Abstract
Background
Alpinia oxyphylla (Zingiberaceae), an herbaceous perennial plant, its capsular fruit is commonly used in traditional Chinese medicine for the treatment of different urinary incontinence symptoms including frequency, urgency and nocturia. These symptoms are similar to the overactive bladder syndrome. In our lab, we found that the 95% ethanol extract of the capsular fruits exhibited significant anti-muscarinic activity. Some constituents in capsular fruits including flavonoids (e.g., izalpinin and tectochrysin), diarylheptanoids (e.g., yakuchinone A and yakuchinone B) and sesquiterpenes (e.g., nootkatone), are regarded as representative chemicals with putative pharmacological activities.
Objective
This study aimed to evaluate the in vitro antagonistic actions of izalpinin on carbachol-induced contraction of the rat detrusor muscle.
Materials and Methods
In vitro inhibition of rat detrusor contractile response to carbachol was used to study the functional activity of izalpinin. The isolated detrusor strips of rats were mounted in organ baths containing oxygenated Krebs' solution. The cumulative consecutive concentration-response curves to carbachol-evoked contractions in strips of rat bladder were obtained.
Results
Carbachol induced concentration-dependent contractions of isolated rat bladder detrusor strips. The vehicle DMSO had no impact on the contraction response. The contraction effects were concentration-dependently antagonized by izalpinin, with a mean EC50 value of 0.35 µM. The corresponding cumulative agonist concentration-response curves shifted right-ward.
Conclusions
Izalpinin exhibits inhibitory role of muscarinic receptor-related detrusor contractile activity, and it may be a promising lead compound to treat overactive bladder.
Keywords: Izalpinin, rat bladder, muscarinic receptor, antagonistic action
Introduction
Overactive bladder syndrome arises from the involuntary activity of the detrusor muscle during bladder filling. This condition is characterized by the symptoms of nocturia, urinary urgency and increased frequency of micturition with or without incontinence (Abrams et al., 2003). In China, overactive bladder syndrome has been estimated to occur in nearly 8% of the population and the prevalence of the syndrome increases with advance in age (Zhu, 2009). Moreover, a questionnaire on the occurrence of overactive bladder syndrome, constructed by the Peking University People's Hospital in 2006, showed that total prevalence was approximately 26.7% in 1036 Chinese women aged between 18–81 years and 36.0% in those aged 50 and over (Chen et al., 2007). Therefore, the potential scale of the health economic challenge is readily apparent when the substantial prevalence of overactive bladder syndrome is considered, especially in the ageing society of China.
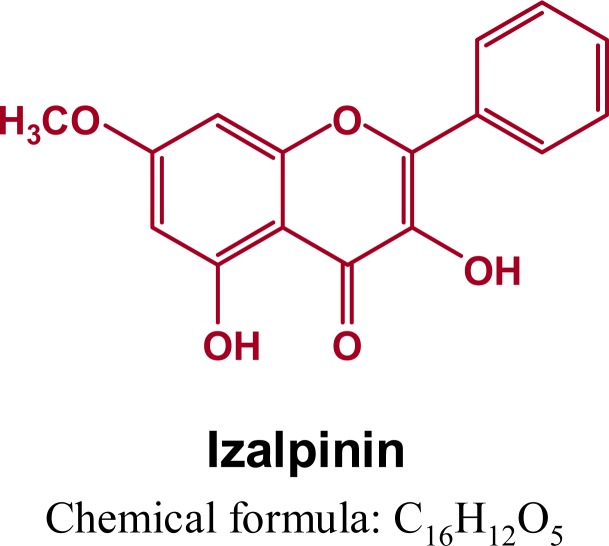
For the therapy of overactive bladder syndrome, many drugs such as anti-muscarinic agents have exhibited significant clinical benefits (Chapple et al., 2008). Meanwhile, some traditional Chinese herbs, such as of Alpinia oxyphylla fruits are documented for the treatment of urinary urgency caused by overactive bladder syndrome in the current Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2010). The history of medicinal use of fruits of A. oxyphylla and its pharmaceutical products in traditional Chinese medicine have attracted considerable attention. In the process of screening for naturally occurring substances with therapeutic effects against bladder over-activity in vitro assay system, we found that the 95% ethanol extract of the fruits of A. oxyphylla exhibited significant antimuscarinic activity. Whereafter, two new natural products and nine known phytochemicals including flavonoid, diarylheptanoid and phenolic acid were isolated from the 95% ethanol extract (Qing et al., 2012). Further investigation is required to clarify whether these isolated compounds can be used to substantiate the pharmacological effects of the ethanol extract? In our preliminary experiments based on the in vitro assay system we found that the chrysin, tectochrysin, kaempherol, yakuchinone A and B, oxyphyllacinol and nootkatone were negative. However, the izalpinin showed the positive results. The chemical structure of izalpinin is shown in Figure 1.
Figure 1.
Chemical structure of izalpinin
This study was undertaken to evaluate the in vitro pharmacological profile of izalpinin on carbachol-induced isolated bladder contraction of rat. Tolterodine was assessed as positive control in the assays. Our results showed that izalpinin was potent in inhibiting carbachol-induced bladder contractions at the designed range of concentrations. These data suggested that izalpinin could be used as a promising lead compound to treat overactive bladder syndrome.
Experimental methods
Materials
Izalpinin was isolated from 95% ethanol extract of A. oxyphylla fruits (Qing et al., 2012) and the purity exceeded 98%. Tolterodine and carbachol were purchased from Hainan HiFly industrial Co.Ltd (Haikou, China). DMSO was obtained from Sigma-Aldrich (St Louis, MO, USA). KCl, NaCl and other chemicals were purchased from Hainan YiGao Instrument Co. Ltd (Haikou, China).
For the in vitro protocol, izalpinin was prepared at 1 mM in DMSO and dilutions made in de-ionized water for appropriate concentration. Tolterodine and carbachol were dissolved in de-ionized water. KCl was prepared in 10% DMSO in de-ionized water. Reported concentrations were the final concentrations in the organ-bath solution.
Animals
All rat experiments were performed in accordance with the Institutional Animal Care and Use Committee at the Hainan Medical University (Haikou, China), as well as the Guidance for Ethical Treatment of Laboratory Animals (The Ministry of Science and Technology of China, 2006). Male Sprague-Dawley rats (280–340 g) were purchased from DongChuang Laboratory Animal Service Department (Changsha, China). Rats were maintained under controlled temperature of 24 ± 2°C and relative humidity of 60 ± 10% with a 12-h light/dark cycle. Commercial rat chows were available ad libitum until to the day of experiment. All rats had free access to water.
Bladder detrusor preparation and functional study
The experimental procedures were performed according to protocols modified from previous published methods (Otsuka et al., 2008; Salcedo et al., 2009; Sinha et al., 2010). Briefly, rats under pentobarbital (50 mg·kg−1, i.p.) anesthesia were killed by bleeding the abdominal aorta (∼10 mL of blood). The rat bladder was isolated and placed in oxygenated Krebs' solution of the following composition (mM): NaCl 114, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, KaH2PO4 1.2, Vit C 1.1 and glucose 11.7. The bladder was quickly removed of adventitia and connective fat tissues and then cut into four longitudinal detrusor strips (∼ 10 × 2 mm). All tissues were then mounted in 25 mL organ baths (ZH-Z type In vitro Organ Measurement System, Huaibei-Zhenghua Biological Instrument, Huaibei, China) containing Krebs' solution, maintained at 37°C and artificially ventilated with 95% O2 and 5% CO2 during the entire period of experiment. A resting tension of the selected bladder detrusor stabilized at 0.5 g. Tissues were equilibrated for 60 min with frequent washes at 15-min intervals and the resting tension was adjusted as necessary. The viability of each tissue was assessed by determining the contractile response to KCl (100 mM) at the start of the experimental protocol. After equilibration, detrusor tissues were stimulated by 1 mM carbachol until resulting two reproducible responses and the isometric tension generated by the tissues were recorded through a force-displacement transducer (JH-2 type, Institute of Space Medical-Engineering, Beijing, China) connected to a BL-420E+ data acquisition system (Chengdu TME Technology, Chengdu, China) and expressed as tension in g.
Next, cumulative concentration-response curves to carbachol in rat bladder detrusor tissues were constructed by cumulatively increasing test compound concentration, which the tissues were allowed to relax to the baseline during the 15-min wash intervals. Tissues were incubated with different concentrations of izalpinin, tolterodine or vehicle for 30 min, after which a second cumulative concentration response curve to carbachol was obtained. To assess the effect of izalpinin and tolterodine, each detrusor tissue was pre-exposed for at least 30 min before the addition of cumulative agonist (i.e., carbachol).
Statistical analysis
In the in vitro study, bladder detrusor strip contractions were recorded as changes in tension from baseline and expressed as a percentage of the maximum response of the highest agonist concentration-response curve. Data were expressed as means ± SD and statistical analysis was carried out with GraphPad Prism software (GraphPad, California, USA). Antagonist potency (pEC50, the negative logarithm of the concentration giving half-maximal effect) was also calculated with the help of this software. Comparisons between groups were carried out with analysis of non-paired Student's t-test. A value of p < 0.05 was considered to be statistically significant.
Results and discussion
The symptoms of overactive bladder syndrome are due, at least in part, to involuntary contractions of the detrusor muscle during the filling phase of the micturition cycle. The involuntary contractions are mediated by acetylcholine-induced stimulation of bladder muscarinic receptors (Robinson and Cardozo, 2010). Antimuscarinic agents (such as tolterodine) serve as the first-line drugs in the clinic management of overactive bladder syndrome by relieving the symptoms of nocturia, urinary urgency and increased frequency of micturition although they have some specific adverse effects (Hegde, 2006). However, cost of medication should be an important consideration. The cost in US dollars of a 30-day supply of oral tolterodine is about $90, or $80 for the long-acting formulation in America (Holroyd-Leduc and Straus, 2004; Shaban et al., 2010). For this reason, some traditional Chinese medicines are often used as alternative therapy to treat overactive bladder syndrome in China, especially for the patients in faraway villages. Unlike the synthetic drugs, however, the underlying mechanisms and active principles of herbal medicines are largely remaining unknown.
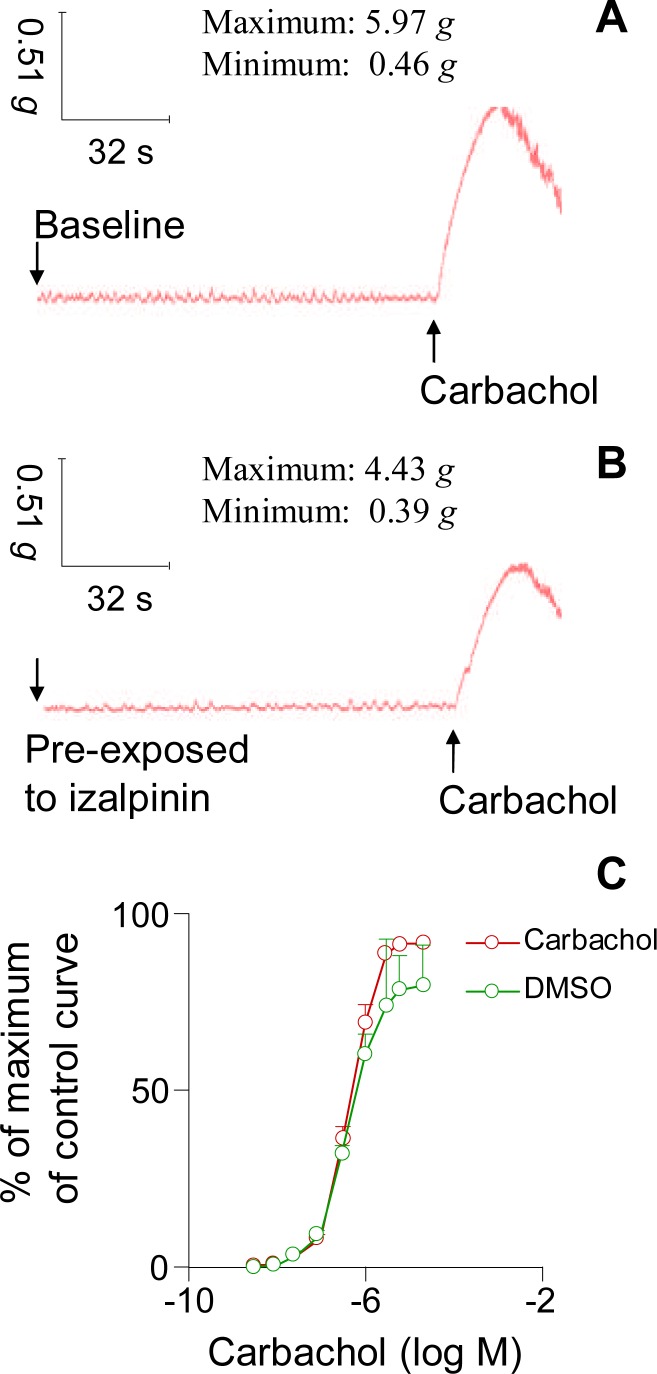
In this study, we investigated the muscarinic receptor antagonistic action of izalpinin, a flavonol isolated from the 95% ethanol extract of fruits of A. oxyphylla. As shown in Fig. 2.A, carbachol induced concentration-dependent contractions of isolated rat bladder detrusor strips. However, the contraction effects stimulated by carbachol at the same concentration (10 µM) were antagonized by the izalpinin which was pre-exposed to the strips (Fig. 2.B). The vehicle DMSO had no influence on the contractions of rat urinary bladder smooth muscle (Fig. 2.C, p>0.05). A consecutive carbachol concentration-response profile (Fig. 2.C) within the assay system without izalpinin yielded a mean pEC50 = 6.10 ± 0.25 (i.e., the mean EC50 = 0.51 µM) with a maximal contraction response (Emax) of 10.2 ± 0.2 g (n = 4). Therefore, the selected detrusor strips responded stably to the stimulation of carbachol with no significant change in agonist potency and maximal response.
Figure 2.
Representative isometric tension curves of isolated rat bladder detrusor strips recorded through BL-420E+ data acquisition system. (A) Baseline curve and the changes stimulated by the addition of carbachol. (B) Pre-exposed to izalpinin and the corresponding changes to the stimulation of carbachol. (C) Vehicle (DMSO) has no influence on the activity of the detrusor strips. Student's t-test was used to compared the two groups data and the p values were more than 0.05, therefore, there were not statistically significant
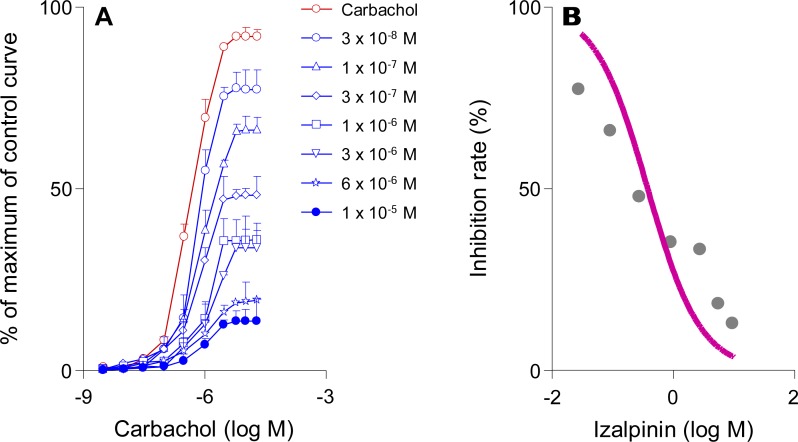
In rat bladder strips, tolterodine had significant impact on the maximum carbachol response at 10 µM (from 3.43% at 25 nM to 99.4% at 10 µM). Moreover, tolterodine shifted the concentration–contraction response curve of carbachol to the right in a concentration-dependent manner (data not shown). The izalpinin concentration-dependently antagonized cumulative agonist concentration-response curves, with parallel right-ward shifts (Fig. 3.A). Izalpinin significantly reduced Emax at 1 µM and 10µM concentrations (to 64.3% and 86.6% respectively) when the contraction effect was induced by carbachol at 10 µM (Figure 3.A). The concentration-response relationship of izalpinin was in shown in Figure 3.B with a mean EC50 of 0.38 µM. Overall, the pEC50 of izalpinin was 0.46 ± 0.07 and the corresponding EC50 was 0.35 ± 0.05 µM. In addition, izalpinin almost yielded Schild regression lines, with slopes close to unity (Figure 3.A).
Figure 3.
Effects of izalpinin on the cumulative consecutive concentration-response curves to carbachol on isolated rat bladder detrusor strips. (A) The effects of izalpinin (30 nM−10 µM) on the carbachol concentration (3 nM−20 µM)-response curves are shown. (B) Dose-response relationship profile of izalpinin (30 nM−10 µM) against the carbachol at 10 µM. Direct contractile effects were expressed as percentages of the maximum response of the control curve. Data are expressed as means ± SD, n = 3–4 animals per concentration.
As well known, the side effects of some antimuscarinic agents (such as tolterodine) mainly stem from a lack of selectivity, which compromise their clinical application. From a physiological point of view, the bladder smooth muscle of most species, including human beings, contains a mixed population of M2 and M3 muscarinic receptors (Hegde and Eglen, 1999; Yamanishi et al., 2002). M3 receptors produce direct smooth muscle contraction, while the M2 receptors, the predominant acetylcholine receptors in bladder, appear to facilitate M3-mediated contractions (Hegde, 2006; Hegde and Eglen, 1999; Yamanishi et al., 2002). Because of this, there are clear potential benefits in terms of efficacy and tolerability to be provided by selective antagonists of M3 receptors subtype. Therefore, binding affinity evaluation of izalpinin for human recombinant muscarinic receptor subtypes is worthy of further research.
There are some limitations in the present study. We did not conduct pharmacological in vivo studies to illustrate the role of izalpinin on bladder function in rats or other animals. It will also be of interest to assess the activity of izalpinin on human detrusor specimens. Thus, further investigation is required to clarify whether izalpinin is an effective and safe therapy for the treatment of overactive bladder syndrome in animals and/or human beings.
Conclusion
In summary, izalpinin demonstrated bladder muscarinic receptor antagonistic action on the isolated rat detrusor smooth muscle. This compound might be used as a promising lead compound for the treatment of overactive bladder syndromes.
Acknowledgements
This work was funded by grants from National 12th Five-Year Plan Regional Base Project (2011BA101B07), Hainan Special Plan for the Modernization of Chinese Medicines (2010ZY012 and 2011ZY004), natural science foundation of Hainan province (812189 and 813196) and research development fund supported by Hainan Medical University (HY2012-006 and HY2012-013).
Conflict of interests: The authors state no conflict of interest.
Abbreviations
- DMSO
dimethyl sulfoxide
- pEC50
potency Negative logarithm of the concentration giving half-maximal effect
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008;54:543–562. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Xu KX, Wang XF. Prevalence of overactive bladder syndrome in women and its effects on quality of life [Conference paper]; CUA 2007 annual meeting (14th); 2007. [Google Scholar]
- 4.Chinese Pharmacopoeia Commission, author. Pharmacopoeia of the People's Republic of China 2010 English edition. Vol. 1. Beijing: China Medical Science Press; 2010. pp. 28–29. 2010. [Google Scholar]
- 5.Hegde SS. Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol. 2006;147(Suppl 2):S80–S87. doi: 10.1038/sj.bjp.0706560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999;64:419–428. doi: 10.1016/s0024-3205(98)00581-5. [DOI] [PubMed] [Google Scholar]
- 7.Holroyd-Leduc JM, Straus SE. Management of urinary incontinence in women: clinical applications. JAM. 2004;291:996–999. doi: 10.1001/jama.291.8.996. [DOI] [PubMed] [Google Scholar]
- 8.Otsuka A, Shinbo H, Hasebe K, Matsumoto R, Ozono S. Effects of a novel beta(3)-adrenoceptor agonist, AJ-9677, on relaxation of the detrusor muscle: an in vitro study. Int J Urol. 2008;15:1072–1076. doi: 10.1111/j.1442-2042.2008.02165.x. [DOI] [PubMed] [Google Scholar]
- 9.Qing ZJ, Yong W, Hui LY, Yong LW, Long LH, Ao DJ, Xia PL. Two new natural products from the fruits of Alpinia oxyphylla with inhibitory effects on nitric oxide production in lipopolysaccharide-activated RAW264.7 macrophage cells. Arch Pharm Res. 2012;35:2143–2146. doi: 10.1007/s12272-012-1211-7. [DOI] [PubMed] [Google Scholar]
- 10.Robinson D, Cardozo L. New drug treatments for urinary incontinence. Maturita. 2010;65:340–347. doi: 10.1016/j.maturitas.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Salcedo C, Davalillo S, Cabellos J, Lagunas C, Balsa D, Perez-Del-Pulgar S, Ballarin M, Fernandez A. In vivo and in vitro pharmacological characterization of SVT-40776, a novel M3 muscarinic receptor antagonist, for the treatment of overactive bladder. Br J Pharmacol. 2009;156:807–817. doi: 10.1111/j.1476-5381.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaban A, Drake MJ, Hashim H. The medical management of urinary incontinence. Auton Neurosci. 2010;152:4–10. doi: 10.1016/j.autneu.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Sinha S, Gupta S, Malhotra S, Krishna NS, Meru AV, Babu V, Bansal V, Garg M, Kumar N, Chugh A, Ray A. AE9C90CB: a novel, bladder-selective muscarinic receptor antagonist for the treatment of overactive bladder. Br J Pharmacol. 2010;160:1119–1127. doi: 10.1111/j.1476-5381.2010.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamanishi T, Chapple CR, Yasuda K, Yoshida K, Chess-Williams R. The role of M2 muscarinic receptor subtypes mediating contraction of the circular and longitudinal smooth muscle of the pig proximal urethra. J Urol. 2002;168:308–314. [PubMed] [Google Scholar]
- 15.Zhu GW. China Pharmaceutical News. 2009 Nov 24;:B07. [Google Scholar]