting enzyme in melanogenesis, tyrosinase, is often a target of the whitening cosmetic products (Maeda at al., 1997). The anti-tyrosinase mechanisms include interfering with transcription and/or glycosylation of tyrosinase prior to melanogenesis, inhibition of tyrosinase, peroxidase and/or byproducts during melanogenesis, degradation of tyrosinase, inhibition of melanosome transfer, increasing of keratinocyte turnover and acceleration of epidermal renewal (Park et al., 2010). There are antioxidants that inhibit several oxidation steps during melanogenesis, reducing agents that reduce dopaquinone back to DOPA and inhibitors for the enzymes tyrosinase and peroxidase that are directly involved in melanin biosynthesis (Piao et al., 2002). In routine screening for potential skin whitening assay, the method often employed is the determination of ability profile of the agent under investigation in inhibiting tyrosinase activity. The test used is a cell-free mushroom tyrosinase assay system, as it is simple and relatively inexpensive to run. However it suffers from the fact that intracellular tyrosinase in melanocyte may respond differently than mushroom tyrosinase (Chan et al., 2011).
A second mechanism for skin whitening is the actual direct cytotoxic effect of the whitening agents on the melanocytes. (Debabrata, 2009).
Algotherapy is a term used for usage of algae, the official name for seaweed and sea grasses, in therapeutic treatments. Seaweed refers to the large marine algae that grow almost exclusively in the sea or in brackish water. Seaweed is widely used as food and as ingredients in cosmetic, pharmaceutical and chemical industries. Mineral-rich seaweed extracts may be found in skincare products such as skin moisturizing agents, facial cleansing products, masks, make-up removers and bath additives. Seaweed may help to improve the skin's elasticity and tone, prevent cellulites, soothe damaged or irritated skin, heal minor skin infections, detoxification, increase the immunity of the skin and enable it to cope with external stress. Employment of chemical constituents of algae was successfully proven by few studies (Hwang et al., 2006). Brown algae poly phenols were used in inhibiting carcinogenesis of skin induced by UV-B in SKH-1 mice (Hwang et al., 2006). Marine algae were also used in attenuating tumors in another study (Hiroyuki et al., 1990). It was reported that seaweed contains high amount of minerals, proteins, vitamins, carbohydrates and other elements which are essential to the human body (Dawczynski et al., 2007; Ruperez, 2002). Sea plants are good for the skin because they have chemical compositions which are similar to human body's plasma, which enables particularly good penetration of the nutrients (Chapman and Chapman, 1980).
In this study, the seaweeds Sargassum polycystum (S. polycystum) and Padina tenuis (P. tenuis) were investigated for their in vitro and in vivo potentials to work as skin whitening agents.
Material and Methods
Chemicals and Reagents
All chemicals used were of Analytical Reagent (AR) grade unless otherwise specified.
Seaweed materials
Seaweeds S. polycystum and P. tenuis were collected from Pulau Sembilang island and Pulau Seri Buat, Endau, Johor, Malaysia. The plants were identified by Professor Dr. Phang Siew Moi, of UMalgae, the Algae Research Laboratory at University of Malaya. The seaweeds were soaked in filtered water and thoroughly washed in demineralized water to remove contaminants such as epiphytes, salts and sands. The seaweeds were then oven-dried at 45°C and ground into powdered form.
Animals
Brown colored guinea pigs (Cavia porcellus) were purchased from the Laboratory Animal Centre, Faculty of Medicine, University of Malaya. The animals were housed in 60 × 40 cm cages (2 per cage) in the animal room of Department of Physiology, Faculty of Medicine, University of Malaya. The ambient temperature of the animal room was set at 27 ± 2 °C and a 12-hour light and dark cycle was provided. The animals received normal chow pellet/high fat diet and water ad libitum. All experimental procedures were approved by the animal ethics committee of the University of Malaya (Ethic number: FIS/27/01/2010/TKK(R)).
Preparation of seaweed extracts and fractions
The ethanol crude extracts were prepared by ethanol extract as described previously (Chan et al., 2011; Dawczynski et al., 2007). Briefly, 1kg of seaweed was macerated in 3liters of ethanol (R&M, UK) for 3 days. The solvent was filtered and evaporated at 45°C using a rotary evaporator to obtain ethanol crude extracts. The process was repeated for two more times, and the extracts were pooled.
The pooled ethanol crude extracts were extracted by soaking with 200ml of n-hexane (RCI Labscan, Thailand) for 3 days, filtered and solvent was evaporated in a rotary evaporator. The n-hexane-soluble fractions were pooled to give hexane fraction. The n-hexane-insoluble residues were separated with ethyl acetate (RCI Labscan, Thailand) and water, 100ml each. The ethyl acetate fractions were evaporated using a rotary evaporator. All extracts and fractions were freeze-dried and stored at 4°C in the dark. For in vitro assays, all extracts and fractions were dissolved in 10% Dimethyl sulfoxide (DMSO): 90% cell culture medium except for the water fractions, which were dissolved in cell culture medium.
Mushroom tyrosinase inhibition assay
Seaweed extracts and fractions were screened for their cell-free anti-tyrosinase activities as described previously with minor modifications (Chan et al., 2011; Sheehan and Hrapchak, 1987). L-3, 4-dihydroxyphenylalanine (L-DOPA) (Nacalai Tesque, Japan) was dissolved in 0.1M phosphate buffer (PB) at a concentration of 2.5mM and used as the substrate. For optimization of mushroom tyrosinase concentration, pure mushroom tyrosinase enzyme (EC 1.14.18.1; Sigma, USA) was dissolved and diluted to 9 different concentrations (25, 50, 75, 100, 200, 300, 400, 500 and 600U/ml) in PB. To each well of a 96-well plate (TPP, Switzerland), 150µl of PB were mixed with 50µl of mushroom tyrosinase in different concentrations and incubated for 10 minutes at room temperature. One hundred µl of L-DOPA were then added into each well and the mixture was further incubated for 20 minutes and the quantity of dopachrome formed was determined by reading the plate at 475nm. The assay was repeated using extracts and fractions at concentrations of 0, 10, 30, 50, 70, 100, 250, 500 and 1000µg/ml in triplicate. Then, 100µl of PB were mixed with 50µl of mushroom tyrosinase at the optimized concentration of 600U/ml and 50µl of the extracts or fractions at different concentrations, incubated for 10 minutes before addition of 100µl of L-DOPA. The mixture further incubated for 20 minutes before the absorbance was read in a microplate reader. Kojic acid (Nacalai Tesque, Japan) at a concentration of 100µg/ml was used as positive control.
Cell culture
The neonatal Human Epidermal Melanocyte (HEM) cells (104-05n, Cell Application, Inc. USA were cultured in Melanocyte Growth Medium (MGM; Cell Application, Inc. USA supplemented with 100U/ml of penicillin and 100µg/ml of streptomycin (Nacalai Tesque, Japan). Chang cells (CCL-13; American Type Culture Collection [ATCC], USA), a normal human liver cell line, were cultured in complete culture medium of high-glucose Dulbecco's Modified Eagle's Medium (DMEM; Nacalai Tesque, Japan) supplemented with 10% of Fetal Bovine Serum (FBS; JR Scientific, Inc., USA), 100U/ml of penicillin and 100µg/ml of streptomycin, 4mM L-Glutamine (Sigma, USA), 110mg/L Sodium Pyruvate (Sigma, USA) and 25mM HEPES (Nacalai Tesque, Japan). Cells were cultured in a humidified incubator at 37°C with 5% CO2/95% fresh air. The culture medium was changed every 3 days. HEM cells used were all below passage 10.
Cell viability assay
Thiazolyl Blue Tetrazolium Bromide (MTT) assay was carried out to investigate the in vitro cytotoxic effects of the extracts and fractions towards both HEM cells and Chang cells. Cells were trypsinized with Trypsin-EDTA (Gibco, Invitrogen, USA) and seeded at 1 × 104 cells per well in 180µl of complete culture medium into a 96-well, flat-bottom cell culture plate, and incubated overnight. Twenty µl of sterile seaweed extracts and fractions were then added into the wells to final concentrations of 0, 10, 30, 50, 70, 100µg/ml in triplicate, and incubated for another 72h. Fifty µl of fresh, pre-warmed MTT solution (Gibco, Invitrogen, USA; 2mg/ml in Phosphate-Buffered Saline) were added into each well and the mixtures further incubated for 3h. The mixtures were then carefully decanted and replaced with 200µl of DMSO (Applichem, Germany). The formazan salts solubilized were quantified in a microplate reader with wavelength of 554nm against reference wavelength of 690nm (Chan et al., 2011).
Whitening cream formulation
Seaweed extracts and fractions which possessed selective melanocytotoxicity were considered to have potential to exhibit de- pigmentation effects and were sent for formulation into cosmetic creams respectively by Healwell Pharmaceuticals, Sdn. Bhd. 4 Jalan Pengacara U1, Temasya Industrial Park, 40151 Shah Alam, Selangor, Malaysia. According to the formulation stated in Table 1, the seaweed extracts and fractions were formulated at double the respective IC50 concentrations of the in vitro experiments. Basal vehicle creams were prepared using the same formulation. The only exception was that they did not contain any seaweed extracts or fractions.
Table 1.
Ingredients and portions in whitening cream formulation
| No | Ingredient | Portion (%) |
| 1 | Distilled Water | 74.85 |
| 2 | Ethylenediaminetetraacetic acid, EDTA | 0.20 |
| 3 | Propylene Glycol | 3.00 |
| 4 | Urea | 1.50 |
| 5 | Methyl Paraben | 0.20 |
| 6 | Imidazolidinyl Urea | 0.30 |
| 7 | Carbomer | 0.05 |
| 8 | Cetearyl Glucoside in Cetearyl Alcohol | 4.00 |
| 9 | Ceteateth-20 in Cetearyl Alcohol | 3.00 |
| 10 | Cetyl Alcohol | 2.00 |
| 11 | Propyl Paraben | 0.10 |
| 12 | Seaweed extracts solubilized in Denatured Alcohol | 5.00 |
| 13 | Polyacrylamide, C13–C14 Isoparaffin, Laureth-7 | 2.00 |
| 14 | Sodium metabisulfate solubilized in Distilled Water | 0.05g in 2.00ml |
| 15 | Ethoxydiglycol | 1.50 |
| 16 | Perfume | 0.30 |
| Total | 100.00 |
Guinea pig studies
The creams with most potent and promising antihyperpigmentation effects were chosen for this guinea pig study. The hyperpigmentation inductions were executed as previously described with minor modifications (Rahman et al., 2001). Three brown male guinea pigs weighing about 400–600g were used. The back of each guinea pig was cleanly shaved using an electronic shaver (epilator). The guinea pigs were treated gently and anesthetized with pentobarbitone (0.03ml/100g) during hyperpigmentation inductions. The selected spots (1cm diameter circles) on the back of each animal were exposed to UVB radiation (emitted maximally at 315nm) by covering the back of each animal with UV opaque film that has separate circular cutouts of 1cm in diameter. The radiation dosage was measured with a UV radiometer. The UVB intensity used was 1.40mW/cm2 and the total energy dose was 800mJ/cm2 per exposure. For hyperpigmentation inductions, the animals were exposed to UVB radiation for 1 min and then stopped for 2 min. This step was repeated 11 times for each spot for each animal, 3 times a week with at least 1 day interval for 3 consecutive weeks. One day after the last exposure, seaweed creams were topically applied to the hyperpigmented spots twice a day for 3 consecutive weeks. The control sites were treated with basal vehicle cream. Three weeks later, biopsies of selected areas of the skin were taken, processed and embedded in paraffin by standard histological methods. After sectioning using a microtome, the tissue was stained by Fontana-Masson stain (Peng et al., 2001) and the effect of the creams on dermal melanin was observed under a light microscope.
Statistical analysis
All data were analyzed using Student's t-test (two-tailed).
Results and Discussion
Effects of seaweed extracts on mushroom tyrosinase inhibition assay and cell viability assay in HEM and Chang cells
From the results obtained in this study, it can be seen that PTHF, SPHF and SPEt possess greater cytotoxic effects on HEM, with lower cytotoxic activity against Chang cells. From the data it was decided to formulate creams containing SPEt, SPHF and PTHF for further in vivo studies in human volunteers.
Skin whitening agents may act in one of two ways - inhibiting melanogenesis in melanocytes or having selective cytotoxicity towards the melanocytes thus preventing their normal function. A commonly used screening method for potential skin whitening agents is the cell -free mushroom tyrosinase inhibition assay, given the central role of tyrosinase in the synthesis of melanin (Chan et al., 2011; Maeda at al., 1997). This assay is simple and relatively inexpensive to use. However, the ability of the extracts to inhibit mushroom tyrosinase did not exactly match the in vivo de-pigmentation results.
SPEt did reduce mushroom tyrosinase activity over the whole range of concentrations used, SPHF extracts had more inconsistent effects and at the highest doses appeared to actually have a stimulatory effect or “negative inhibition” (Table 2). PTHF too inhibited mushroom tyrosinase activity at the lower doses but higher doses of 500 and 1000µg/ml the effect was again stimulatory instead. Based on these mushroom tyrosinase inhibition studies alone, SPHF and PTHF would have most likely to be excluded from further study. Even though SPEt reduced mushroom tyrosinase activity, however the reduction was modest reaching less than 12% at the maximum, even at high doses of extract, and so would probably be considered only a modestly promising agent at most.
Table 2.
Effects of Sargassum polycystum and Padina tenuis extracts and fractions on mushroom tyrosinase inhibition assay.
| Extracts a | Mushroom tyrosinase activity inhibition (%) b | ||||||||
| 0µg/ml | 10µg/ml | 30µg/ml | 50µg/ml | 70µg/ml | 100µg/ml | 250µg/ml | 500µg/ml | 1000µg/ml | |
| Kojic Acid c | 0.00 | - | - | - | - | 87.15±1.25** | - | - | - |
| SPEt | 0.00 | 5.19±4.60 | 4.81±4.55 | 4.08±3.48 | 6.30±3.97 | 7.06±3.55* | 9.83±2.12** | 11.89±1.88** | 11.63±1.80** |
| SPHF | 0.00 | 3.26±2.71* | 1.08±4.83 | 1.08±4.50 | 5.57±3.14** | 3.60±3.65 | 10.14±8.43* | 3.12±5.19 | −19.34±16.92* |
| SPEA | 0.00 | 4.73±1.61** | 2.47±2.30 | 2.68±0.97* | 5.44±2.22* | 9.63±12.13 | 6.54±1.16** | 2.32±3.44 | 18.72±11.23* |
| SPWF | 0.00 | 7.38±3.32* | 8.50±1.92** | 7.40±2.22** | 7.85±2.33** | 8.24±4.35* | 0.95±7.38 | 0.77±7.74 | 1.79±6.57 |
| PTEt | 0.00 | 6.13±3.42** | 7.31±2.55*** | 7.34±3.17** | 10.07±3.93** | 8.98±3.99** | 11.49±2.37*** | 9.98±3.84** | 11.91±6.54** |
| PTHF | 0.00 | 15.94±4.21** | 14.99±5.68* | 16.34±1.96*** | 18.44±3.28** | 17.30±1.05*** | 9.47±5.56* | −15.95±4.35** | −62.39±12.27** |
| PTEA | 0.00 | 8.36±2.95* | 6.91±2.83* | 9.39±2.82** | 1.14±1.86 | −0.07±5.92 | 2.48±3.05 | −0.71±7.94 | −17.09±20.59 |
| PTWF | 0.00 | 4.47±2.06** | 2.06±2.51 | 3.86±3.03** | 4.87±2.14** | 5.41±6.49 | 6.57±2.07** | 7.19±1.52** | 6.19±3.50* |
Data are presented as mean ± SD of 4 independent experiments. *p<0.05, **p <0.01, ***p<0.001.
SPEt: Sargassum polycystum Ethanol crude extract; SPHF: S. polycystum Hexane fraction; SPEA: S. polycystum Ethyl Acetate fraction; SPWF: S. polycystum Water fraction; PTEt: Padina tenuis Ethanol crude extract; PTHF: P. tenuis Hexane fraction; PTEA: P. tenuis Ethyl Acetate fraction; PTWF: P. tenuis Water fraction.
Activity (%) = Percentage of sample versus control.
Kojic acid was used as positive control at the concentration of 100µg/ml.
The effects of different extracts of S. polycystum and P. tenuis on mushroom tyrosinase activity are elucidated in Table 2. Of all the fractions analyzed, only ethanol extract of S. polycystum (SPEt-Sargassum polycystum Ethanol crude extract) and P. tenuis (PTEt-Padina tenuis Ethanol crude extract) induced possessed consistent inhibition of tyrosinase activity up to extract concentration of 1000µg/ml. However the inhibition was slight, only up to a maximum of below 12 % even at the highest doses. Other fractions had more inconsistent inhibitory activity, and in two fractions, hexane extract of S. polycystum (SPHF-S. polycystum Hexane fraction) and P. tenuis (PTHF-P. tenuis Hexane fraction) there was inhibition at lower concentrations and stimulation (“negative inhibition”) at higher concentrations.
The cytotoxic effects of the extracts of Human Epidermal Melanocyte (HEM) cells and control Chang cells are illustrated in Table 3 and Table 4. With HEM cells, Sargassum polycystum Ethanol crude extract (SPET), S. polycystum Hexane fraction (SPHF) and P. tenuis Hexane fraction (PTHF) had the greatest cytotoxic activity with IC50 values of 36 µg/ml, 65µg/ml and 76.5 µg/ml, respectively. In Chang cells, SPEt also showed some cytotoxicity but the toxicity was lower than that in HEM cells (IC50 61 µg/ml vs 36 µg/ml). PTHF showed considerable toxicity towards HEM cells had little effect on Chang cells. On the other hand, PTEt and PTEA showed greater toxicity towards HEM cells than Chang cells.
Table 3.
Effects of Sargassum polycystum and Padina tenuis extracts and fractions on cell viability assays in HEM cells. Control wells without treatment were set as 100% and results were expressed as percentage to their respective controls
| Extracts a | Cytotoxicity in HEM cells (%) b | IC50 (µg/ml) c | |||||
| 0µg/ml | 10µg/ml | 30µg/ml | 50µg/ml | 70µg/ml | 100µg/ml | ||
| SPEt | 0.00 | 30.2±3.65** | 47.6±10.00* | 54.0±15.61* | 72.5±1.21*** | 77.4±3.79*** | 36 |
| SPHF | 0.00 | −7.4±9.14 | 3.2±10.15 | 30.6±6.09* | 55.3±3.47** | 75.8±17.51* | 65 |
| SPEA | 0.00 | 3.1±5.05 | 18.1±1.80** | 26.0±18.08 | 31.8±10.62* | 18.3±13.56 | >100 |
| SPWF | 0.00 | 31.1±12.51 | 15.8±14.34 | 1.4±14.45 | 22.1±7.70* | 10.5±17.24 | >100 |
| PTEt | 0.00 | −9.0±4.98 | −1.4±4.06 | 0.2±20.58 | 19.7±8.24 | 44.0±12.29* | >100 |
| PTHF | 0.00 | 18.9±19.56 | 38.4±4.09** | 41.5±7.76* | 47.3±9.69* | 60.5±5.97** | 76.5 |
| PTEA | 0.00 | 2.5±19.84 | 13.1±17.85 | 8.7±8.03 | 21.9±4.04* | 20.8±19.72 | >100 |
| PTWF | 0.00 | −4.6±9.28 | 4.7±6.90 | 2.0±8.16 | 13.3±15.92 | 5.1±17.74 | >100 |
Data are presented as mean ± SD of 3 independent experiments. *p <0.05, **p <0.01, ***p<0.001.
SPEt: Sargassum polycystum Ethanol crude extract; SPHF: S. polycystum Hexane fraction; SPEA: S. polycystum Ethyl Acetate fraction; SPWF: S. polycystum Water fraction; PTEt: Padina tenuis Ethanol crude extract; PTHF: P. tenuis Hexane fraction; PTEA: P. tenuis Ethyl Acetate fraction; PTWF: P. tenuis Water fraction.
Activity (%) = Percentage of sample versus control.
IC50 (the concentration which will exert 50% inhibitory effect) was calculated based on the graph plotted using Microsoft Excel 2013.
Table 4.
Effects of Sargassum polycystum and Padina tenuis extracts and fractions on cell viability assays in Chang cells. Control wells without treatment were set as 100% and results were expressed as percentage to their respective controls
| Extracts a | Cytotoxicity in Chang cells (%) b | IC50 (µg/ml) c | |||||
| 0µg/ml | 10µg/ml | 30µg/ml | 50µg/ml | 70µg/ml | 100µg/ml | ||
| SPEt | 0.0 | 9.1±9.98 | 21.8±2.85** | 41.7±7.55* | 54.7±3.34** | 61.0±3.33** | 61 |
| SPHF | 0.0 | −3.9±2.69 | −0.6±3.80 | 5.1±2.98 | 20.2±8.23 | 76.8±13.96* | 86.5 |
| SPEA | 0.0 | −12.3±4.88* | −11.5±3.46* | −0.5±6.86 | 10.2±5.53 | 24.0±8.92* | >100 |
| SPWF | 0.0 | 11.9±2.35* | 9.0±1.62* | 14.0±1.24** | 14.4±1.80** | 19.1±0.14*** | >100 |
| PTEt | 0.0 | 16.4±4.84* | 50.1±1.65*** | 52.2±2.64*** | 48.4±2.84** | 58.7±1.93*** | 30 |
| PTHF | 0.0 | −6.0±6.42 | 4.5±10.59 | 16.3±5.07** | 14.1±7.33 | 39.3±5.87** | >100 |
| PTEA | 0.0 | 3.1±3.57 | 17.8±6.40* | 26.8±6.86* | 40.8±7.77* | 58.6±1.06*** | 85 |
| PTWF | 0.0 | 2.6±4.21 | 5.5±6.43 | 8.9±2.38* | 9.5±6.86 | 12.0±3.42* | >100 |
Data are presented as mean ± SD of 3 independent experiments. *p <0.05, **p <0.01, ***p<0.001.
SPEt: Sargassum polycystum Ethanol crude extract; SPHF: S. polycystum Hexane fraction; SPEA: S. polycystum Ethyl Acetate fraction; SPWF: S. polycystum Water fraction; PTEt: Padina tenuis Ethanol crude extract; PTHF: P. tenuis Hexane fraction; PTEA: P. tenuis Ethyl Acetate fraction; PTWF: P. tenuis Water fraction.
Activity (%) = Percentage of sample versus control.
IC50 (the concentration which will exert 50% inhibitory effect) was calculated based on the graph plotted using Microsoft Excel 2013.
Another consideration for the use of skin whitening agents is to ensure that it should have less cytotoxicity towards normal cells than melanocytes. From the cell viability studies, SPEt, SPHF and PTHF had lower IC50 values on HEM cells than on the Chang cells, and thus showed some degree of selective cytotoxicity (Tables 3 and 4). It was decided to formulate the cream based on these results. The PTEt extract was not used because although it inhibited mushroom tyrosinase (Table 2), it has also showed high cytotoxicity towards the Chang cells (Table 4) which was used to represent normal cells. The doses of extracts used were decided upon following consultation with the formulators of the cream. A dose of double the IC50 was decided upon to ensure that the melanocytes were exposed to an adequate dose of the extract. Melanocytes in the skin are located on the stratum basale which means that the active components of the extract will have to penetrate through several layers, including the stratum corneum, stratum lucidum, stratum granulossum and stratum spinosum before reaching the target cells.
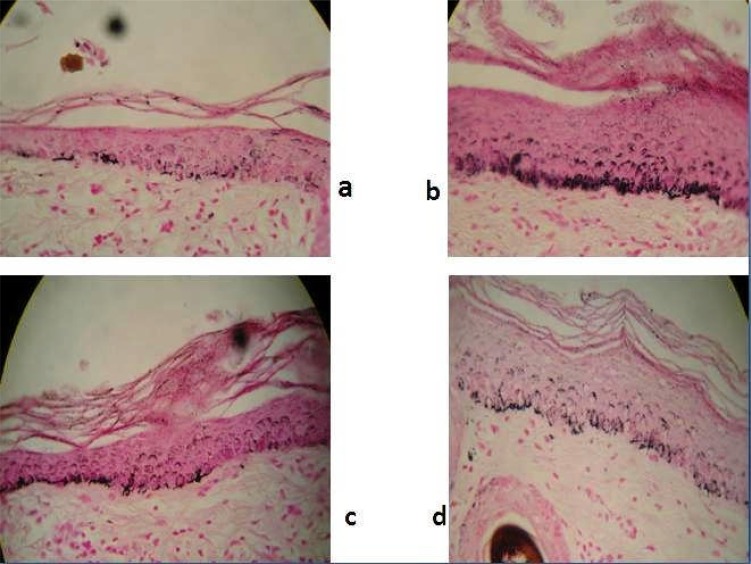
Figures 1 a ∼ d illustrates the melanin distribution in the skin of one guinea pig following exposure to UVB radiation and the effects of treatment with cream containing SPEt and SPHF compared with controls.
Figure 1.
Histopathology images of skin of guinea pig (40X magnification)
Figure 1 a shows that the skin from an area of the skin away from the cut-out region of the UV opaque film that was shielded from UVB exposure. There is a small amount of melanin, which stains black. Fig 1 c is from an area of the guinea pigs skin treated with cream only. This area shows more extensive patches of black melanin staining. Skin from areas that had been treated with cream containing SPHF is been represented in (Fig. 1 c) and that of SPEt is illustrated in (Fig. 1 d) which had less staining for melanin than in Figs 1 a and 1b. A similar pattern was seen in two other guinea pigs. Therefore, the in vivo results need not necessarily be reflective of the in vitro tests, and depending on the in vitro screening tests alone may result in some skin good whitening agents to be overlooked.
The exposure of guinea pig skin to UVB radiation was successful in causing hyperpigmentation after three weeks of exposure. Moreover, the fur which was unevenly colored, continued to overgrow the pigmentated patches and even when shaven it was difficult to assign a score with confidence. Thus it was decided to examine the melanin formation histologically. Although a score could not be assigned here, the dark granules of melanin stained by the Fontana-Masson method in the exposed area of the skin treated with cream base only (control) was noticeably higher than the skin from shielded area, indicating that the UVB radiation induced melanognesis. Treatment with cream containing SPHF and SPEt over 3 weeks after the cessation of UVB irradiation reduced the melanin staining compared to control. The active substances may act by directly inhibiting the tyrosinase, or by affecting the function of the melanocytes by their cytotoxic effects. The mechanism of action and composition of the active ingredients in the extracts are under active investigation.
In conclusion, it appears that the seaweeds S. polycystum and P. tenuis may contain substances that may be useful for the use of skin whitening formulations. Of these S. polycystum appears to be more promising, especially the hexane extracts. Further research is underway to identify the active substances.
Acknowledgements
We would like to thank University Malaya for providing the University Malaya Research Grant (UMRG) RG357/11HTM and Postgraduate Research Fund (PPP) PV061/2011B for this research. Besides, we would like to express our gratitude towards research students Chan Ying Ying, Lee Kar Keh and Ng Wai Jinn for their valuable assistance in this research project.
Abbreviations
- HEM
human epidermal melanocyte
- DOPA
Dihydroxyphenylalanine
- DMSO
Dimethyl sulfoxide
- MGM
Melanocyte Growth Medium
- SPEt
Sargassum polycystum Ethanol crude extract
- PTEt
Padina tenuis Ethanol crude extract
- SPHF
S. polycystum Hexane fraction
- PTHF
P. tenuis Hexane fraction
References
- 1.Chan YY, Kim KH, Cheah SH. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J Ethnopharmacol. 2011;137:1183–8. doi: 10.1016/j.jep.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 2.Chapman VJ, Chapman DJ. Seaweeds and their Uses. Springer Netherlands; 1980. Sea Vegetables (Algae as Food for Man) pp. 62–97. [Google Scholar]
- 3.Dawczynski C, Schubert R, Jahreis G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007;103:891–899. [Google Scholar]
- 4.Debabrata B. Topical Treatment of Melasma. Indian J of Dermaology; 2009;54:303–309. doi: 10.4103/0019-5154.57602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiroyuki N, Hideomi A, Koichi A. Antitumor activity of marine algae. Hydrobiologia. 1990;204:577–584. [Google Scholar]
- 6.Hwang H, Chen T, Nines RG. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int J of Cancer. 2006;119:2742–2749. doi: 10.1002/ijc.22147. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Yokokawa Y, Hatao M, Naganuma M, Tomita Y. Comparison of the melanogenesis in human black and light brown melanocytes. J Dermatol Sci. 1997;14:199–206. doi: 10.1016/s0923-1811(96)00575-0. [DOI] [PubMed] [Google Scholar]
- 8.Miyamura Y, Coelho SG, Wolber R, Miller SA, Wakamatsu K, Zmudzka BZ. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20:2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 9.Park KOC, Huh SY, Choi HR, Kim DS. Biology of melanogenesis and the search for hypopigmenting agents. Dermatologica Sinica. 2010;28:53–58. [Google Scholar]
- 10.Peng LH, Liu S, Xu SY, Chen L, Shan YH, Wei W. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomed. 2013;20:1082–1087. doi: 10.1016/j.phymed.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Piao LZ, Park HR, Park YK, Lee SK, Park JH, Park MK. Mushroom tyrosinase inhibition activity of some chromones. Chem & Pharma Bulletin. 2002;50:309–311. doi: 10.1248/cpb.50.309. [DOI] [PubMed] [Google Scholar]
- 12.Rahman A, Choudhary MI, Thomson WJ. Bioassay Techniques for Drug Development. The Netherlands: Harwood Academic Publishers; 2001. p. 223. [Google Scholar]
- 13.Ruperez P. Mineral content of edible marine seaweeds. Food Chem. 2002;79(1):23–26. [Google Scholar]
- 14.Sheehan D, Hrapchak B. Theory and Practice of Histotechnology. Ohio: Battelle Press; 1987. pp. 226–227. [Google Scholar]
- 15.Tan KK, Kim KH. Evidence-Based Complementary and Alternative Medicine. 2013. Alternanthera sessilis Red Ethyl Acetate Fraction Exhibits Antidiabetic Potential on Obese Type 2 Diabetic Rats; pp. 8–16. Article ID 845172. [DOI] [PMC free article] [PubMed] [Google Scholar]