Abstract
Background
According to the traditional view, we depend on three methods to treat tumors; surgery, chemotherapy and radiotherapy. However, these methods have its own limitations in application. Traditional Chinese Medicine (TCM) is one of the oldest healing systems. Astragalus mongholicus (AMs) that is the common herbal medicine, the biggest part of TCM, have been proved to be effective in treating cancers from lots of clinical cases. However, we have not fully understood the anti-tumor mechanism of AMs, and this has lead to some doubt for some Western-Medicine scholars and restricts its wide use. The main objective of this research is to discuss the effect and mechanism of AMs to human stomach cancer.
Materials and Methods
To observe the effect and mechanism of tumor treatment by AMs, we have done the research from three major aspects, the influence of DCs, the inhibition of tumor in vitro as well as the animal studies in vivo after treatment. First, we culture the mouse dendritic cells (DCs) from bone marrow of mouse hind legs according to the method using Interleukin-4(IL-4) and Granulocyte-macrophage colony stimulating factor (GM-CSF), which refer to the way established by Inaba (Inaba K, 1992). And then we investigate the growth-rate of the DCs co-cultured with AMs injection. We analyze the expression of the Toll-like-receptor 4 (TLR4), with SYBR-Green I Real-time PCR and the I-kappa-B-alpha (IκB-α) with Western-Blot, the main regulatory protein to control nuclear factor NFκB-p65 nuclear translocation. Second, we choose the human gastric cancer cell lines MKN 45 as the target cell, which was co-cultured with DCs, T cells from spleen of mouse and AMs injection, and use MTT assay to judge the amount of cell lines and Immnunoflurescene to analyze the expression of anti-active caspase 3 pAb anti-PARP P85 fragment pAb, the mark of apoptosis of cells. Third, we have conducted the animal studies beside the basic experiment in vitro. The nude mouse developed stomach cancer, due to intra-preritoneal injection with MKN45 have been divided into two groups: the treatment group challenged with AMs injection and the control group with saline injection. We took the average of the diameter of each group as the y axis and the days after administered with AMs as x axis. After 40 days, all animals were killed by detruncation, and the tumor were removed and measured. We compare the diameter (<40 days) and weight (>40 days) of the tumor as well as the survival days between different groups to investigate the effect of inhibition of cancer.
Results
All results show that AMs is effective in treating human stomach cancer and the mechanism might be regulated by TLR4 mediated signal transduction of DCs. The results are briefly introduced as follows: First, we succeed in culturing the DCs induced by IL-4 and GM-CSF and find the positive rate of CD11c expression, the mark of DCs, is beyond 90% (Fig-1). We detect AMs can precipitate DCs maturation by upregulating TLR4 in SYBR-Green I Real-time PCR (Fig-2) and suppressing I.B-aby Western-Blot (Fig-3). Second, after the MKN45 co-cultured with DCs, T cells and AMs injection, the result show that AMs can great reduce the amount of cell lines by MTT assay (Fig-4) and induce apoptosis with Immunofluorescence (Fig-5). Finally, we have conducted animal studies beside the experiment in vitro, and the result in vivo show that AMs can delay tumor development from the diameter and weight of the tumor (Fig-6, Fig-7), prolong life-span and improve life-quality.
Conclusion
Ams Can play a great role in treating human stomach cancers as a good Chinese herbal medicine by precipitating DCs maturation, which is probably due to its effects by regulating the TLR4 mediated signal transduction.
Keywords: Astragalus mongholicus, huaman stomach cancer, Traditional Chinese Medicine (TCM), Chinese herbal medicine, Toll-like-receptors 4, NFκB-p65, IκB-α
Introduction
It is well-known that we depend on three methods to treat cancers: surgery, chemotherapy and radiotherapy. However, these three ways have its own pros and cons. The greatest weakness is its side effects and even to accelerate the growth after receiving these methods due to irritation of remnants of cancer. Traditional Chinese medicine(TCM) is one of the oldest healing systems. TCM includes herbal medicine, acupuncture, food therapy and physical exercise. According to some research in recent years, TCM especially herbal medicine, is superior to these three methods cited above in treating cancers in some respects, what is the most prominent is less side effect.
Astragalus mongholicus (AMs) is the most common herbal medicine in treating Hypertension, Ischemic heart disease, Acute glomerular nephritis, tumors after surgery or radiotherapy, Osteoporosis, diabetes and so on .These days, it have been reported that AMs is effective in treating some cancers, especially stomach cancer from lots of clinical cases, but fail to know about the mechanism, leading to some doubt for Western-medicine doctors and restriction of wide use.
The dendritic cells (DCs) is one of the most powerful antigen presenting cells (APC), which can link the innate and adaptive immune system. It is obvisous that DCs make great difference to be against tumors. Toll-like-receptors (TLRs), especially Toll-like-receptor 4 (TLR4), are key initiators of innate and adaptive immune response by TLR4 signaling to regulate antigen-presenting cells (APCs) (Akira and Takeda, 2004). The imbalance of Immune system is the most important cause in many of the mechanisms involved in tumor tumor rigenesis. We speculate that AMs can suppress some molecular expression on the TLR4 mediated signaling pathway which make a significant difference on molecular level for the occurrence and development to tumors. We undertook this research to delineate the anti-tumor mechanism of AMs to human stomach cancer in order to make better use of AMs as good Chinese Herbal Medicine.
Materials and Methods
The culture and phenotypic identification of mouse dendritic cells (DCs)
The C57/BL female mouse (4–6 weeks) were killed by detruncation and the rear legs were taken, and then the bone marrow were pushed with saline and collected by 15mL centrifuge tube. It is emphasized that all animals conducted in all the experiments of this research were purchased from Military Medical Science Academy, Beijing, China and all kinds of experiments involved animals have been approved by the ethics committee of Anhui Medical University, Hefei, Anhui Province, in accordance with the principle of life ethics. The protocol of cell culture refer to the method of Inaba K (Inaba, K., 1992) were cited as follows: 1)To split the red blood cells: we put 5 mL Tris-NH4Cl (PH 7.2–7.4) into the bone marrow and mix with pipette, and then let stand 10 minutes. 2) To discard the supernatant: After lysate, we wash the remnants twice using 1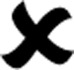 phosphate-buffered saline (PBS). 3) To separate the cells according to the adherent ability: the number of the cells were adjusted into 106/mL with basic medium RPMI 1640 and put into the flask, which have been put into a humidified, 5% CO2 incubator at 37°C for three hours. And then we discard the suspension. 4) To culture DCs: We put the same amount of complete medium (the basic medium RPMI1640 including fetal bovine serum (FBS) 10% and pen/strap 1% plus granulocyte/macrophage colony-stimulating factor (GM-CSF) 10ng/mL and recombinant human Interleukin-4 protein (rhIL-4) 5ng/mL) into the flask, and the medium have been changed semiquantitatively for each other day. 5) To identify DCs: After six days, we collect the DCs and adjust the number into 107/mL, and added 100 uL 1
phosphate-buffered saline (PBS). 3) To separate the cells according to the adherent ability: the number of the cells were adjusted into 106/mL with basic medium RPMI 1640 and put into the flask, which have been put into a humidified, 5% CO2 incubator at 37°C for three hours. And then we discard the suspension. 4) To culture DCs: We put the same amount of complete medium (the basic medium RPMI1640 including fetal bovine serum (FBS) 10% and pen/strap 1% plus granulocyte/macrophage colony-stimulating factor (GM-CSF) 10ng/mL and recombinant human Interleukin-4 protein (rhIL-4) 5ng/mL) into the flask, and the medium have been changed semiquantitatively for each other day. 5) To identify DCs: After six days, we collect the DCs and adjust the number into 107/mL, and added 100 uL 1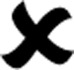 PBS to suspend the cells. We put 10 uL anti-mouse CD11C antibody linked with PE (ebioscience, cat#12-0114), the specific mark of DCs, and place the mix in 4°C for 10 minutes and then washed by 1
PBS to suspend the cells. We put 10 uL anti-mouse CD11C antibody linked with PE (ebioscience, cat#12-0114), the specific mark of DCs, and place the mix in 4°C for 10 minutes and then washed by 1 PBS at 1200 rpm for 10 minutes. Discard the supernatant and put 500 uL 1
PBS at 1200 rpm for 10 minutes. Discard the supernatant and put 500 uL 1 PBS to resuspend the cells. After the work have been done, we check the positive rate of CD11C by Flow Cytometry (FCM).
PBS to resuspend the cells. After the work have been done, we check the positive rate of CD11C by Flow Cytometry (FCM).
Real-time PCR to detect TLR4 mRNA expression of DCs
We added AMs injection (Shineway, ShiJiaZhuang city, HeBei Province, China) into the DCs cultured for three days at different concentration of 0ng/mL, 20ng/mL, 40ng/mL, 60ng/mL and 80ng/mL for 24 hours. The DCs were collected into a clean tube and washed twice by 1 PBS and then the RNA extraction, the cDNA sythesis and real-time PCR have been done one by one. The protocol of the technique is as follows: 1). RNA extraction: The DCs cultured with and without AMs were collected respectively, the RNA was extracted by Quock -RNA ™ MicroPrep (ZYMO Research, Cat#R1050) 2). The synthesis of cDNA: we produce the cDNA with Reverse Transcription Shots (PrimerDesign Cat#RT-NanoScript). 3). SYBR-Green I Real-time PCR reaction: SYBR-Green I Real-time PCR was done using Precisionã 2*Real-time PCR Mastermix (PrimerDesign Cat#Precision-iC) on 7700 Sequence Detection System (PE Applied Biosystem). The forward primer and reverse primer of TLR4 and beta-actin of mouse DCs are cited from the reference (Xiaoyi yan and Xuetao Cao, 2005). The reverse primer and forward primer of TLR4 are 5′GCT TTC ACC TCT GCC TTC AC-3′ and 5′-CGA GGC TTT TCC ATC CAA TA-3′.In parallel, the forward and upward primer of beta-actin are 5′ TGG AAT CCT GTG GCA TCC A 3 and 5′TAA CAG TCC GCC TAG AAG CA 3. PCR threshold cycle (Ct) is recorded when the fluorescence is first detected and is about 15 cycles. The PCR reaction was in a total volume of 25 uL containing 2.5 ul of 10*PCR buffer, 5 uL of 25 mM MgCL2 solution, 0.75 uL 10 mM of each deoxyguanosine triphosphate (dGTP), deoxyadenosine triposphate (dATP), deoxythymidine triposphate (dTTP), and deoxycytidine triphosphate (dCTP), 0.125 uL of Taq Polyease, 0.25 uL of each primer (10 uM), 0.5 uLSYBR-Green I, and 0.1 to 1 ug of DNA. The mouse beta-actin is performed in parallel as a positive control in the same reaction. The parameter is 40 cycles with pre-denaturation at 95°C for 15 minutes and denaturatin at 94°C for 30 seconds, annealing at 60°C for 30 seconds and extending at 72°C for 30 seconds. The steps above are repeated for 30 cycles. The temperature to read the template is 78°C.
PBS and then the RNA extraction, the cDNA sythesis and real-time PCR have been done one by one. The protocol of the technique is as follows: 1). RNA extraction: The DCs cultured with and without AMs were collected respectively, the RNA was extracted by Quock -RNA ™ MicroPrep (ZYMO Research, Cat#R1050) 2). The synthesis of cDNA: we produce the cDNA with Reverse Transcription Shots (PrimerDesign Cat#RT-NanoScript). 3). SYBR-Green I Real-time PCR reaction: SYBR-Green I Real-time PCR was done using Precisionã 2*Real-time PCR Mastermix (PrimerDesign Cat#Precision-iC) on 7700 Sequence Detection System (PE Applied Biosystem). The forward primer and reverse primer of TLR4 and beta-actin of mouse DCs are cited from the reference (Xiaoyi yan and Xuetao Cao, 2005). The reverse primer and forward primer of TLR4 are 5′GCT TTC ACC TCT GCC TTC AC-3′ and 5′-CGA GGC TTT TCC ATC CAA TA-3′.In parallel, the forward and upward primer of beta-actin are 5′ TGG AAT CCT GTG GCA TCC A 3 and 5′TAA CAG TCC GCC TAG AAG CA 3. PCR threshold cycle (Ct) is recorded when the fluorescence is first detected and is about 15 cycles. The PCR reaction was in a total volume of 25 uL containing 2.5 ul of 10*PCR buffer, 5 uL of 25 mM MgCL2 solution, 0.75 uL 10 mM of each deoxyguanosine triphosphate (dGTP), deoxyadenosine triposphate (dATP), deoxythymidine triposphate (dTTP), and deoxycytidine triphosphate (dCTP), 0.125 uL of Taq Polyease, 0.25 uL of each primer (10 uM), 0.5 uLSYBR-Green I, and 0.1 to 1 ug of DNA. The mouse beta-actin is performed in parallel as a positive control in the same reaction. The parameter is 40 cycles with pre-denaturation at 95°C for 15 minutes and denaturatin at 94°C for 30 seconds, annealing at 60°C for 30 seconds and extending at 72°C for 30 seconds. The steps above are repeated for 30 cycles. The temperature to read the template is 78°C.
Western-Blot to detect the expression of IκB-α
The DCs treated with and without AMs injection at the dose 0ng/mL, 20ng/mL, 40ng/mL, 60ng/mL and 80ng/mL for 24 hours were collected and washed twice with 1*phosphate-buffered saline(PBS). We added 1mL RIPA buffer (thermo Cat#89901) containing 50mM Tris, 150mM NaCl, 0.1% SDS, 0.5% deoxycholate, 2mM EDTA, 0.1% Triton X-100, 10% glycerol, 1mM phenylmethylsulfonyl fluoride and 10ug/mL aprotinnin into the tube. The proteins were quantified with BCA protein Assay kit and separated by 12% SDS polyacrylamide gel electrophoresis. After separation, the proteins on the gel were transferred onto a nitrocellulose membrane (0.45 um) and then probed with the primary antibody IκB-α (Santa Cruz Biotech, Cat#SC-373893) at the dilution 1:500, followed by the goat anti-mouse IgA-HRP (Santa Cruz Biotech, Cat#SC-3791) at the dilution 1:2000. The immunblots were visualized with enhanced chemiluminescence (ECL) western blotting regents (Pierce Cat#32109) and exposed to Kodak X-Omat K film. The membranes were then reprobed with the primary anti-body beta-actin (Santa Cruz Biotech, Cat#SC130300) for normalization. The rest of the operation is the same as the protocol as cited above.
MTT assay to observe human gastric cancer cell lines viability and proliferation
The human gastric cancer cell line, MKN45, was purchased from Perking Union Medical University, Beijing, China. The line was cultured with the medium Dulbecco modified Eagle medium (DMEM) including Fetal bovine serum (FBS) 10% plus pen/strap 1% in a humidified, 5% CO2 incubator at 37°C. After two or three days of incubation in a 37°C, 5% CO2 incubator, harvest the cells by centrifugation at 300*g for 5 minutes and pass cells at the ration 1:2 as much as 80% for the area of the flask. Other cells were preserved after programmed cooling in liquid nitrogen using frozen stock solution (DMEM 70%+20% FBS+10% DMSO). MKN45 on a good state observed by microscope was collected, counted and adjusted into 1*105 cells/mL for further use. The C57/BL mice were killed by detrunction, whose spleens were taken and grinded into single cell-suspension (named spleen T cells) with 200 orders of strainers. The spleen T cells were counted and adjusted into 103 cells/mL. The DCs treated with AMs injection at the dose 0 ng/mL, 20 ng/mL, 40 ng/mL, 60 ng/mL and 80 ng/mL for 24 hours were harvested and counted and adjusted into 103 cells/mL. The control group is treated with corresponding saline.
We add 100 uL spleen T cells and 100 uL DCs of each group into 1mL MKN45 cell suspension, which constitute the cell mix. And then the cell-mix were divided into 6-well culture plate for further use. Finally, we use MTT assay (ATCC, Cat#30-1010K) to observe the viability and proliferation of MLN45 as follows: 1). The cell-mix of each group were adjusted into 2*105/mL and inhaled into 96 cell culture plate; 2). The plates were incubated for 6–24 hours and add 10 uL MTT regent and incubate for 2–4 hours until purple precipitate is visible. 3). Add 10 uL detergent reagent and leave it at room temperature in the dark for 2 hours. 4). To record absorbance at 570 nm on enzymes labelling instrument and calculate the cell number of each group.
Fluorescene Microscope to observe cell apoptosis
Anti-Active Caspases-3 pAb (Promega, Cat#G7481) and anti-PARP p85 fragment pAb (Promega, Cat#G7341) are the most important marker of cell-apoptosis, which specially detect apoptotic cells. And we use these markers to observe whether AM can induce the apoptosis.
We co-culture the cell lines MKN45 with DCs treated with AMs 60ng/mL for 24 hours and spleen T cells. And then we attached the cell mix to HTC 8-well frosted slides (Fisher Cat#10-154), fix 12 hours and then the slides were preserved in 2% formaldehyde solution in protein-free 1 PBS. The next day, these slides were permeabilized with 0.2% Triton X-100 in 1
PBS. The next day, these slides were permeabilized with 0.2% Triton X-100 in 1 PBS and blocked with blocking buffer (PBS/0.1%Tween 20+5% horse serum). Wash the slides in 1
PBS and blocked with blocking buffer (PBS/0.1%Tween 20+5% horse serum). Wash the slides in 1 PBS for 5 minutes at room temperature and repeat. Drain the slide and add 100uL Anti-Active Caspase −3 pAb (Promega cat#G7481) at a 1:250 dilution in blocking buffer, and meantime, prepare a slide with no anti-Active Caspase-3 pAb as a negative control. The following day, wash the slides and add 100 ul of donkey anti-rabbit conjugate diluted 1:500 in 1
PBS for 5 minutes at room temperature and repeat. Drain the slide and add 100uL Anti-Active Caspase −3 pAb (Promega cat#G7481) at a 1:250 dilution in blocking buffer, and meantime, prepare a slide with no anti-Active Caspase-3 pAb as a negative control. The following day, wash the slides and add 100 ul of donkey anti-rabbit conjugate diluted 1:500 in 1 PBS (Jackson Cat#711-165-152). Drain the liquid and mount the slides in a permanent or aqueous mounting medium and observe under a fluorescence microscope. The operation of anti-PARP P85 pAb (the dilution 1:100) is the same as the protocol cited above.
PBS (Jackson Cat#711-165-152). Drain the liquid and mount the slides in a permanent or aqueous mounting medium and observe under a fluorescence microscope. The operation of anti-PARP P85 pAb (the dilution 1:100) is the same as the protocol cited above.
Animal Studies
Twenty C57/BL6 female nude mice (3–5 week old) were randomly divided into the treatment group and the control group. Each group have ten mice. First, all mouse were injected with the human gastric cell lines MKN45 (105 cells/kg) intraperitoneally and make sure all animals were developed the stomach caner due to transplantion. Second, after seven days, the treatment group were administered 250 mg/kg intraperitoneally for 6 consecutive days. By contrast, the control group were done as the same operation with an equivalent volume of saline. Every three days, we measure the diameter of the tumor of each mouse for the two groups. Meanwhile, we observe the hair brightness and active degree as well as the survival days between different groups for consecutive 40 days. After 40 days, all animals besides some mouse already dead, were killed by detruncation, and the tumor were removed and measured. We compare the diameter and the weight of the tumor as well as the survival days between different groups. And then we calculated the inhibition rate of tumor according to the formula (the weight of treatment group-the weight of control group/the weight of the control group) in order to analyse the effect of tumor-treatment of AMs.
Statistical analysis
We use statistical software SPSS ver.11.5 and rank-test to analyse all the data. We consider P <0.05 to be statistically significant.
Results
The morphology and phenotypic identification of DCs
We observed the state of the mice DCs use microscope (the field is 20 ) and find obvious branches (fig1-a), which is typical for DCs. And then we check the expression rate of CD11C with FCM, specific marker of the cells, and find that the positive rate of CD11 cultured by the method 1 is beyond 80% (fig 1-b). All results show that the cells cultured by our method are indeed DCs.
) and find obvious branches (fig1-a), which is typical for DCs. And then we check the expression rate of CD11C with FCM, specific marker of the cells, and find that the positive rate of CD11 cultured by the method 1 is beyond 80% (fig 1-b). All results show that the cells cultured by our method are indeed DCs.
Figure 1.
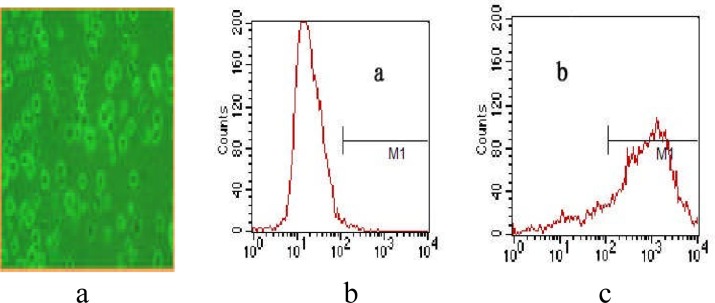
the morphology and phenotypic identification of DCs.
- The form of DCs observed by microscope with field 20*.
- The isotype antibody control using FCM.
- The positive rate of CD11c expression.
The TLR4 mRNA expression
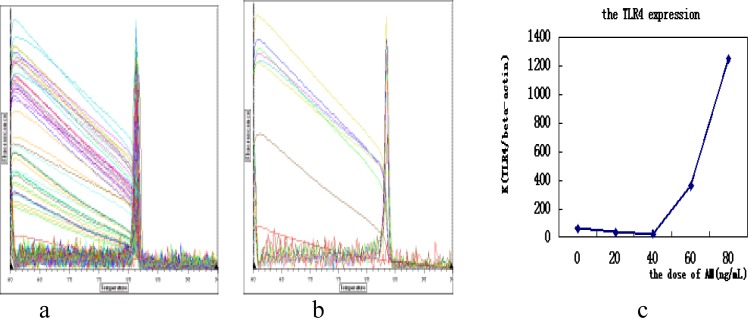
We use SYBR-Green I Real-time PCR to detect the change of TLR4 mRNA expression, and find the melting curve has no misvellaneous peaks and non-specific signal interference (fig 2-a, b). After that, we divide the value of TLR4 expression of DCs (fig 3-b) by the value beta-actin expression (fig 2-a). We take the ratio as the y axis and the dose of AMs as the x axis. The results show that AMs can stimulate DCs to express TLR4 (fig 2-c) with dose dependent.
Figure 2.
the melting curve and the chart of TLR4 expression
a) the melting curve of beta-actin; b)the melting curve of TLR4;c)the TLR4 expression of DCs stimulated with AM at different dose. There is significant statistic difference between the 60ng/mL and 80ng/mL group and other group (P<0.05 by rank test)
Figure 3.

the IκB-α expression of DCs with different dose of AMs
L0: 0ng/mL; L1:20ng/mL; L2:40ng/mL; L3:60ng/mL; L4:80ng/mL
The expression of IκB-α
We use western-blot to analyse the expression of IκB-α of DCs stimulated with AMs at different dose and find that AMs can suppress the expression (fig 3) and the effect is dose dependent.
The viability and proliferation of MKN45
We use MTT assay to analyse the viability and proliferation of MKN45 and find that AMs can reduce the amount of the cells (fig 6).
Figure 6.
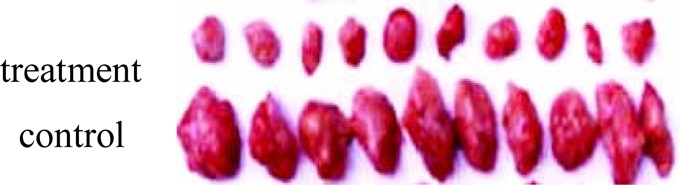
The difference of tumor between treatment group and control group.
The cell apoptosis
The caspase family of cysteine proteases plays a key role in apoptosis. Anti-Active Caspases-3 pAb (Fig 5-a) and anti-PARP p85 fragment pAb (Fig 5-b) were stimulated in the group of DCs treated with AMs at dose 60ng/mL for 24 hours, which indicate that the tumor were induced into apoptosis by AMs.
Figure 5.
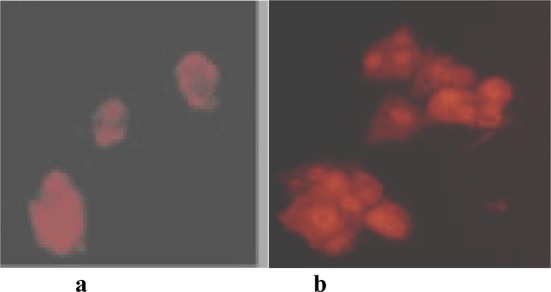
the anti-active caspase-3 pAb (a) and anti-PARP P85 fragment pAb (b) actived by immunofluorescence.The cell mix were treated with 100uL anti-active caspase-3 pAb at a 1:250 dilution and anti-PARP P85 fragment pAb at a 1:100 dilution, and the secondary Ab was donkey anti-rabbit Cy®3 conjugate diluted 1:500 in PBS (Jackson Cat#711-165-152). From the photo, we find that anti-active caspase-3 pAb and anti-PARP P86 fragment pAb can express which is very important to indicate cell-apoptosis.
Animal studies
We used a quanlitative and quantitative method to evaluate the effect of tumor-treatment. From the quanlitative perspective, we find both the diameter and the weight of the tumor of the treatment group were much smaller than the control group (Fig 6). In addition, we discovered that the treatment groups are much active and the hairs are much brighter during the survival period of the mouse. From the quantitative angle, We compare the diameter (0.7±0.14 versus 1.46±0.12) and the weight (10.5±0.25 versus 24.5±0.31) between the treatment and the control group and the two index are significantly different by rank-test (p<0.05) (Table 1). Conversely, the survival days of the treatment group (40d) are much longer than the control group (30.5d) (Table 1). According to the weight, we calculate the inhibition rate of tumor is 57.1%. According to the proof listed, we could reasonably conclude that AMs can prolong life span from the survival days and improve life quality from the diameter and weight as well as hair brightness and active degree. The inhibition rate of tumor (57.1%) proves our assumption from other angle.
Table 1.
The effect of inhibition to tumor of AMs
| group | number | diameter(x +SD)(cm) | weight( x +SD)(g) | survival time(d) |
| treatment | 10 | 0.7±0.14 | 10.5±0.25 | 40 |
| control | 10 | 1.46±0.12 | 24.5±0.31 | 30.5 |
All mice were injected with the human gastric cell lines MKN45 (105cells/kg) intraperitoneally and make sure all animals were developed the stomach caner due to transplantion. The treatment group were administered 250 mg/kg intraperitoneally for 6 consecutive days. By contrast, the control group were done as the same operation with an equivalent volume of saline. Every three days, the diameter of the tumor have been recorded for consecutive 40 days. After 40 days, all animals besides some mouse already dead, were killed by detruncation, and the tumor were removed and measured. We compare both the diameter and the weight of the tumor between two groups.
Discussion
AMs is effective in the clinical application from lots of cases, however, the mechanism to which is unknown so that some western-medicine doctors have some doubt. The lack of study on the mechanism restricts its wide use. In this study, we want to find AMs how to act in regulating immunity and treating cancers.
The DCs are the most powerful antigen presenting cells, which link the adaptive and innate immune-system. Toll-like-receptors (TLRs) is an important group of pattern recognition receptor (PRR) presented on the surface of innate immune cells such as DCs and macrophages. In the family of TLRs, TLR4 mainly control the DCs whether matured. The immatured DCs become matured after TLR4 stimulation by kinds of external signals, such as lipopolysaccaride, inflammatory factors and some Chinese Traditional herb and so on. In the TLR4 signal transduction, NF-kB is the most important in its down stream molecules, especially in the anti-tumor process (Wang, R.F., 2008). As for NF-kB, the I-kB α is the major regulatory protein, which can inhibit its nuclear translocation (Wenlin, H., 2005). It is well know that one of the mechanism to tumor start and development is immune-balance. So we speculate that AMs can precipitate the DCs maturation by stimulating TLR4 mediated NF-kB signaling transduction. To confirm this speculation, we first culture the DCs from mouse bone marrows and after seven days, we use FCM to identify the positive rate of CD11C, and the result show that the CD11C expression rate of DCs is beyond 80% (fig 1-c). And then, the DCs were treated with AMs at different dose, we find AMs can precipitate the TLR4 expression by FCM (fig 2-c) and suppress the the I-kB αexpression with Western-Blot (fig 3) at the dose 60 ng/mL or 80 ng/mL (P<0.05 compared with other group). The I-kBα suppression means the enhancement of NF-kB nuclear translocation. From the result, we consider the speculation to be corresponded to the experiments.
To be further studied in the research, we conclude that AMs can precipitate DCs maturation to improve T cells to suppress tumor cells development. It is common knowledge that the spleen is the biggest source of T cells. So we take the spleen from the mice killed by destruction, which was grinded into single cell suspension. We co-culture the tumor cell lines MNK 45 with T cells from spleen plus AMs and find that the amount of MKN45 were greatly reduced with MTT assay (Fig 4) and the anti-active caspase-3 (fig 5-a) and the anti-PARP P85 fragment pAb (fig 5-b) were both stimulated by immunofluorescence. The result show that AMs can induce MNK45 into apoptosis at the dose of 60 ng/mL or 80 ng/mL (Fig 4) (P<0.05 compared with the control group).
Figure 4.
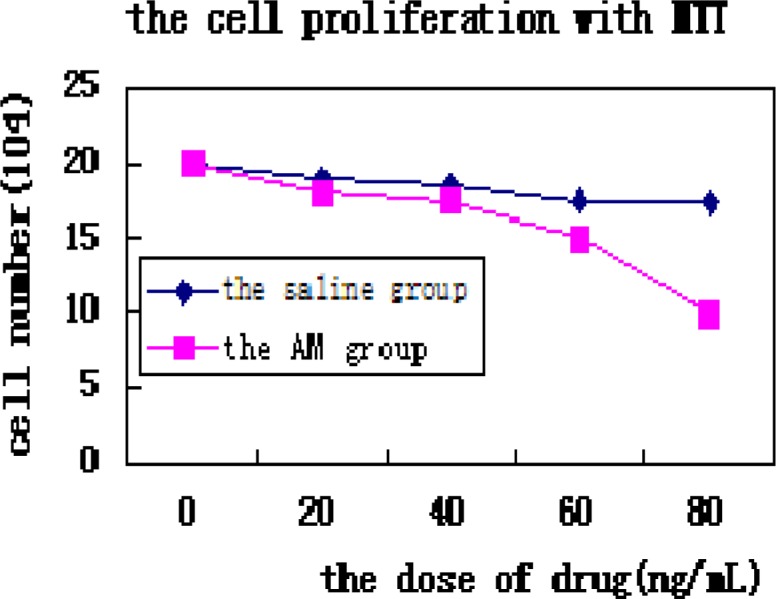
MTT assay to analyse the viability and proliferation of the two cell lines (P<0.05 between the group with the dose of 60ng/mL and 80ng/mL and other group). the horizontal axis is the group treated with AM and saline at different dose, the vertical axis is the cell number.
After basic experiment in vitro, we conduct the animal studies to observe the effectiveness of treatment of AMs. First, the nude female C57/BL6 mouse were intrapreritoneal injected with MKN45 to establish the stomach cancer model. And then the mouse cultivated with MKN45 were divided into two group, the treatment group with AMs injection (250mg/kg) and the control group with the same volume of saline, which were observed for consecutive 40 days. We evaluate the anti-tumor effect from qualitative and quantitative aspect. Both the diameter and the weight of the tumor of the treatment group were much smaller than the control group (Fig 6). Beside this, the treatment groups are much active and the hair are much brighter during the survival period. According to the data, we find that the diameter and the weight between the treatment and the control group are significantly different by rank-test (p<0.05) (Table 1). Meanwhile, the survival days of the treatment group (40d) are much longer than the control group (30.5 d)(Table 1). And we calculate the inhibition rate of tumor is 57.1%.In sum, it is assumed that AMs can prolong life span from the survival days and improve life quanlity from the diameter and weight as well as hair brightness and active degree. More closely, The inhibition rate of tumor (57.1%) prove the effectiveness of anti-tumor of AMs.
In conclusion, the result of this study show that AMs is effective in treating stomach cancer and it might precipitate DCs maturation by regulating TLR4 mediated NF-kB signal transduction against tumor, which offers a kind of novel perspective and beneficial exploration to the anti-tumor mechanism.
References
- 1.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Xiaoyi Y, Xuetao C. Fever promotes TLR4 expression and signalling in dendritic cells [D] Zhengjiang University; 2005. [Google Scholar]
- 3.Wang RF, Miyahara Y, Wang HY. Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene. 2008;27(2):181–189. doi: 10.1038/sj.onc.1210906. [DOI] [PubMed] [Google Scholar]
- 4.Wenlin H, Xiaofeng Z, Xiaoning W, Xiaofeng Z. Signal Transduction. Beijing: Chinese People Press; 2005. 2005. [Google Scholar]
- 5.Inaba K, Inaba M, Romanni H, Aya M, Deguch S, Ikehara S, Muramatsu R, Steinman M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulation factor. J Exp Med. 1992;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]