Abstract
Background
Yanang (Tiliacora triandra) has been known as vegetable and herbal in northeast Thailand and Lao People's Democratic Republic. Extracts from Yanang leaves contain high amounts of polyphenol constituents possessing antioxidant activity.
Materials and Methods
This work investigated bioactive compounds of Yanang extracts prepared by infusion with water, ethanol and acetone. Furthermore, this paper reports the design of the experimental method for optimization of Yanang encapsulation using three independent variables: the ratio of core material (Yanang), to wall material (gum Arabic), gum Arabic concentration and inlet temperature of spray drying on bioactive compounds stability. The stability of bioactive compounds was evaluated using phenolic compounds, total antioxidant, carotenoids and chlorophyll.
Results
The study of the bioactivity of Yanang extracts found that extraction with water was the appropriate application. The study of Yanang encapsulation demonstrated that gum Arabic, as coating agents, protected bioactive compounds of Yanang. Optimized condition for the encapsulation was at the ratio of core to wall {1:4}, in gum Arabic concentration 10% (w/v), and inlet temperature at 160▯C. The results show that the bioactive compounds were mainly affected by the ratio of core to wall material. Besides, moisture content and particle size of encapsulation depend on inlet temperature of spray drying, and gum Arabic concentration, respectively. This optimization reveals that the encapsulation process did not lose the bioactive compounds.
Conclusion
Yanang extract with water was the main phenolic compound and showed high antioxidant activities. This study demonstrates the potentials of using spray drying process and optimization for the encapsulation of herbal products.
Keywords: Yanang, Tiliacora triandra, Encapsulation, Spray drying, bioactive compounds
Introduction
Yanang, Tiliacora triandra (Colebr.) Diels belongs to the family of Menispermaceae. It is a species of flowering plant, native to mainland Southeast Asia, and is widespread within northeast Thailand. Yanang is common in deciduous and dry evergreen forests (Smitinand and Larson, 1991). It is a climbing plant with deep green leaves and yellowish flowers. It is used particularly in the cuisines of northeast Thailand and Lao PDR, especially in bamboo shoot soup. The extract from Yanang root is used as herbal medicine for the treatment of fever and malaria, and is also an antipyretic (Wiriyachita and Phuriyakorn, 1981). A number of alkaloids, especially bisbenzylisoquinoline alkaloids have been identified in Yanang, which includes; tiliacorinine, tiliacorine and nortiliacorinine (Mahidol, et al., 1994; Wiriyachita and Phuriyakorn, 1981).
Since consumers are more aware of the relationships between diet and health, the demand for functional foods, or foods that promote health beyond providing basic nutrition, is at a constant increase. Increased vegetable consumption has been found to aid the prevention of human diseases, hence intensified interest in plant food containing phytochemicals as potential physiologically active agents. Considering the growing use of vegetables or herbs in medicinal applications, it is perhaps the potential role of these active agents in preventing human diseases that has aroused more interest in them. The world Bank had projected that the global market for herbal products will grow from USD 200 billion in 2008 to USD 5 trillion in 2050 (Chan, et al., 2010). A key factor for this rapid growth of the market would be consumer's growing knowledge and confidence in natural products or medicines. In addition, bioactive compounds of herbal plants such as antioxidant, phenolic compounds, carotenoids and chlorophyll have been shown to have multiple functional and remedial properties (Bae and Suh, 2007). However, the stability of bioactive compounds were unstable, depending on the pH, light, oxygen, temperature, and enzymatic activities (Saénz, et al., 2009). It is possible that the stabilization of bioactive compounds could be improved using the encapsulation technologies.
Encapsulation is a process in which thin films, generally of polymeric materials is applied to little solid particles, liquid or gases droplets. This method is used to trap bioactive compounds and release them under controlled conditions. Several materials have been encapsulated in the food industry such as amino acids, vitamins, minerals, antioxidants, colorants, enzymes and sweeteners (Deladino, et al., 2008). In addition, encapsulation may promote better product stability by isolating bioactive compound from the detrimental effects of oxygen or moisture (Chan et al., 2010). Spray drying encapsulation has been used in the food industry to provide active ingredient with the removal of water, and to convert liquids to powders. By decreasing water contents and activity, spray-drying is generally used in food industry to ensure a microbiological stability of products, avoid the risks of chemical and/or biological degradation, reduce the storage and transportation costs, and finally to obtain a product with specific properties like instantaneous solubility (Gouin, 2004; Gharsallaoui, et al., 2007). However, there is no available information in literature on any previous encapsulation of Yanang extract. Therefore, the aim of this research was to investigate bioactive compounds and to optimize the encapsulation process of Yanang leaves extract and to characterize its bioactive compounds stability.
Materials and methods
Materials
Yanang leaves, procured from the local market within northeast of Thailand (Ubon Ratchathani Province), were cleansed with water to remove dust, and infected leaves were separated. The prepared leaves were then dried at 60°C for 3 hrs. The dried leaves were ground and stored at room temperature in a vacuum-packed container before use (Singthong, et al., 2009).
Preparation of Yanang leaves extracts
Approximately 100 mg Yanang leaves powder was extracted with three 12 mL portion of boiling water, ethanol, or acetone in a shaking water bath at 25°C for 15 min. Samples were centrifuged at 3000 x g for 3 min after which solvent layers were collected and filtered by vacuum. Filtrates were combined and final volume was adjusted volumetrically (with representative extraction solvent), to 50 ml. Aliquots of 2 ml were dried under vacuum and stored frozen at −20°C until use (Oonsivilai, et al., 2007).
Encapsulation optimization
The experimental design of encapsulation was carried out under various conditions, according to the central composite design (CCD). The effect of three independent variables; gum Arabic concentration, X1 (10–20%w/v), the ratio of core material (5% (w/v), (Yanang leaves extract), to wall material (gum arabic), X2 (1:6–1:4), and Inlet temperature of spray drying, X3 (140–160°C), were determined, using the detailed experimental design shown in Table 1. The independent variables were coded in three levels of −1, 0 and +1. The optimization condition for encapsulation of Yanang leaves extract was determined using response surface methodology. All experimental data were statistically analyzed with Design-Expert® software (Version 6.0.10, Stat-Ease, Inc., and Minneapolis, MN).
Table 1.
Central composite design (CCD) for the optimization of Yanang encapsulation
| Treatment | Coded variablea | Process variableb | ||||
| X1 | X2 | X3 | X1 | X2 | X3 | |
| 1 | −1 | −1 | −1 | 10 | 1:6 | 140 |
| 2 | −1 | −1 | +1 | 10 | 1:6 | 160 |
| 3 | −1 | +1 | −1 | 10 | 1:4 | 140 |
| 4 | −1 | +1 | +1 | 10 | 1:4 | 160 |
| 5 | +1 | −1 | −1 | 20 | 1:6 | 140 |
| 6 | +1 | −1 | +1 | 20 | 1:6 | 160 |
| 7 | +1 | +1 | −1 | 20 | 1:4 | 140 |
| 8 | +1 | +1 | +1 | 20 | 1:4 | 160 |
| 9 | 0 | 0 | 0 | 15 | 1:5 | 150 |
| 10 | 0 | 0 | 0 | 15 | 1:5 | 150 |
| 11 | 0 | 0 | 0 | 15 | 1:5 | 150 |
| 12 | 0 | 0 | 0 | 15 | 1:5 | 150 |
| 13 | −2 | 0 | 0 | 5 | 1:5 | 150 |
| 14 | +2 | 0 | 0 | 25 | 1:5 | 150 |
| 15 | 0 | −2 | 0 | 15 | 1:7 | 150 |
| 16 | 0 | +2 | 0 | 15 | 1:3 | 150 |
| 17 | 0 | 0 | −2 | 15 | 1:5 | 130 |
| 18 | 0 | 0 | +2 | 15 | 1:5 | 170 |
Coded level of variables: + = upper level, − = lower level and 0 = center points
X1, X2 and X3 were gum arabic concentration (%), the ratio of core material to wall material (w/w), and outlet temperature (°C), respectively.
Encapsulation was prepared as follows: Yanang leaves extract were mixed with water and gum arabic with constant stirring and heated to 60°C for 1 hr. The mixture was filtered through a vacuum filter. The supernatant was fed to the spray-dryer (EYELA spray-dryer SD-100, Japan). The spray-dryer was operated at inlet temperature ranging from 140–160°C and the outlet temperature was ∼90°C. The blower, flow rate and atomization pressure was 9.9m2/min, 1.9ml/min and 9.0x10KPa, respectively. The powders obtained were stored in a vacuum container at room temperature for further analysis
Analytical methods
Total phenolic content (TPC)
Total soluble phenolic constituents of the Yanang extracts and encapsultion using the Folin-Ciocalteu reagent with gallic acid as standard. 20 µl of the solution sample was added into a 1.5 ml cuvette, to which 1.58 ml of water and 100 µl Folin-Ciocalteu reagent were added. The sample was throoughly mixed and incubated for 5 min at room temperature. Following incubation 300 µl of Na2CO3 (2% w/v), solution was added and the mixture allowed to stand at room temperature for 2 hrs. Absorbance rate was measured at 765 nm. Results were expressed as gallic acid equivalents (GAE), (Oonsivilai et al., 2007).
The DPPH method
Antioxidant activity was evaluated in accordance with the DPPH method. Free radical scavenging activity was determined using the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH), radical according to the procedure described by Brand-Williams, et al. (1995), with slight modification. Briefly, 50 µl of standard samples were added to 1.9 µl DPPH methanolic solution. The reaction mixture was covered and left in the dark at room temperature. After 15 min, the absorption rate was measured at 515 nm. Ascorbic acid and BHT were used as standard control.
IC50 value was the concentration of sample required to scavenge 50% DPPH free radical and was calculated from a calibration curve by a linear regression.
The FRAP method
This method was proposed by Bae and Suh (2007), and involved the presence of antioxidants in extract to reduce the ferricyanide complex to the ferrous form. The FRAP solution (1.5 ml), was added to 50 µl of standard samples. The reaction mixture was covered and left in the dark at room temperature for 4 min. The absorption rate was measured at 593 nm. Trolox and BHT were used as standard control. IC50 value was estimated and calculated.
The ABTS method
The antioxidant activity was evaluated using ABTS+ method (Bae and Suh, 2007). 50 µl of standard samples were added to 950 µl ABTS+ solution. The reaction mixture was covered and left in the dark at room temperature. After 6 min, the absorption rate was measured at 734 nm. Trolox and BHT were used as standard control. IC50 value was estimated and calculated.
Total carotenoids
Total carotenoids was carried out using the method of Wongsa, et al. (2010), with slight modification. Sample solution of about 10 ml was extracted with 10 ml of acetone:ethanol (1:1 v/v). It was centrifuged at 1500 × g for 15 min at 4 – 5°C. The supernatant final volume was adjusted to 100 ml. The absorbtion rate was measured at 450 nm and calculated.
Total chlorophyll
Total chlorophyll was carried out using the method of Ramesh and Devasenapathy (2006), with slight modification. 10 ml of sample solution was extracted with 10 ml acetone. The supernatant final volume was adjusted to 50 ml. The absorbtion rate was measured at 652 nm and calculated.
Moisture content
Moisture determination was performed according to AOAC method (1995).
Color measurment
The color of the encapsuled powder was determined using a Hunter Lab (Color Flex Model 45/0, USA).
Scanning electron microscopy (SEM)
The outer structures of the encapsuled powders obtained by optimal conditions were studied by SEM. The samples were coated with gold using a sputter coater, model SPI-MODULE™ Sputter Coat, and examined by a JEOL, JSM-5410LV scanning microscope.
Statistical analysis
All measurements were carried out in at least three replicate experiments, and the results are reported as the means with standard derivation. Means of each parameter were analyzed by analysis of variance (ANOVA). Differences between treatments at the 5% (p≤0.05) level were considered significantly.
RSM was focused on determining the regression coefficients and statistical significance of the model terms. RSM helps in fitting the regression models to the experimental data to achieve an overall optimal region for all response variables studied. The generalized response surface model is given below:
Y= β0 + ∑βiXi + ∑βiiXi2 + ∑βijXij
Where, Y is the response, which is calculated by the model; β0 is a constant; βi, βii and βij are linear, squared and interaction coefficients, respectively. According to Myer (1995), R2 should be at least 0.80 for a good fit for a model. Larger values of absolute t-value and smaller values of p-value show that the variables would be more significant (p≤0.05). The terms found to be statistically non-significant (p>0.05), were removed and the experimental data was refitted to only the significant (p≤0.05), factors to obtain the final reduced model. The experimental design, data analysis and optimization procedure were carried out using the Design-Expert® software (Version 6.0.10, Stat-Ease, Inc., Minneapolis, MN).
Results
Bioactive compounds of Yanang extracts
Water, ethanol and acetone extracts of Yanang were examined to determine the best extraction solvent to be used in this study. Among the three solvents, the water extract yielded the highest TPC (97.899 mg GAE/g). The TPC values of the ethanol and acetone extracts were lower, being 26.703 and 16.456 mg GAE/g, respectively (Table 2). Water was found to be the most suitable solvent in the extraction in polyphenolic compounds from this plant.
Table 2.
Biological activities of Yanang extracts.
| Yanang extracts | TPC (mgGAE/g) |
DPPH (IC50, mg/g) |
ABTS (IC50, mg/g) |
FRAP (mmol Fe2+/g) |
| Water | 97.899±1.735 | 0.197±0.018 | 0.077±0.011 | 0.054±0.0015 |
| Ethanol | 26.703±1.642 | 0.333±0.024 | 0.191±0.059 | 0.034±0.0012 |
| Acetone | 16.456±2.968 | 0.419±0.091 | 0.312±0.056 | 0.014±0.0006 |
| Standard | ||||
| Ascorbic acid | 0.36±0.01 | 0.041±0.026 | 0.059±0.028 | |
| BHT | 0.34±0.04 | 0.090±0.010 | 0.254±0.143 |
Values are given as mean ± SD (n=3).
For improving antioxidant effectiveness, several analytical methods were used. The results of antioxidant activity determined by three complementary test system via DPPH, ABTS and FRAP assay are shown in Table 2. The reduction capability of DPPH radical was determined by the decrease in absorbance by reduced DPPH to plant antioxidants (Ksouri, et al., 2009). Free radical scavenging property of water extract was characterized by a higher free radical scavenging property (IC50 of 0.197 mg/ml), with comparison to that of ethanol and acetone extracts (IC50= 0.333 and 0.419 mg/ml, respectively). This antiradical activity could be due to the phenolic compounds. The water extract of Yanang displayed the most favorable activity against DPPH and was significantly more active than the reference compound, ascorbic acid and BHT (IC50= 0.36 and 0.34 mg/ml, respectively).
The reducing power (FRAP assay), serves as a significant indicator of its antioxidant activity. The reducing power of Yanang extracts ranged from 0.014 to 0.054 mmol Fe2+/g. The water extract showed the highest reducing power, which also indicated their potential as electron donors to scavenge free radicals. However, Yanang extracts showed weak antioxidant activity as compared to standards (ascorbic acid and BHT). Free radical scavenging activity for ABTS radical expressed as IC50 ranged from 0.077 to 0.312 mg/ml. The water extract was observed as the most powerful ABTS radical scavenger as compared to the other extracts as evidenced by low IC50 (0.077 mg/ml) followed by ethanol and acetone extracts with IC50 values of 0.191 and 0.312 mg/ml, respectively. ABTS radical scavenging activity for Yanang extracts were comparable with ascorbic acid and BHT (IC50= 0.041 and 0.090 mg/ml, respectively). The water extract as the most suitable activity against antioxidant activity. Yanang extracts with high phenolic content had the most promising antioxidant activity. The higher TPC value corresponded with higher FRAP values and lower IC50 of DPPH and ABTS values.
Yanang encapsulation
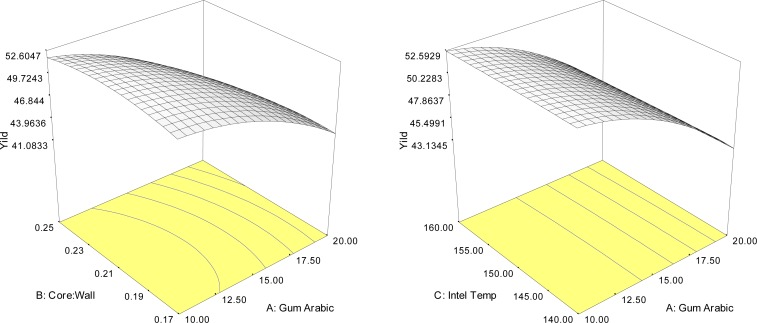
The main effects of gum arabic concentration and the quadratic effects of gum arabic and core to wall material ratio, significantly (p<0.05), affected the yield value of Yanang encapsulated using spray dryer. Thus, an increase of the core material lead to increased yield. Figure 1 shows how significant (p<0.05), interaction effects of independent variables influenced the yield of Yanang encapsulation. The quadratic effects of independent variables could be explained by the curvature in three-dimensional response surface plots. The interaction between gum Arabic concentration, the ratio of core to wall material and inlet temperature gave rise to a higher yield at low gum Arabic concentration and a wide range of the ratio and inlet temperature. The individual optimum region led to a maximum yield value (R2=0.93), that was predicted to be achieved in the spray drying system by a combined level of 10% gum arabic, the ratio of core to wall material 1:4 and inlet temperature of spray dryer 160°C.
Figure 1.
Response surface plots indicating the interaction effect of encapsulation conditions on yields (%).
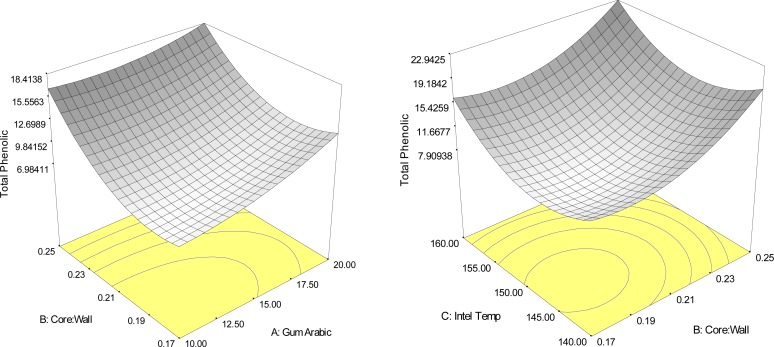
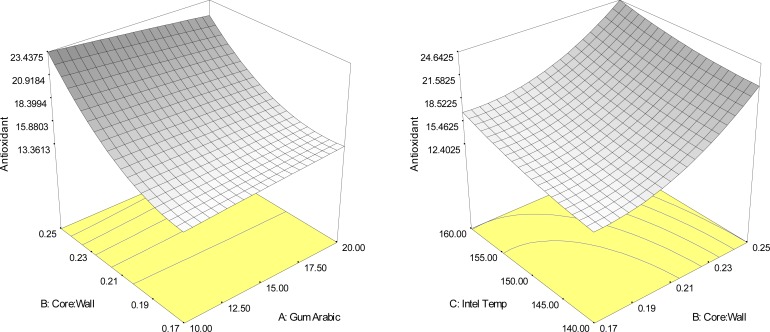
The ratio of core material to wall material, gum arabic concentrate and inlet temperature of spray dryer were have effected to bioactive compounds contents in Yanang encapsulation as shown in Fig. 2–5 and Table 3. The result indicated that the main effect of the ratio of core to wall material, the quadratic effects of the ratio of core to wall material and inlet temperature of spray dryer, significantly (p<0.05) affected the total phenolic compounds of Yanang encapsulation using spray drying. Fig. 2 showed the response surface plots illustrating the effects of the ratio of acore to wall material, gum rabic concentration and inlet temperature of spray dryer on total phenolic compounds. All high ratio of core to wall material gave a rise to the high total phenolic compounds. The individual optimum region led to a high total phenolic value (R2=0.83) that was predicted to be achieved in the spray drying system by a combined level of 10% gum arabic, the ratio of core to wall material 1:4 and inlet temperature of spray dryer 160°C. Total antioxidant value showed the result similar as total phenolic compounds as shown in Fig. 3. Increasing the ratio of core to wall material effected in increased bioactive compounds of encapsulation system. Spray drying of Yanang encapsulation showed that the main effects of the ratio of core to wall material indicated the significant (p<0.05) effect on the total antioxidant of Yanang encapsulation. Total phenolic increased with increase in the ratio of core to wall material. The increased total phenolic content might be due to interference of core material (Yanang extract) with the phenolic compounds during analysis.
Figure 2.
Response surface plots indicating the interaction effect of encapsulation conditions on total phenolic content (mg GAE/g sample).
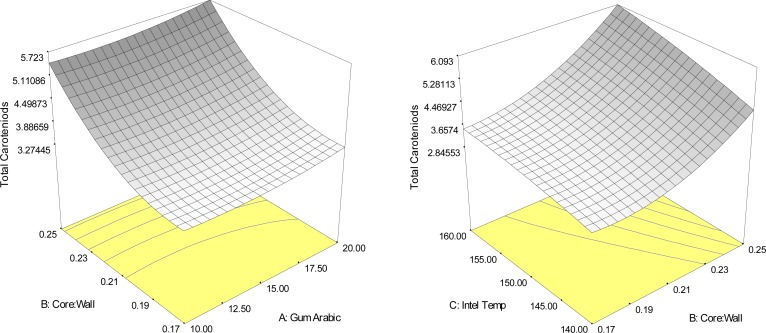
Figure 5.
Response surface plots indicating the interaction effect of encapsulation conditions on total carotenoid content (mg/g sample).
Table 3.
Bioactive compounds of Yanang encapsulation using combinations of various levels of the ratio of core to wall material, gum arabic concentration and inlet temperature.
| Treatment | Total Carotenoids (mg/g sample) |
Total Chlorophyll (mg/g sample) |
Total Phenolic (mg GAE/g sample) |
Total Antioxidant (mg AEAC/g sample) |
| 1 | 4.24±0.06d* | 0.35±0.01ef | 4.67±0.35j | 15.10±0.90e |
| 2 | 4.46±0.06d | 0.37±0.01ef | 8.36±0.69i | 22.48±0.74c |
| 3 | 5.28±0.10c | 0.39±0.01de | 16.23±0.70fg | 25.40±014b |
| 4 | 8.07±0.93a | 0.84±0.05a | 20.71±0.89e | 30.42±0.25a |
| 5 | 2.49±0.31f | 0.35±0.04ef | 11.31±0.69h | 13.84±0.00f |
| 6 | 4.44±0.21d | 0.31±0.00fg | 16.72±1.39fg | 20.59±0.26d |
| 7 | 5.32±0.25c | 0.44±0.01cd | 22.13±0.70de | 20.35±0.44d |
| 8 | 6.35±0.34b | 0.37±0.02ef | 23.11±0.69d | 26.47±0.92b |
| 9 | 3.50±0.09e | 0.48±0.04c | 10.57±0.35h | 14.76±0.26e |
| 10 | 3.46±0.11e | 0.44±0.02cd | 10.93±0.82h | 14.03±0.26e |
| 11 | 3.44±0.06e | 0.45±0.02cd | 11.10±0.48h | 14.76±0.26e |
| 12 | 3.41±0.01e | 0.46±0.04cd | 10.70±0.20h | 14.39±0.25e |
| 13 | 2.24±0.25f | 0.46±0.01cd | 21.14±0.69e | 10.38±0.25f |
| 14 | 4.99±0.52cd | 0.43±0.01cd | 30.98±0.70b | 15.37±0.74e |
| 15 | 3.54±0.23e | 0.54±0.01b | 15.24±0.69g | 12.74±0.01f |
| 16 | 7.45±0.45a | 0.86±0.06a | 17.45±1.74f | 31.88±0.26a |
| 17 | 2.11±0.09f | 0.27±0.00g | 26.06±0.69c | 14.47±0.41e |
| 18 | 3.33±0.33e | 0.36±0.01ef | 36.22±0.75a | 16.34±0.29e |
Mean values (n=3) with the superscript alphabets in each column are significantly different (p<0.05).
Figure 3.
Response surface plots indicating the interaction effect of encapsulation conditions on antioxidant activity (mg AEAC/g sample).
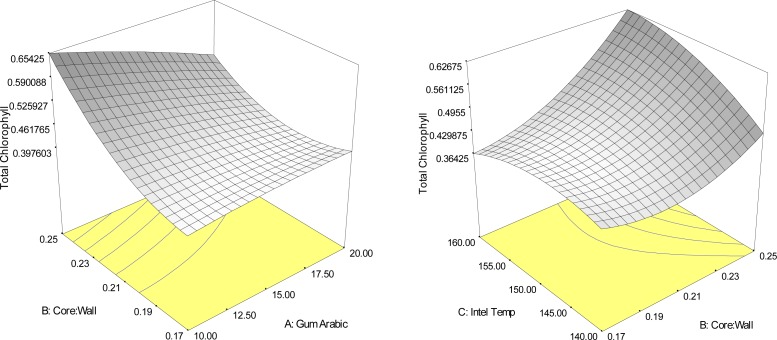
The results (Table 3), indicated that the main effect of the ratio of core to wall material, in addition to the quadratic effects of the ratio of core to wall material and the interaction effects of gum arabic concentration and inlet temperature of spray dryer significantly (p<0.05), influenced the chlorophyll content of Yanang encapsulation using spray drying (Figure 4). The highest total chlorophyll value (R2=0.88), was predicted to be a combined level of 10% gum Arabic concentration, the ratio of core to wall material 1:4 and 160°C inlet temperature of spray dryer. Figure 5 displayed response surface plots of the effects of gum Arabic concentration, the ratio of core to wall material and inlet temperature of spray dryer on total carotenoids, the maximum total carotenoids content was high the ratio of core to wall material and a wide range of gum arabic concentration and inlet temperature. In addition, total chlorophyll value showed the result similar as total carotenoids (Fig. 4).
Figure 4.
Response surface plots indicating the interaction effect of encapsulation conditions on total chlorophyll content (mg/g sample).
Moisture content
Spray drying of Yanang encapsulation showed that the main effects of inlet temperature and the quadratic effects of inlet temperature indicated the significant (p<0.05), effects on moisture content of Yanang encapsulation (Table 4). The optimum inlet temperature is 160°C which can safely be used without damaging the product.
Table 4.
Moisture, color, size and yield of Yanang encapsulation using combinations of various levels of the ratio of core to wall material, gum arabic concentration and inlet temperature.
| Treatment | Moisture (%) | Color (L*) | Color (b*) | Size microencapsule (µm) | Yield (%) |
| 1 | 6.07±0.37defgh* | 88.78±0.05b | 8.23±0.04h | 5.80±0.84de | 50.18±0.43h |
| 2 | 5.74±0.22fgh | 89.10±0.03a | 8.10±0.15h | 5.80±1.10de | 50.43±0.14g |
| 3 | 6.07±0.38defgh | 86.95±0.05g | 9.18±0.09d | 4.40±0.55e | 50.08±0.23i |
| 4 | 5.49±0.18h | 87.32±0.06e | 9.51±0.16c | 4.60±0.89e | 50.03±0.15k |
| 5 | 7.18±0.80ab | 88.56±0.03c | 8.24±0.05h | 9.00±1.58abc | 43.41±0.50n |
| 6 | 5.92±0.37efgh | 88.81±0.14b | 8.15±0.03h | 8.00±1.87abc | 41.49±0.46o |
| 7 | 7.29±0.45a | 87.93±0.24d | 8.83±0.14e | 7.80±1.48abc | 38.65±0.33q |
| 8 | 5.51±0.38h | 87.40±0.15e | 8.65±0.16f | 5.80±0.45de | 39.81±0.23p |
| 9 | 6.40±0.25bcdefg | 86.78±0.17h | 9.23±0.16d | 9.00±1.58abc | 50.68±0.48d |
| 10 | 6.37±0.43cdefg | 86.41±0.01i | 9.43±0.02c | 9.60±2.19a | 50.05±0.15j |
| 11 | 6.56±0.14abcde | 87.23±0.01ef | 8.52±0.03fg | 9.00±1.22abc | 50.48±0.36f |
| 12 | 6.86±0.38abcd | 86.92±0.23fg | 8.96±0.22e | 8.40±1.14abc | 50.74±0.15c |
| 13 | 6.66±0.72abcde | 86.46±0.01i | 10.25±0.02a | 4.60±0.55e | 52.95±0.38a |
| 14 | 6.34±0.20cdefg | 85.98±0.14k | 9.54±0.09c | 7.60±1.52bc | 34.98±0.16r |
| 15 | 6.48±0.34bcde | 88.35±0.10c | 7.88±0.05i | 9.40±1.14ab | 47.14±0.42l |
| 16 | 6.96±0.71abc | 86.23±0.23j | 9.79±0.09b | 7.40±0.55cd | 46.48±0.39m |
| 17 | 6.17±0.06cdefgh | 86.89±0.25fg | 8.90±0.23e | 8.20±0.84abc | 51.24±0.50b |
| 18 | 6.29±0.07cdefgh | 87.91±0.05d | 8.43±0.01g | 8.20±0.84abc | 50.60±0.42e |
Mean values (n=3, n=5 for color and size) with the superscript alphabets in each column are significantly different (p<0.05).
Color values
The Hunter color parameter of Yanang encapsulation with different encapsulation conditions (Table 4). There were no significant difference in L* and b* values. However, L* value decreased with increase the ratio of core to wall material. On the other hand, b* value increased with increase the ratio of core to wall material. The changes of color values were attributed to concentration of Yanang extract.
Microstructure
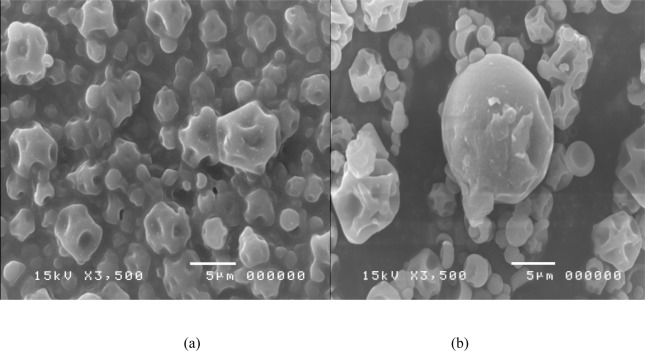
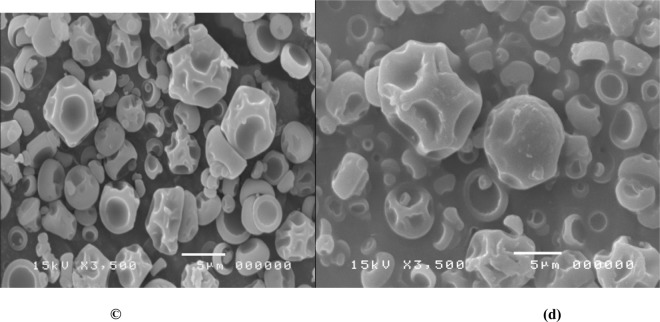
Under an electron microscope, the Yanang encapsulation using spray dryer was observed (Fig. 6). The results (Table 4), indicated that main effect of gum arabic and the quadratic effects of gum arabic showed the significant (p<0.05), effects on size of Yanang encapsulation. The mixer of different encapsulation conditions influenced on micro-particles morphology. The combination of low gum arabic, high ratio of core to wall material and high inlet temperature resulted in microspheres with smoother surface and fewer teeth or roughness.
Figure 6.
Scanning electron microstructure of spray-dried Yanang encapsulation produced from different wall material (gum arabic) concentration.
(a) 5%gum arabic (b) 10%gum arabic
(b) 20%gum arabic (d) 25%gum arabic
3.2.6 Optimized encapsulation
The statistical analysis indicated that the proposed model was adequate, processing insignificant lack of fit and with statistically R2 values for yield and bioactive compounds of 0.9 and 0.8, respectively. The optimized condition of Yanang encapsulation was 10%, 1:4 and 160°C for gum arabic concentration, the ratio of core to wall material and inlet temperature of spray drying, respectively.
Discussion
The results of Yanang extraction seem similarly to the study made by Cai, Luo, Sun, & Corke (2004), whereby water extraction of the powder sample of P. oleracea at 80°C for 20 min yielded the highest total phenol content. The reason could be due to the hydrolysis of the glycosidic and ester bonds of the condensed phenolic compounds at near boiling temperature (Oboh, 2005). Phenolic phytochemicals are thought to promote optimum health partly through their antioxidant and free scavenging effects which protects cellular components against free radical induced damage (Dapkevicius, et al., 2002; Jabri-Karoui, Bettaieb, Masada, Hammami, & Marzouk, 2012).
The antioxidant activity may be due to different mechanisms, such as the prevention of chain initiation, decomposition of peroxides and prevention of continued hydrogen abstraction, free radical scavenging, reducing capacity and binding of transition-metal ion catalysts (Mao, Pan, Que, & Fang, 2006; Jabri-Karoui et al., 2012). DPPH was the free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule (Brand-Williams et al.1995). The reduction capability of DPPH radical was determined by the decrease in absorbance by reduced DPPH to by plant antioxidants (Ksouri, et al., 2009). The reducing power (FRAP assay), and free radical scavenging activity for ABTS radical serves as a significant indicator of its antioxidant activity. The reduction of the Fe3+/ferricyanide complex to the ferrous form (Fe2+), due to antioxidants was monitored (Joshi, Verma, & Mathela, 2010). The water extract showed the highest reducing power, which also indicated their potential as electron donors to scavenge free radicals. The water extract is the most suitable activity against antioxidant activity.
Chen et al., (2012) reported that the developmental stage of Prunella vulgaris L. at harvest might influence the concentration of secondary metabolites in the aerial parts of the plant and affect the quality of the harvested plant material. The study was to analyze the rosmarinic acid (RA), ursolic acid (UA), and oleanolic acid (OA), content of different aerial parts of P. vulgaris at various stages of development and the results found that the concentrations of RA, UA and OA in samples of the whole plant increased during the course of ontogenesis.
Encapsulation of Yanang in gum arabic provided an example of a swelling-controlled system for the controlled release of bioactive compounds (Mehrnoush, Tan, Hamed, Azis, & Ling, 2011). When the core material increased in the optimum wall material concentration (gum Arabic), the core material that is protected against oxidation by a wall material also increased (Gharallaoui et al., 2007). Thus, an increase of the core material lead to increased yield.
Bioactive compounds in many plants and fruits were the important such as phenolic compounds, chlorophyll, etc. It is well known that the amounts of phenolic contents may affect the antioxidant activity of food (Ahmed, Akter, Lee, & Eun, 2010). Similar results were report by Ahmed et al. (2010), and Jabri-Karoui et al. (2012), that the total antioxidant activity was highly correlated with total phenolic content. Increasing the ratio of core to wall material effected in increased bioactive compounds of encapsulation system. The increased total phenolic content and antioxidant activity might be due to interference of core material (Yanang extract), with bioactive compounds during analysis.
Air inlet temperature is directly proportional to the microcapsule drying rate and the final water content (Gharsallaoui et al., 2007). The external morphology of particles showed a spherical shape and various sizes with no apparent cracks of fissures, which is an advantage, since it implies that capsules have lower permeability to gases, increasing protection and retention of the active material. Moreover, the variety in size is a typical characteristic of particles produced by spray drying (Gharsallaoui et al., 2007). The mixer of different encapsulation conditions influenced on micro-particles morphology. This is more evident when comparing the images from the combination of low gum arabic, high ratio of core to wall material and high inlet temperature with the others, since this condition resulted in microspheres with smoother surface and fewer teeth or roughness. The formation of the dented surfaces of the spray-dried particles was attributed to the shrinkage of the particles during the drying process (Saénz, 2009).
Finally, majority of the plants affect the amount of bioactive activities, resulting in its antioxidant activities. The selection of the majority range of raw materials should be concerned during raw materials preparation.
Conclusion
In conclusion, it was found that Yanang extracted with water showed the main phenolic compound and high antioxidant activities. The antioxidant activity of Yanang showed strong antioxidant activities suggesting higher utilization potential for use as natural additives in food. Optimized Yanang encapsulation was identified as 1:4 ratio of core material to wall material, 10% gum arabic concentration and 160°C inlet temperature of spray dryer. From all 3 parameters chosen, the parameter found to effect encapsulation of Yanang most was the ratio of core material to wall material. Spray drying method can give the high yield encapsulation efficiency and stabilize bioactive compounds. Encapsulation of Yanang is an interesting food addition for incorporation into functional foods.
Acknowledgements
The authors would like to thank Thailand Research Fund (TRF) and Ubon Ratchathani University, Thailand for financial supports.
References
- 1.Ahmed M, Akter S, Lee J, Eun J. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT-Food Science and Technology. 2010;43:1307–1313. [Google Scholar]
- 2.AOAC, author. Official Methods of Association of Analytical Chemists. 16th ed. Washington, DC: Association of Analytical Chemists; 1995. [Google Scholar]
- 3.Bae S, Suh H. Antioxidant activities of five different mulberry cultivars in Korea. LWT -Food Science and Technology. 2007;40:955–962. [Google Scholar]
- 4.Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT -Food Science and Technology. 1995;28:25–30. [Google Scholar]
- 5.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with cancer. Life Sciences. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan ES, Yim ZH, Phan SH, Mansa RF, Ravindra P. Encapsulation of herbal aqueous extract through absorption with ca-alginate hydrogel beads. Food and Bio-products Processing. 2010;8:195–201. [Google Scholar]
- 7.Chen Y, Zhu Z, Guo O, Zhang L, Zhang X. Variation in concentrations of major bioactive compounds in Prunella vulgaris L. related to plant parts and phenological stages. Biological Research. 2012;45(2) doi: 10.4067/S0716-97602012000200009. [DOI] [PubMed] [Google Scholar]
- 8.Dapkevicius A, Van Beek TA, Lelyveld GP, Van Veldhuizen A, De Groot AE, Linssen JPH, Venskutonis R. Isolation and structure elucidation of radical scavengers from Thymus vulgaris leaves. Journal of Natural Products. 2002;65:892–896. doi: 10.1021/np010636j. [DOI] [PubMed] [Google Scholar]
- 9.Deladino L, Anbinder P, Navarro A, Martino M. Encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydrate Polymers. 2008;71:126–134. [Google Scholar]
- 10.Gouin S. Microencapsulation: industrial appraisal of existing technologies and trends. Trends in Food Science and Technology. 2004;15:330–347. [Google Scholar]
- 11.Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Research International. 2007;40:1107–1121. [Google Scholar]
- 12.Joshi SC, Verma AR, Mathela CS. Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food and Chemical Toxicology. 2010;48:37–40. doi: 10.1016/j.fct.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Jabri-Karoui I, Bettaieb I, Masada K, Hammami M, Marzouk B. Research on the phenolic compounds and antioxidant activities of Tunisian Thymus capitatus. Journal of Functional Foods. 2012;4:661–669. [Google Scholar]
- 14.Ksouri R, Falleh H, Megdiche W, Trabelsi N, Mhamdi B, Chaieb K, Bakrouf A, Magné C, Abdelly C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food and Chemical Toxicology. 2009;47:2083–2091. doi: 10.1016/j.fct.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Mao LC, Pan X, Que F, Fang XH. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze dried daylily flowers. European Food Research and Technology. 2006;222:236–241. [Google Scholar]
- 16.Mahidol C, Sahakitpichan P, Ruchirawat S. Bioactive natural products from Thai plants. Journal of Pure and Applied Chemistry. 1994;66:2353–2356. [Google Scholar]
- 17.Myers RH. Response surface methodology: Process and product in optimization using designed experiments. New York: Wiley; 1995. [Google Scholar]
- 18.Mehrnoush A, Tan CP, Hamed M, Azis N, Ling TC. Optimisation of freeze drying conditions for purified serine protease from mango (Mangifera indica Cv. Chokanan) peel. Food Chemistry. 2011;128:158–164. doi: 10.1016/j.foodchem.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Oboh G. Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT-Food Science and Technology. 2005;38:513–517. [Google Scholar]
- 20.Oonsivilai R, Cheng C, Bomser J, Ferruzzi M, Ningsanond S. Phytochemical profiling and phase II enzymeinducingproperties of Thunbergia laurifolia Lindl. (RC) extracts. Journal of Ethnopharmacology. 2007;114:300–306. doi: 10.1016/j.jep.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh T, Devasenapathy P. Physiological response of cowpea in a rainfed alfisol ecosystem to the impulse of soil moisture conservation pracyives. General Applied Plant Physiology. 2006;32:181–190. [Google Scholar]
- 22.Saénz C, Tapia S, Chávez J, Robert P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica) Food Chemistry. 2009;114:616–622. [Google Scholar]
- 23.Singthong J, Ningsanond S, Cui S. Extraction and physicochemical characterization of polysaccharide gum from Yanang (Tiliacora triandra) leaves. Food Chemistry. 2009;114:1301–1307. [Google Scholar]
- 24.Smitinand T, Larsen K. Flora of Thailand (Vol5 Part 3) Bangkok, Thailand: The Forest Herbarium, Royal Forest Department; 1991. [Google Scholar]
- 25.Wiriyachitra P, Phuriyakorn B. Alkaloids of Tiliacora triandra. Australian Journal of Chemistry. 1981;34:2001–2004. [Google Scholar]
- 26.Wongsa P, Takeaw R, Deerod O. Proceedings of Food Innovation Asia Conference: Indigenous Food Research and Development to Global Market. Bangkok, Thailand: 2010. Some phytochemical compounds and antioxidant activity of Thai pungency chili genotype during maturity stages; pp. 284–293. [Google Scholar]