Abstract
Background
Natural products such as herbs, fruits, spices, beverages, vegetables are becoming more popular among scientific community and consumers because of their potential to arrest the effect of free radicals in human system. This study determined the total antioxidant capacity of ten selected species of Zingiberaceae (Ginger) used as spices and for medicinal purposes in Southeast Asia.
Materials and Methods
Methanol was used as the extraction solvent, 2,2 - diphenyl-1-picrylhydrazil (DPPH) for free radical scavenging activity and ferric reducing antioxidant power (FRAP) assays. Phenolic compounds were measured using Total flavonoid, Phenolic acid and Polyphenols content assay to evaluate the quality of the antioxidant capacity of the rhizomes and vitamin C as positive control.
Results
The results obtained revealed that Curcuma longa and Zingiber officinale had the highest free radical scavenging capacity of 270.07mg/TE/g DW and 266.95mg/TE/g DW and FRAP assay, Curcuma longa and Zingiber officinale also gave the highest ferric reducing power of 231.73mg/TE/g DW and 176.26mg/TE/g DW respectively. For Phenolic compounds, Curcuma longa and Curcuma xanthorrhiza gave the highest values of flavonoid (741.36mg/NGN/g DW and 220.53mg/NGN/g DW), phenolic acid (42.71mg/GAE/g DW and 22.03mg/GAE/g DW) and polyphenols (39.38mg/GAE/g DW and 38.01mg/GAE/g DW) respectively. Significant and positive linear correlations were found between Total antioxidant capacity and Phenolic compounds (R = 0.65 – 0.96).
Conclusion
This study provides evidence that extracts of Zingiberaceae (Ginger) rhizomes are a potential source of natural antioxidants and could serve as basis for future drugs and food supplements
Keywords: Zingibearaceae, Antioxidant, Free Radical, Oxidative stress, DPPH, Flavonoid, Phenolic acid, Polyphenol
Introduction
Plants with medicinal properties have been found to contain various antioxidants that are present as secondary metabolites which are synthesized as defense mechanism. Antioxidant capacity is related with compounds capable of protecting a biological system against potential harmful effects of processes or reactions involving reactive oxygen and nitrogen species (ROS and RNS), (Ayse et al., 2008).
These reactive species are produced as a result of metabolic activities in the body which must be at equilibrium with the antioxidant. An equilibrium shift to reactive species leads to increase in the concentration of these reactive species which cause anti-oxidative stress. Oxidative stress results in the damage of biopolymers including nucleic acids, protein, polyunsaturated fatty acids and carbohydrate (Badarinath et al., 2010). Oxidative stress result in serious health complications like cancer, atherosclerosis, aging, immuno-suppression, diabetes, hair loss, inflammation, heart disease, ischemic and neurodegenerative disorder (Beal et al., 1995., Maxwell, 1995., Poulson et al., 1998). Antioxidants play a major role in mopping up these reactive oxygen and nitrogen species by scavenging for free radicals in biological system.
Zingiberacaeae family is a plant specie endowed with anti-oxidative properties. It is the largest family of the order Zingiberales. It is widely distributed throughout the tropics particularly in Southeast Asia. It is estimated that there are 150 species of ginger belonging to 23 genera found in peninsular Malaysia (Holttum, 1950). Zingiberaceae species grow naturally in damp, shaded part of the lowland or on hill slopes, as scattered plants or thicket. Zingiberaceae produces aromatic rhizomes that are above the ground or subterranean (Soepadmo, 1976). Most members of the family are easily recognized by the characteristic aromatic leaves and fleshy rhizome when both are crushed and also by the elliptic to elliptic-oblong leaves arranged in two ranks on the leaf shoot. In Southeast Asian region, several species of Zingiberaceae are used as spices, condiment, traditional medicines, flavoring agents and as the source of certain dyes (Burkill, 1996), (Larsen et al., 1999). The work of (Ibrahim et al., 2007) reviewed different types of gingers grown in Peninsular Malaysia. Several species from the genera Apinia, Amomum, Curcuma, Costus, Kaempferia and Zingiber are major ingredients in traditionally prepared tonics locally called “Jamu” which are commercially available in Indonesia and Malaysia (Habsah et al., 2000). The Rhizomes are either eaten raw or cooked as vegetables (Larsen et al., 1999). Some usually grown species are Zingiber officinale, Curcuma longa and Alpinia galanga.
Rhizomes of Z. officinale are usually used as flavour and additives in beverages and food industry. Curcuma longa rhizomes are commonly used as spice in curries for coloring and flavoring. Rhizomes of galanga are used as spice for meat dishes. Women consumed the rhizome of ginger traditionally during illness, ailment and confinement. They are also consumed as a carminative for relieving flatulence (Eric et al., 2011). In Thailand, C. longa, B. rotunda, A. galanga and Z. officinale rhizomes have been used as traditional medicine for treating diarrhea, stomach ache and as carminative (Oonmetta-aree et al., 2006). Z. zerumbet rhizomes are also used for de-worming of children, used as cure for loss of appetite, sores and swelling (Somchit and Shukriyah., 2003), (Faizah et al., 2002).
The aims of this study is to evaluate the antioxidant capacity and the free radical scavenging activity of ten selected species of Zingiberaceae using 2,2-diphenyl-1-picrylhydrazil (DPPH), and Ferric reducing capacity antioxidant power assay, to determine the Total flavonoid content (TFC), Total phenolic acid (TPA), Total Polyphenols (TPP), and investigate the correlations between flavonoid, phenolic acids polyphenols and antioxidant activity.
Materials and Methods
Chemicals and reagents
DPPH, 2, 4, 6-tri (2-pyridyl)-S-triazine (TPTZ), Trolox, Folin-Ciocalteu's phenol reagent, and Naringenin were purchased from Sigma Co. St. Louis, Missouri, USA. Sodium carbonate, Methanol, Aluminum Chloride, Iron (III) chloride hexahydrate, Gallic acid, Sodium nitrite and Acetic acid and Sodium hydroxide were purchased from Merck, Darmstadt, Germany. All chemicals and reagents were of analytical grade.
Plant
Ten selected Zingiberaceae species (Gingers) Rhizomes commonly used as spices, anti-skin aging and medicinal purposes in Southeast Asia were planted at Tanam Pertanian Universiti, Universiti Putra Malaysia (University Agricultural Park), and the rhizomes were harvested after one month of growth. These rhizomes were identified and confirmed by the Herbal Garden Department, Universiti Putra Malaysia (UPM).
Sample preparation
Samples were washed cleaned in running tap water, cuts into small pieces and dried to a constant weight using the oven at 70°C. The samples were pounded into powder form using pestle and mortar; it was stored in an air tight container till when needed.
Extraction
0.5g of each sample was weighed using full analytical balance, GR 200 model into a round bottom flask and 25mL of 100% methanol was added. The mixture was refluxed at 60°C for 2hrs. The mixture was filtered using the Whatman No.1 filter paper and the filtrate was stored in 15mL Amber bottle in −20°C refrigerator (Norhaiza et al., 2009).
Determination of Total Antioxidant Capacity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) which is stable was used to determine the free radical scavenging capacity of each sample using the method described by (Wong et al., 2006). 0.1mM solution of methanol DPPH was prepared and the absorbance was measured at 515nm using (spectrophotometer UV-2602, Labomed, Inc. USA). 60uL of each sample was added to 3mL of 0.1mM DPPH in methanol and were incubated for 30mins at room temperature in the dark. The change in absorbance was then measured at 515nm. The experiment was done in three replicates. Percentage inhibition was calculated using the formula (%) inhibition = [(A515 control - A515 sample)/ A515 control] x 100 where A515 is absorbance at 515nm. The antioxidant capacity of the extracts using DPPH for free radical scavenging ability was expressed as mg Trolox equivalent per gram of dry weight of sample. Vitamin C was used as control.
Ferric Reducing Antioxidant Potential (FRAP)
The ability of the extracts to reduce the yellow TPTZ complex was conducted using Benzie and Strain (1996), method. The FRAP reagent is made in ratio of 10:1:1 from the following reagent respectively 300mM sodium acetate buffer at pH 3.6, 10mM TPTZ solution, and 20mM Fecl.6H2O. 20uL of extracts was added to 3mL of FRAP reagent that was prepared. The mixture was incubated in a water bath which has been maintained at 37°C for 30mins. The experiment was done in three replicates. A change in absorbance was measured at 593nm using (spectrophotometer UV-2602, Labomed, Inc. USA). The antioxidant capacity was calculated using the formula Antioxidant (%) = [(A593 sample - A593 control) / A593 sample] × 100 where A593 is absorbance at 593nm and FRAP reagent was used as control. Total antioxidant capacity was expressed as mg Trolox equivalent per gram of dry weight of sample. Vitamin C was used as positive control.
Determination of Phenolic Compounds
Total flavonoid Assay
Total flavonoid of extracts was estimated using (Marinova et al., (2005). Aluminum chloride colorimetric method was used to determine the Total flavonoid content of the extracts. 200uL of extracts to 4mL of distilled water, 0.3mL 5% NaNO2 was then added and after 5mins 0.3mL of 10% AlCl3 was added. After 6mins, 2mL of 1M NaOH was then added. 3.2mL of distilled water was added to the mixture to make a total volume of 10mL. The mixture was mixed vigorously and the absorbance was taken at 510nm. Total flavonoid was expressed as mg Naringenin equivalents (Ng)/g samples.
Total Phenolic acid Assay
The Total phenolic content in the extracts was estimated using Folin - Ciocalteu method by Singleton and Rossi (1965). 100uL of extracts was added to 9mL of distilled water. 1mL of Folin - Ciocalteu's phenol reagent was then added and the mixture was mixed well. After 5mins, 10mL of 7% of Na2CO3 was added. 4.9mL of distilled water was added to make a total volume of 25mL; the mixture was then incubated at room temperature for 90mins. Absorbance was taken at 750nm and total phenolic acid content was expressed as mg gallic acid equivalents (GAE)/g of dry samples.
Total Polyphenol Assay
The Total Polyphenol content in the extracts was estimated using Folin - Ciocalteu method by (Hakiman et al., 2012). The Folin - Ciocalteu was diluted 10 times. 100uL of extracts was added to 2.5mL of Folin - Ciocalteu's phenol reagent and 2.5mL of 7% of sodium carbonate was added after 5mins. The mixture was incubated at room temperature for 1hr. Absorbance was taken at 725nm and total polyphenol content was expressed as mg gallic acid equivalents per gram of dry samples (mg GAE/g DW).
Statistical analysis
All experiments were replicated thrice and results are presented as mean ± standard deviation (SD) of three replicates and analyzed using One-way ANOVA, the difference between samples were determined by Duncan's Multiple Range test (p<0.05) using SPSS Statistics version 21. Correlation coefficients (R) were calculated using Microsoft Excel 2010.
Results
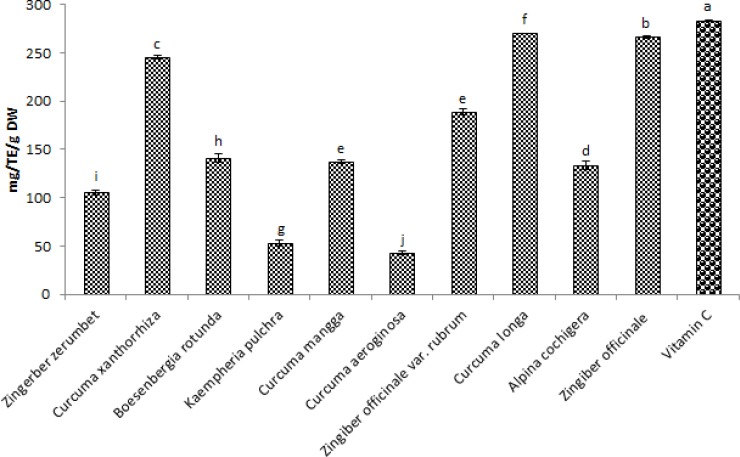
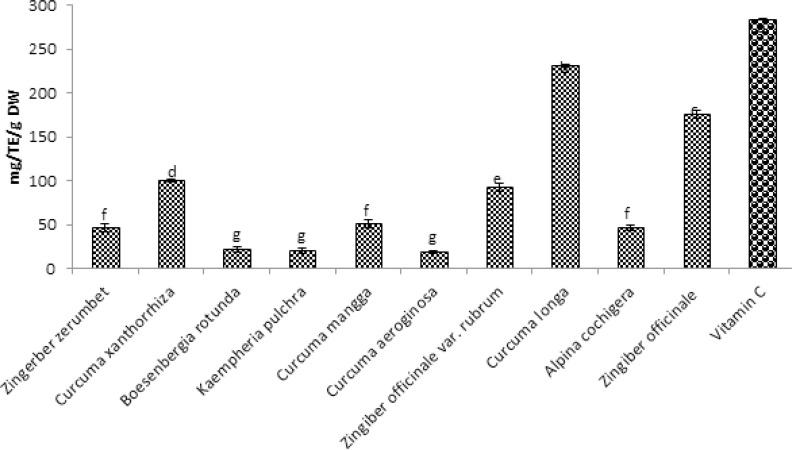
The Total antioxidant capacity of methanol extracts of ten selected species of Zingiberaceae (ginger) using DPPH and FRAP were evaluated. The phenolic content (Total flavonoid, Total Phenolic acid and total polyphenols), was also evaluated. The results of DPPH and FRAP for total antioxidant capacity of the ten samples are shown in Figure 1 and 2.
Figure 1.
Total antioxidant capacity of ten selected Zingiberaceae species using DPPH method for free radical scavenging assay expressed as Trolox equivalent in methanol extractions. Values are means ± SD (n = 3). Bar represents standard error.
Figure 2.
Total antioxidant capacity of ten selected Zingiberaceae sp. using FRAP method for Ferric reducing antioxidant power assay expressed as Trolox equivalent in methanol extractions. Values are means ± SD (n = 3). Bar represents standard error.
In figure 1, the ten species of Zingiberaceae extracts tested for free radical scavenging capacity shows a significant results with the extract of Curcuma longa having the highest scavenging activity with the value 270.07mg/TE/g DW, follow by Zingiber officinale with value of 266.95mg/TE/g DW and the lowest free radical scavenging activities were seen in Curcuma aeroginosa and Kaempheria pulchra with the values 43.21mg/TE/g DW and 52.81mg/TE/g DW respectively and Vitamin C which serves as positive control has 283.69mg/g as its free radical scavenging activity.
In figure 2, the ability to reduce yellow ferric tripyridyl triazine complex by the ten Zingiberaceae extracts was observed with extract of Curcuma longa having the highest activity with the value 231.73mg/TE/g DW and followed by Zingiber officinale with value of 176.26mg/TE/g DW. The lowest extracts with the Ferric reducing activities were observed in Curcuma aeroginosa and Kaempheria pulchra with 18.94mg/TE/g DW and 19.77mg/TE/g DW values respectively. Vitamin C which serves as positive control has 284.23 mg/g as its ferric reducing power activity.
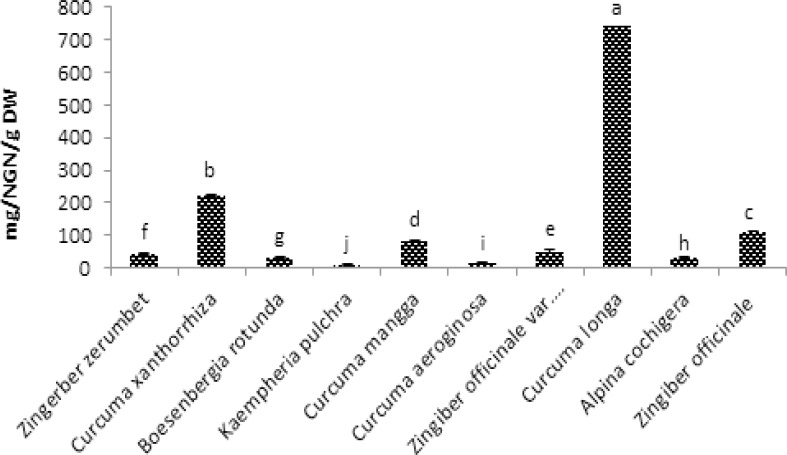
Figure 3, shows the Total flavonoid content of the methanol extract of the ten species of Zingiberaceae. Curcuma longa and Curcuma xanthorrhiza shows the highest Total flavonoid content with the values of 741.36mg/NGN/g DW and 220.53mg/NGN/g DW respectively while the lowest flavonoid content was seen in the extract of Kaempheria pulchra with value of 8.3mg/NGN/g DW then follow by Curcuma aeroginosa with 14.97mg/NGN/g DW flavonoid content.
Figure 3.
Total flavonoid content of ten selected Zingiberaceae sp. in methanol extractions expressed as Naringenin equivalent. Values are means ± SD (n = 3). Bar represents standard error.
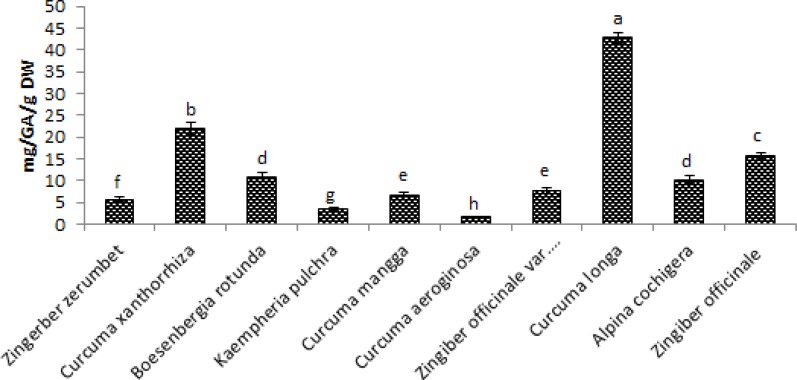
Figure 4, shows the results of Total Phenolic acid of the methanol extract of the ten species of Zingiberaceae. All extracts shows significant activities' with Curcuma longa and Curcuma xanthorrhiza showing the highest Total Phenolic acid with the values of 42.71mg/GAE/g DW and 22.03mg/GAE/g DW respectively while the lowest Total Phenolic acid was seen in the extract of Curcuma aeroginosa with value of 1.80mg/GAE/g DW then follow by Kaempheria pulchra with 3.43mg/GAE/g DW Total Phenolic acid content.
Figure 4.
Total Phenolic acid content of ten selected Zingiberaceae sp. in methanol extractions expressed as Gallic acid equivalent. Values are means ± SD (n = 3). Bar represents standard error.
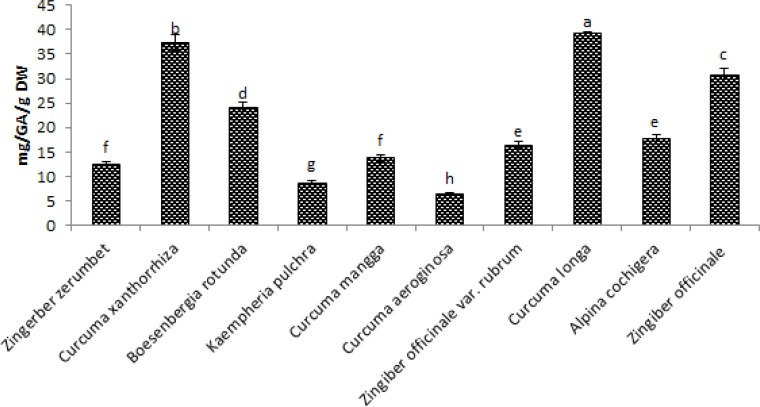
Figure 5, shows the results of Total Polyphenol content of the methanol extract of the ten species of Zingiberaceae. All extracts shows significant activities' with Curcuma longa and Curcuma xanthorrhiza showing the highest Total Polyphenol content with the values of 39.38mg/GAE/g DW and 38.01mg/GAE/g DW respectively while the lowest Total Phenolic acid was seen in the extract of Curcuma aeroginosa with value of 6.41mg/GAE/g DW then follow by Kaempheria pulchra with 8.78mg/GAE/g DW Total Polyphenol content.
Figure 5.
Total Polyphenol content of ten selected Zingiberaceae sp. in methanol extractions expressed as Gallic acid equivalent. Values are means ± SD (n = 3). Bar represents standard error.
From table 1, it can be observed that all antioxidant and phenolic compounds (Total flavonoid, Total phenolic acid and Total Polyphenols), investigated correlate with each other positively. The highest correlation from table 1 can be found between flavonoid and phenolic acid with r = 0.96. Other high correlations can also be seen between DPPH and Polyphenol with r = 0.92 and between DPPH and FRAP with r = 0.88.
Table 1.
Correlation between total antioxidant content (DPPH and FRAP), against Phenolic compounds (Total flavonoid, Total phenolic acid and Total Polyphenol) in methanol extracts of ten selected sp. of Zingiberaceae. All values of correlation are significant at p < 0.05.
| FRAP | FLAVONOID | POLYPHENOL | PHENOLIC ACID | |
| DPPH | 0.88 | 0.65 | 0.92 | 0.80 |
| FRAP | --- | 0.83 | 0.80 | 0.86 |
| FLAVONOID | --- | --- | 0.72 | 0.96 |
| POLYPHENOL | --- | --- | --- | 0.88 |
Discussion
The role of free radical has been established in different diseases such as atherosclerosis, cancer, diabetes, cirrhosis, aging etc (Halliwell and Gutteridge, 1985). Therefore, compound that can scavenge these free radicals have great potentials to prevent or inhibit these degenerative diseases. Antioxidants are such compound with radical scavenging activity. From figure 1, all the extracts investigated using DPPH exhibited free radical scavenging capacity with the highest observed in Curcuma longa and Zingiber officinale which is in tandem with the previous work of (Aruoma et al., 1997) that Zingiber officinale contain high antioxidants such as β-Carotene, ascorbic acid (vitamin C), terpenoids, alkaloids and polyphenols. Zingiber officinale is confirmed to be rich in antioxidants (Kikuzaki and Nakatani, 1993).
The observed antioxidant of extracts may be due to the neutralization of free radical (DPPH), either transfer of hydrogen atom or by transfer of an electron (Naik et al., 2003). In DPPH method, the plant extracts behaved as radical scavengers and inhibit Perioxidation radical to terminate per oxidation chain reactions that is caused by lipid peroxidation process (Liu and Yao, 2007). In the presence of radical scavenger (extracts), as hydrogen donor, DPPH will be transformed into non-radical form and their reduction can be measured at 515nm (Prior et al., 2005). The correlation analysis reveals a close relationship between the results obtained by DPPH and polyphenol content ( r = 0.92) and between the results obtained by DPPH and FRAP (0.88), DPPH and Phenolic acid (0.80), DPPH and flavonoid (0.65).
In figure 2, reduction of ferric - tripyridyltriazine to the ferrous complex which form a deep blue color can be measured at 593nm. The intensity of the color is directly proportional to the amount of antioxidant reductants in the extracts. Curcuma longa and Zingiber officinale possess the highest FRAP activity suggesting both to be richer in antioxidant reductants. Thaipong et al., (2006) reported that FRAP method can be used to determine antioxidant activity. A strong correlation is observed between FRAP and flavonoid (0.83), FRAP and polyphenols (0.80) and FRAP and phenolic acid (0.86). This is a strong indication that the reduction of ferric tripyridyltriazine in the extracts is due to the presence of flavonoid.
The Total flavonoid content as indicated in figure 3 revealed Curcuma longa and Curcuma xanthorrhiza to have most abundant flavonoid content. This supports why Curcuma longa have numerous biological activities such as anti-microbial and as protective compounds against plant diseases (Wang et al., 1989). Antioxidant activities of flavonoid are influence by hydroxylation and presence of sugar moiety (Bravo, 1998). Flavonoids can make complexes with metal and inhibit metal initiating lipid oxidation (Hendrich et al., 1999). Strong correlation between flavonoid, polyphenol and phenolic acid exist (0.72 and 0.96).
Figure 4 and 5 shows the Total phenolic acid and total polyphenols content with similarities in activity. Curcuma longa and Curcuma xanthorrhiza have the highest activities in both methods with 42.71mg/GAE/g DW and 22.03mg/GAE/g DW respectively for total phenolic acid and 39.38mg/GAE/g DW and 38.01mg/GAE/g DW respectively for total polyphenol. Strong correlations exist between the two methods (0.88). This activities is due to the reduction of ferric complex which is corroborated by the high correlation that exist between FRAP and phenolics.
Conclusion
All Zingiberaceae species evaluated revealed strong antioxidant capacity with Curcuma longa and Zingiber officinale having the strongest free radical scavenging activities and high antioxidant reductants capacity while there is consistency in the activities of Curcuma longa and Curcuma xanthorrhiza. This study has provided evidence that these three Zingiberaceae species are potential source of antioxidants and could serve as basis for future drugs and food supplements.
List of abbreviations
- NGN
Naringenin
- GAE
Gallic acid equivalent
- TE
Trolox equivalent
- TPTZ
2,4,6 - tri-(2-pyridyl)-1,3,5-triazine
References
- 1.Aruoma OI, Spencer JP, Warren D, Jenner P, Butler J, Halliwell B. Characterization of food antioxidants, illustrated using commercial garlic and ginger preparation. Food Chem. 1997;60:49–156. [Google Scholar]
- 2.Ayse K, Beraat O, Samin S. Review of methods to determine antioxidant capacities. Food Anal Method. 2009;2:41–60. [Google Scholar]
- 3.Badarinath AV, Mallikarjuna K, Madhu SOC, Ramkanth S, Rajan TV, Gnanaprakash K. A review on in vitro antioxidant methods: Comparisons, correlations and considerations. Intl Jour Pharmtech Res. 2010;2(2):1276–1285. [Google Scholar]
- 4.Beal MF. Aging, energy and oxidative stress in neurodegenerative diseases. Annals Of Neurology. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 5.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal Biochem. 1996;23(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 6.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 7.Burkill IH. A dictionary of the economic products of the Malay Peninsula. London: Crown agent; 1966. [Google Scholar]
- 8.Eric C, Lim Y Y, Wong SK. Antioxidant properties of ginger leaves: An overview. Free Rad And Antiox. 2011;1(1):6–16. [Google Scholar]
- 9.Faizah S, Somchit MN, Shukryah MH. Zingiber zerumbet (Lempoyang): A potential anti - inflammatory agent. In: Omar R, Ali Rahman Z, Latif MT, Lihan T, Adam Jh, editors. Proceedings of Regional symposium on environmental and Natura Resour. Kuala Lumpur, Malayasia: Hotel Renaissance; 2002. Apr 10 – 11, pp. 516–520. [Google Scholar]
- 10.Habsah M, Amran M, Mackeen MM, Lajis NH, Kikuzaki H, Nakatani N, Rahman AA, Ghafer AM, Ali Screening of Zingiberaceae extracts for antimicrobial and antioxidant activities. Jour Of Ethnpharm. 2000;72:403–410. doi: 10.1016/s0378-8741(00)00223-3. [DOI] [PubMed] [Google Scholar]
- 11.Hakiman M, Mohd AS, Ahmad S, Mahmood M. Total antioxidant, polyphenol, phenolic acid and flavonoid content in Ficus deltoidea varieties. Jour Plant Res. 2002;6(33):4776–4784. [Google Scholar]
- 12.Halliwell B, Gutteridge JMOC. Free Rad Biol And Med. 2nd editn. Oxford, UK: Clarendron press; 1985. Free radicals aging and disease; pp. 279–315. [Google Scholar]
- 13.Hendrich S, Wang GJ, Lin HK, Xu X, Tew BY, Wang HY, Murphy PA. Isoflavone metabolism and bioavailabilty. In: Papus Am., editor. Antioxidant status, diet, nutrition and health. Boca Raton, Fla: CRC Press; 1999. pp. 211–230. [Google Scholar]
- 14.Holttum RE. The Zingiberaceae of the Malay Peninsula. Gard Bullen Of Singapore. 1950;13:1–249. [Google Scholar]
- 15.Ibrahim H, Khalid N, Hussin K. Cultivated gingers of Peninsula Malayasia: Utilization, Profiles and Micropropagation. Gard Bull Sing. 2007;59:77–88. [Google Scholar]
- 16.Kikuzaki H, Nakatani N. Antioxidant effect of some ginger constituents. Jour Food Sci. 1993;578:1407–1410. [Google Scholar]
- 17.Larsen K, Ibrahim H, Khaw SH, Saw LG. Ginger of Peninsula Malaysia and Singapore. Kota Kinabalu: Natura. History publicatns; 1999. (Borneo) [Google Scholar]
- 18.Liu Q, Yao H. Antioxidant activities of barley seeds extracts. Food Chem. 2007;102:732–737. [Google Scholar]
- 19.Marinova D, Ribarova F, Antanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. Jour Uni Chem Tech Metallurgy. 2005;40:255–260. [Google Scholar]
- 20.Maxwell SRJ. Prospects for the use of antioxidant therapies drugs. 1995;45:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 21.Naik GH, Priyadersini KL, Satav JG, Bansvalikar MM, Sohoni PP, Biyani MK. Comparative antioxidant activity of individual herbal component used I Ayurvedic medicine. Phytochem. 2003;63:97–104. doi: 10.1016/s0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- 22.Norhaiza M, Maziah M, Hakiman M. Antioxidative properties of leaf of popular herb, Labisia pumila. Jour Plant Res. 2009;3(4):217–223. [Google Scholar]
- 23.Oonmetta-aree J, Suzuki T, Gasaluck P, Eumkeb G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT - Food sci tech. 2006;39:1214–1220. [Google Scholar]
- 24.Poulson HE, Preime H, Loft S. Role of oxidative DNA damage in cancer initiation and promotion. Euro Jour Of cancer Preven. 1998;7:9–16. [PubMed] [Google Scholar]
- 25.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in food and dietary supplement. Jour Agricult Food Chem. 2005;53:4290–4303. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 26.Singlton VL, Rossi JA. Colorimetry of total phenolics with Phoshosmolybdic - phostungstic acid reagent. Ame Jour Soc Enol Viticult. 1995;16:144–158. [Google Scholar]
- 27.Soepadmo E. Ginger plants. Natur Malay. 1976;1:32–39. [Google Scholar]
- 28.Somchit MN, Shukryah MH. Anti - inflammatory property of ethanol and water extracts of Zingiber zerumbet (Lempoyang) Indian Jour Pharm. 2003;35:181–182. [Google Scholar]
- 29.Thaipong K, Boonprakab U, Crosby K, Cisneroszevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruits extracts. Jour Food Compostn And Anal. 2006;19:669–675. [Google Scholar]
- 30.Wang Y, Hamburger M, Gueho, Hosttermann K. Antimicrobial flavonoids from Psiadia trinervia and their methylated and acetylated derivatives. Phytochem. 1989;28:2323–2327. [Google Scholar]
- 31.Wong SP, Lai PL, Jen HWK. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783. [Google Scholar]