Abstract
Background
Ficus exasperata Vahl-Holl (Moraceae) leaves are used for infectious and inflammatory conditions in many West African Countries. However, there is need for more phytochemical studies to justify the ethnomedicinal uses of the plant.
Material and Methods
The crude 50% aqueous ethanolic extract of the leaves was partitioned successively between water and; n-hexane, ethyl acetate and n-butanol. The fractions were subjected to antimicrobial activity using agar diffusion test. n-Butanol fraction, which showed both antimicrobial and radical scavenging activities was subjected to repeated chromatographic fractionation on both silica and Sephadex LH-20 columns. Each stage of the purification was monitored by thin layer chromatographic diphenylpicryl hydrazyl autographic assay. Three compounds were isolated. The structures of the compounds were elucidated using spectroscopic methods, shift reagent studies, acid hydrolysis, and by comparison with literature data.
Results
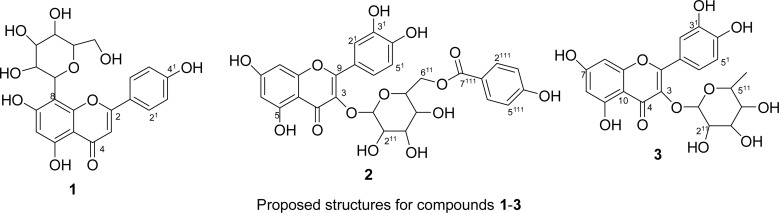
The compounds were identified as apigenin C-8 glucoside (1), isoquercitrin-6-O-4-hydroxybenzoate (2) and quercetin-3-O-β-rhamnoside (3). The solvent fractions and isolated compounds were found to inhibit the growth of Gram +ve organisms only.
Conclusion
These flavonoid glycosides are being reported in this plant species for the first time. Their weak in vitro antimicrobial activity suggest the flavonoids may be acting as pro-drug. The radical scavenging activity of the compounds may justify some of the ethnomedicinal uses of the plant as free radicals are implicated in the aetiology of many inflammatory diseases.
Keywords: Ficus exasperata, flavonoid glycosides, radical scavenging activity, antimicrobial activity
Introduction
During infection, Inflammation is the first step in the cascade of events that lead to release of free radicals, culminating in the destruction of invading organisms to bring about healing. However, exceeding the concentration of free radicals the body's endogenous anti-radical mechanisms can cope with has been linked with the aetiologies of many diseases (Emery, 2006). Many of the rural populace and people with low socioeconomic status had developed and relied heavily on traditional medicine which revolves around the use of herbs to treat many of these diseases, due to the inadequate access to orthodox medical practice. One of such herbs is Ficus exasperata Vahl-Holl (Moraceae), popularly referred to as “Sandpaper leaf tree” owing to the rough surface of the leaves.
Ficus exasperata is a deciduous African tree that grows up to about 20 m in height and prefers evergreen and secondary forest habitats (Berg, 1989). The leaf extract has been used ethno-medicinally to treat high blood pressure, rheumatism, arthritis, intestinal pains and colics, epilepsy, bleeding and wounds (Irvin, 1961). Some biological studies indicate that leaves of F. exasperata exhibit anti-ulcer, hypotensive, hypoglycemic, hypolipidemic, anti inflammatory, anxiolytic, oxytocin inhibiting, anticonvulsant, antinociceptive, antipyretic, anti microbial, anti candidal, insecticidal, pesticidal activities, antiarthritic and antioxidant (Akah, 1998; Ayinde, 2007; Odunbaku, 2008; Taiwo, 2010; Adewole, 2011; Bafor, 2011; Ogunleye, 2011;Woode,2011a–b). Antioxidant lignans had previously been reported from the leaves extract of the plant (Taiwo et al., 2006), while recently, bergapten and oxypeucedanin hydrate were reported from the stem bark and sitosterol and sitosterol-3-Obeta-D-glucopyranoside were reported from the leaf extract of the plant (Amponsah, 2012).
Widespread medicinal use and significant biological activities of the extracts from the plants justified continued investigation of F. exasperata. We report here the isolation of flavonoid glycosides from the leaves of the plant.
Experimental
General
One dimensional (1D), NMR data were obtained on a Varian 200 MHz NMR spectrometer (Varian Inc., Palo Alto) at the Central Science Laboratory, Obafemi Awolowo University, while Two-dimensional (2D), experiments were carried out on a Bruker AV600 spectrometer at the Department of Chemistry, University of Potsdam, Germany. Chemical shifts were expressed in parts per million (ppm). Ultra violet (UV) spectra and UV absorbance were measured in methanol, on a Cecil UV/Visible spectrophotometer (Cecil Instruments Ltd, Cambridge). Adsorption column chromatography was performed using Accelerating Gradient Chromatography (AGC).The ascending mode was employed using silica gel 60 (ASTM 230–400 mesh, 0.040–0.063 mm particle size, Merck). The equipment for the AGC work station was from Baeckstrom Separo AB, Lidingo, Sweden. Adsorption chromatography (open column) was performed with Kieselgel 60 (ASTM 230–400 mesh, 0.040–0.063 mm particle size, Merck). Size exclusion column chromatography was achieved using Sephadex LH-20 (Pharmacia) pre-swollen in the specified solvent before loading onto the column. All Thin Layer Chromatography (TLC) analyses were performed at ambient temperature using analytical silica gel 60 GF254 + 366 pre-coated aluminium backed plates (Merck, 0.25 mm thick). The resulting spots on TLC plates were visualized under UV light (254 nm) and detected by the use of vanillin/H2SO4 and DPPH spray reagents. Solvents used for extraction and chromatography were distilled before use.
Plant Material
The leaves of Ficus exasperata (Vahl-Holl) (Moraceae) were collected on Obafemi Awolowo University Campus premise in October, 2010, and were identified by Mr. Gabriel Ademoriyo of the Department of Botany, Obafemi Awolowo University with a voucher specimen (number UHI 16,399) deposited at the IFE herbarium, Department of Botany, Obafemi Awolowo University. The plant materials were air-dried at room temperature and powdered. The milled plant material (5.3 Kg) was extracted with 50% aqueous ethanol (12 L) by maceration at room temperature for 24 hours. The mixture was filtered and the filtrate (crude extract) was concentrated to dryness in-vacuo at 40°C to give 268 g of crude extract. The crude extract was suspended in water and partitioned successively between n-hexane and water, ethyl acetate and water and n-butanol and water. Each fraction was concentrated in-vacuo at 40°C to give n-hexane (20.9 g), ethyl acetate (7.5 g), n-butanol (14.4 g) and aqueous (198 g) fractions respectively.
Antimicrobial activity
Antimicrobial activity was determined using the agar diffusion method. A stock solution of 20 mg/ml in 50% aqueous methanol was used for the crude extract while 5 mg/ml was used for isolated compounds with 50 % aqueous methanol as the blank. A 24 hour broth culture of each of the following test organisms was used: Escherichia coli (National Collection of Typed Cultures, NCTC 10418), Staphylococcus aureus (NCTC 6571), Pseudomonas aeruginosa (American Typed Culture Collection, ATCC 10145), Bacillus subtilis (NCTC 8236) Candida pseudotropicalis (National Collection of Yeast Cultures, NCYC 6). Each culture (0.5 ml) was inoculated into 50 ml of molten and cooled nutrient agar (Saboraud agar for the fungi). The inoculated nutrient broth was poured into Petri dish (14 cm), and allowed to set. Holes with diameter of 8 mm were cut in the nutrient plates, with each hole filled with 100 µl of the plant extract and the extract was allowed to diffuse for 1 hour before incubation at 37°C for 24 hours for bacteria and 25 °C for 7 days for the fungi. After incubation, the zones of inhibition observed were measured in millimetre.
Isolation of compounds 1, 2 and 3
The crude extract as well as solvent fractions were tested for activities using the TLC DPPH autographic assay for free radical scavenging screening (Burits and Bucar, 2000), and the agar diffusion test for antimicrobial assay. n-Butanol fraction gave spots that strongly bleached the purple colour of the DPPH solution to yellow while also showing clear zone of microbial growth inhibition. Therefore n-butanol fraction (14.6 g), was adsorbed on silica gel and successively eluted through an open column (3.5 × 40 cm) with solvents of increasing polarity using 300 ml each of 0, 10, 20, 40, 60, 80 and 100% ethyl acetate in n-hexane and 200 ml each of 0, 10, 20, 40, 80 and 100% of methanol. Eluate of about 15 ml was collected in each test tube. TLC analysis of the column eluate was carried out using ethyl acetate: MeOH : H2O : AcOH (10 : 2 : 1: 0.2solvent mixture 1). Test tube fractions with similar TLC profile were bulked together to give four fractions coded A-D. Fraction C (4.54 g), which gave positive result with the DPPH spray was subjected to repeated chromatography on silica gel using medium pressure on an AGC (3 x 30 cm, flow rate − 1 ml per minute) with solvent mixtures of increasing polarities using 100 ml each of 0, 10, 20, 40, 60, 80 and 100 ethyl acetate in n-hexane and 200 ml each of 0, 10, 20, 40, 80 and 100% of methanol in ethyl acetate to give six sub-fractions coded C1-C6. Fraction C5 (1.80 g) was dissolved in 5 ml of toluene: ethanol (7:3) and subjected to open column chromatographic using Sephadex-LH 20 (3.5 × 30 cm), pre-swollen overnight in a mixture of toluene : ethanol (7:3). The column was eluted isocratically with 9 x 50 ml of toluene: ethanol (7:3). The TLC analysis of the column eluate on silica using solvent mixture 1 above led to three fractions C5a (0.8 g), C5b (compound 1, 41 mg Rf =0.47, solvent 1) and C5c (156 mg). C5c was subjected to repeated purification on Sephadex LH-20 column to obtain compound 2 (31 mg, Rf = 0.64, solvent mixture 1) and compound 3 (23 mg, Rf = 0.58, solvent mixture 1).
UV shift reagent studies
UV shift reagent study on the isolated compounds was carried out according to the method described by Markham et al., (1982).
Acid hydrolysis of compounds 1 and 3
The isolated compounds (10 mg each), were separately refluxed in 2N HCl (10 ml), for 2 hours. The reaction mixture was allowed to cool and extracted with chloroform (5 ml twice). The chloroform extract was concentrated to dryness. The aqueous portion was also concentrated to dryness and reconstituted in methanol. The solution was spotted on TLC plate on silica alongside reference samples of glucose, galactose, fructose and rhamnose and eluted with solvent system 1 above.
Results and Discussion
The λmax of 271 (Band II) and 343 (Band I) in methanol in the UV spectrum of compound 1 (Table 3) indicated the presence of a flavone nucleus. The bathochromic shift of the spectrum upon the addition of NaOMe to the solution is an indication of a momosubstituted ring B with a free 4′hydroxyl group. A bathochromic shift of about 60 nm after the addition of aqeous solution of NaOAc to the methanolic solution of compound 1 in the UV spectrum indicated a free 7-OH group. The 1H NMR (DMSO) spectrum of compound 1 showed two sets of doublets, which integrate for two symmetrical protons, for H-3′ and H-5′). This was confirmed by the strong COSY correlation of the symmetrical protons proton which are ortho related and appeared at δ 6.90 ppm (d, J=8.6 Hz for H-2′ and H-6′) and at δ 8.00 ppm (d, J= 8.6 Hz δ 6.90 ppm with that at δ 8.00 ppm indicating a para substituted ring B. The proton signal at δ 13.2 ppm is consistent with proton signals of a free 5-OH hydrogen bonded to the C-4 ketone group always observed when DMSO is used as a solvent (Markham and Chari, 1982). In the 1H NMR spectrum, the signal at δ 6.30 ppm, a singlet, assigned to H-6 showed no correlation in the COSY spectrum. Also, the olefinic proton signal at δ 6.85 ppm attributable to H-3 did not couple with any otherproton. An anomeric proton signal at δ 4.75 (d, J=8.6 Hz H-1′) and other proton signals between δ 3.30–4.60 ppmindicated compound to be a glycoside. The Attached Proton Test (APT) experiment showed 9 quaternarycarbon,11 methine and one methylene carbon atom. The 13C NMR data of compound 1 was in good agreement with literature values (Table 1), therefore it was identified as apigenin glycoside. The position of glycosylation was determined by observing a 10 ppm downfield shift of the C-8 signal (glycosylated carbon) leaving the signals of other carbons relatively unaffected (Eldahshan et al., 2009) The TLC of the aqueous portion of the product of hydrolysis on comparison with reference sugars indicated the sugars to be glucose. The spectroscopic data of compound 1 are in good agreement with literature data and was therefore identified as apigenin-C-8 glucoside (Arot-Manguro et al., 2004). Compound 2 was isolated as a yellow powder. The 1H NMR spectrum showed a de-shielded signal at 12.63 ppm for a free 5-OH hydrogen chelated to the C-4 ketone group. There were set of doublets at 6.39 ppm (d, J=1.8 Hz, H-8) and 6.19 ppm (d, J=1.4 Hz, H-6). 7.58 ppm (d, J=2.0 Hz, H-2′), 7.56 ppm (dd, J=1.8, 9.4 Hz, H-6′) and 6.83 ppm (d, J=9.4 Hz, H-5′). There was an additional doublet at 7.59 ppm (dd, J=8.4, 2.4 Hz, H-2‴/6‴) and 7.577 ppm (dd, J=8.4, 2.4 Hz, H-3‴/5‴) for an ABX coupling pattern. The Carbon 13 spectrum of compound 2 displayed 28 signals which in the Attached Proton test spectrum (APT) showed 3 methine carbon signals at 93.9, 99.1, 122.0 ppm. The remaining methine carbon signals at 115.7, 116.6, and 132.0 ppm each integrated for two carbons. The spectrum also showed oxygenated methine carbons at 101.2, 78.0, 76.9, 74.5, 70.3 and 61.4 ppm. The remaining signals include oxygenated aromatic carbon signals at 156.6, 156.8, 145.3, 148.9, 133.8, 161.7 (x3), 164.6 ppm; while the carbonyl carbon signals were 167.4 and 177.9 ppm. The spectroscopic data are in good agreement with the literature data (Table 1) for compound 2 and was identified as isoquercitrin-6″-O-4‴-hydroxylbenzoate (Marzouk et al., 2006).
Table 3.
Results of the UV shift reagent studies.
| Comp | Shift reagents | MeOH | NaOMe | AlCl3 | AlCl3/HCl | NaOAc | NaOAc/H3BO3 |
| 1 | Band 1 | 343 | 381 | 384 | 346 | 404 | 354 |
| Band II | 271 | 276 | 277 | 273 | 279 | 269 | |
| 3 | Band 1 | 365 | 399 | 388 | 370 | 387 | 371 |
Keys:- λmax values read in nm. 1 =apigenin C8-glucoside, 3 =quercetin rhamnoside.
Table 1.
Comparison of the signals of compound 1 and 2 with literature data
| Carbon | 13C data(DMSO) | |||
| 1 50MHz |
Lit1* | Comp 2 50MHz |
Lit2* | |
| 2 | 163.3 | 163.9 | 156.6 | 156.3 |
| 3 | 103.1 | 102.4 | 133.8 | 133.1 |
| 4 | 182.8 | 181.0 | 177.9 | 177.0 |
| 5 | 161.8 | 161.0 | 161.7 | 161.4 |
| 6 | 98.8 | 98.1 | 99.1 | 99.7 |
| 7 | 164.6 | 162.5 | 164.6 | 164.5 |
| 8 | 104.7 | 104.6 | 93.9 | 94.51 |
| 9 | 156.7 | 155.9 | 156.8 | 156.2 |
| 10 | 105.3 | 104.0 | 104.4 | 103.8 |
| 1′ | 122.3 | 121.5 | 121.6 | 121.2 |
| 2′ | 129.6 | 128.8 | 115.7 | 115.2 |
| 3′ | 116.5 | 115.7 | 145.3 | 145.1 |
| 4′ | 161.1 | 160.3 | 148.9 | 149.0 |
| 5′ | 116.5 | 115.7 | 115.7 | 115.9 |
| 6′ | 129.6 | 128.8 | 122.0 | 122.8 |
| 1′ | 74.1 | 73.3 | 101.3 | 101.6 |
| 2′ | 71.5 | 70.8 | 74.5 | 73.5 |
| 3′ | 79.3 | 78.8 | 78.0 | 77.0 |
| 4′ | 71.2 | 70.5 | 70.3 | 68.8 |
| 5′ | 82.5 | 81.7 | 76.9 | 76.6 |
| 6′ | 62.0 | 61.3 | 61.4 | 63.4 |
| 1″ | 121.6 | 121.0 | ||
| 2‴/6‴ | 132.0 | 133.1 | ||
| 3‴/5‴ | 116.6 | 116.6 | ||
| 4‴ | 161.7 | 163.5 | ||
| 7‴ | 167.4 | 167.6 | ||
Table 2.
NMR (CD3OD) data of compound 3 compared with literature data.
| C | ppm | * | DEPT | H-H COSY | HSQC | HMBC |
| 2 | 159.0 | 158.3 | Cq | - | - | |
| 3 | 135.6 | 135.6 | Cq | - | - | |
| 4 | 179.5 | 178.7 | Cq | - | - | - |
| 5 | 163.1 | 162.3 | Cq | - | - | |
| 6 | 99.9 | 98.8 | CH | 6.20 (d, J = 2.4Hz)/6.39 | 6.20/99.9 | 94.7, 105.7 |
| (d, J=1.8Hz) | ||||||
| 7 | 166.0 | 163.7 | Cq | - | - | |
| 8 | 94.7 | 94.0 | CH | 6.39. (d, J=1.8Hz)/ 6.20 | 6.39/94.7 | 99.9, 105.7 |
| (d, J= 2.4Hz) | ||||||
| 9 | 158.4 | 157.4 | Cq | - | - | - |
| 10 | 105.7 | 104.5 | Cq | - | - | - |
| 1′ | 123.2 | 122.4 | Cq | - | - | |
| 2′ | 116.0 | 116.6 | CH | 7.58 (dd, 8.4, 2.4 Hz)/ | 7.584/117.6 | 117.6, 159.0, |
| 6.87ortho 7.71 (d, 2.4Hz) meta | 149.9 | |||||
| 3′ | 149.9 | 149.1 | CH | 6.87 (d, 8.4 Hz)/7.58-ortho | 6.87/116.0 | 123.2,145.9, |
| 4′ | 145.9 | 148.2 | Cq | - | - | - |
| 5′ | 117.6 | 116.0 | Cq | - | - | - |
| 6′ | 123.0 | 123.5 | CH | 7.71 (d, 2.4 Hz)/ 7.58 meta | 7.71/117.6 | 159.0, 123.0, |
| 145.9 | ||||||
| 1″ | 104.3 | 102.1 | CH | 5.26 (d, 7.8 Hz)/3.43 | 5.26/104.3 | 135.6, 78.4 |
| 2′ | 75.7 | 70.5 | 3.43 (triplet) | 3.49/75.7 | 104.3 | |
| 3′ | 78.1 | 71.9 | 3.35 (triplet) | 3.43/78.1 | ||
| 4′ | 78.4 | 71.3 | CH | 3.49 (triplet) | 3.23/78.4 | |
| 5′ | 71.2 | 69.7 | 3.23 (multiplet) | 3.35/71.2 | ||
| 6′ | 19.5 | 17.8 | CH3 | 0.99 (d, J=6.6 Hz) | 0.992/19.5 | 71.2 |
The absorbance of the MeOH solution of compound 3 at 365 nm in the UV spectrum (Table 3) indicated a 3-O-substituted flavonol. The bathochromic shift in band II after the addition of NaOAc in the shift reagent studies indicated a free 7-OH while the increased wave lenght of absorption of band I on addition of NaOMe indicated a free 4′-OH. The 1H NMR spectrum showed an ABX system for an ortho-dihydroxyl group for ring B with the two sets of doublet at 6.39 ppm (d, J=1.8 Hz, H-8) and 6.20 ppm (d, J=2.4 Hz, H-6), Which are meta related aromatic protons. The COSY spectrum showed a cross peak between the H-8 and H-6 protons. These two protons were correlating with 94.7 and 99.9 ppm in the HSQC while they showed a 3J correlation with 99.9, 107.5 and 94.7 and 107.5 ppm in the HMBC spectrum respectively. 13CNMR spectrum showed 21 signals, 15 for a flavonol nucleus and six for a hexose sugar. Anomeric carbon signal at 104.3 ppm was observed to have a cross peak with a proton signal at 5.26 ppm in the HSQC spectrum, which in the 1H NMR spectrum was observed to be a doublet with a J value of 7.8 Hz. There was a signal at 0.99 ppm in the 1H NMR spectrum, a doublet with a coupling constant of 6.6 Hz characteristic of rhamnose methyl signal. The signal was having a 3J correlation with a signal at 71.2 ppm in the HMBC spectrum. The TLC of the aqueous fraction of the hydrolytic product of the compound compared with reference sugars indicated the presence of rhamnose while glucose and galactose were absent. The point of attachment of the sugar was deduced by observing a long range correlation between the signal of the anomeric proton at 5.26 ppm and 135.6 ppm for the C-3-O of the aglycone indicating glycosylation to have taken place at the 3-OH of the quercetin nucleus. The large J value for the anomeric proton (7.8 Hz) indicated a β-orientation of the rhamnose sugar. Thus the compound 3 was proposed to be a quercetin-3-O-β-rhamnoside (Waage and Hedin, 1985).
The compounds (1–3) demonstrated very strong radical scavenging activity in TLC DPPH autographic assay. Their radical scavenging ability is well reported in literature. The screening of F. exasperata fractions indicated they have activity against Gram-positive bacteria only (Table 4.0 above). Structural elucidation of the compounds from the n-butanol fraction indicated the presence of flavonoid glycosides. Phenolic compounds and extracts of various medicinal plants containing flavonoids have been reported to possess antimicrobial activity (Basilea et al., 1999; Li et al., 1997; Tereschuk et al., Rhoca et al., 1995; Shutz et al., 1995 and Vaughn, 1995). The isolated compounds' antibacterial activity was weak unlike the activity reported in literature for both quercetin and apigenin aglycones. It is possible that their glycosilation causes a reduction in lipophilicity and the consequent diminished ability to penetrate bacterial membrane (Basilea et al., 1999). The flavonoid glycosides may be acting as pro-drug, a common concept with some orthodox medicines. Once administered, they may be cleaved to their respective aglycones inside the human body by enzymatic activity.
Table 4.
Results of the antimicrobial test of the Fractions and isolated compounds from F. exasperata
| Zones of inhibition (mm) | |||||||||
| Test organisms | Hex. | EtOAc | BuOH | Aq | 1 | 3 | Strept. | Acrifl. | Blank |
| E. coli | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 9.0 | NT | 0.0 |
| NCTC 10418 | |||||||||
| S. aureus | 6.3 | 6.0 | 6.0 | 0.5 | 1.5 | 2.0 | 17.0 | NT | 0.0 |
| NCTC 6571 | |||||||||
| Ps. earuginosa ATCC | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NT | 0.0 |
| 10145 | |||||||||
| B. subtilis | 6.0 | 5.0 | 6.0 | 0.3 | 1.2 | 2.5 | 17.0 | NT | 0.0 |
| NCTC 8236 | |||||||||
| C.pseudotropicalis. | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NT | 7.8 | 0.0 |
| NCYC 6 | |||||||||
Key:- Test concentration for each fraction =20 mg/ml. NT= Not tested, Hex. =hexane fraction, EtOAc = ethyl acetate fraction, n-BuOH= butanol fraction, Aq.= aqueous fraction, Strept.= streptomycin, Acrif =acriflavine, Aq.Me = aqueous methanol, 1 apigenin C-glucoside, 2 =quercetin rhamnoside.
Conclusion
Compounds 1–3 are reported from this plant for the first time. They have very strong radical scavenging activity, while they exhibited weak in-vitro antibacterial activity against Gram +ve organisms. The observed activity may contribute to the reasons why the leaves are used for infectious and inflammatory conditions in ethnomedicine. This study has demonstrated the presence of stachydrine in the leaves, stem and root bark of Ritchea capparoides var longipedicellata.
Acknowledgements
The authors wish to thank Prof. A.O. Ogundaini, Director, Central Science Laboratory of the Obafemi Awolowo University, Nigeria for spectra acquisition on compound 1 and 2, also Dr. Mathias Heydreyrich of the Department of Chemistry, University of Potsdam, Germany for the acquisition of the NMR spectra for compound 3.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Abotsi WMK, Woode E, Ainooson GK, Amo-Barimah AK, Boakye-Gyasi E. Antiarthritic and antioxidant effects of the leaf extract of Ficus exasperata P. Beauv. (Moraceae) Pharmacognosy Res. 2010;2(2):89–97. doi: 10.4103/0974-8490.62958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adewole SO, Adenowo T, Naicker T, Ojewole JA. Hypoglycaemic and hypotensive effects of Ficus exasperata Vahl. (Moraceae) leaf aqueous extract in rats. Afr J Tradit Complement Altern Med. 2011;8:275–283. doi: 10.4314/ajtcam.v8i3.65290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed F, Mueen KK, Abedin MZ, Karim AA. Traditional uses and pharmacological potential of Ficus exasperata vahl. Systematic Review in Pharmacy. 2012;3(1):15–23. [Google Scholar]
- 4.Akah PA, Wanbembe CN, Shittu A, Kapu SD, Kunle OO. Studies on gastrointestinal properties of Ficus exasperata. Fitoterapia. 1997;68:7–20. [Google Scholar]
- 5.Arot-Manguro LO, Ivar U, Lemen P. Further flavonoid glycosides of Embelia schimperi leaves. Bulletin of Chem Soc of Ethiopia. 2004;18(1):51–57. [Google Scholar]
- 6.Ayinde BA, Omogbai EK, Amaechina FC. Pharmacognosy and hypotensive evaluation of Ficus exasperata Vahl (moraceae) Leaf. Acta Pol Pharm. 2007;64:543–546. [PubMed] [Google Scholar]
- 7.Amponsah I. Anti-inflammatory and antimicrobial properties of Ficus exasperata: Anti-inflammatory, antioxidant and antimicrobial Coumarins and sterols from the leaves and stem bark of Ficus exasperata Paperback. 2012. ISBN 13 9783844307344. [Google Scholar]
- 8.Bafor EE, Omogbai EK, Ozolua RI. Oxytocin inhibiting effect of the aqueous leaf extract of Ficus exasperata (Moraceae) on the isolated rat uterus. Acta Pol Pharm. 2008;68:541–547. [PubMed] [Google Scholar]
- 9.Basilea A, Giordanoa S, Antonio JA, Lopez-Saez JA, Cobianchia RC. antibacterial activity of pure flavonoids isolated from mosses. Phytochem. 1999;52:1479–1482. doi: 10.1016/s0031-9422(99)00286-1. [DOI] [PubMed] [Google Scholar]
- 10.Berg CC. Classification and distribution of Ficus. Experientia. 1989;45:605–611. [Google Scholar]
- 11.Burits M, Bucar R. Antioxidant activity of Nigelia sativa essential oil. Phytotherapy res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Eldahshan OA, Ayoub NA, Singab AB, Al Aziz MM. Potential antioxidant phenolic metabolites from doum palm leaves. Afr J of Pharm and Pharmacol. 2009;3(4):158–164. [Google Scholar]
- 13.Emery P. Treatment of rheumatoid arthritis. BMJ. 2006;332:152–155. doi: 10.1136/bmj.332.7534.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irvine FR. Woody plants of Ghana. 1 ed. London: Oxford University Press; 1961. [Google Scholar]
- 15.Li XC, Cai L, Wu CD. Antimicrobial compounds from Ceanothus americanus against oral pathogens. Phytochem. 1997;46:97–102. doi: 10.1016/s0031-9422(97)00222-7. [DOI] [PubMed] [Google Scholar]
- 16.Markham KR, Chari VL. Techniques of flavonoids identification in Advances. In: Harbonne RJ, Marbry TJ, Marbry II, editors. Flavonoids Research. London: Chapman and Hall; 1982. p. 9. 1982. [Google Scholar]
- 17.Marzouk MS, Eman GH, Fatma AM, Magda TI, Osama AB. Antioxidant flavonol glycosides from Schinus molle. Phytother Res. 2006;20:200–205. doi: 10.1002/ptr.1834. [DOI] [PubMed] [Google Scholar]
- 18.Odunbaku OA, Ilusanya OA, Akasoro KS. Antibacterial activity of ethanolic leaf extract of Ficus exasperata on Escherichia coli and Staphylococcus albus. Sci Res Essay. 2008;3:562–564. [Google Scholar]
- 19.Ogunleye RF. Effectiveness of the leaf powder of Ficus exasperata Vahl. (Moraceae) in suppressing the population of two major storage insect pests. Continental J Biol Sci. 2011;4:6. [Google Scholar]
- 20.Rhoca L, Marston A, Potterat O, Kaplan MA, Stoeckli-Evans H, Hostettmann K. Antibacterial phloroglucinols and flavonoids from Hypericum brasiliense. Phytochem. 1995;40:1447–1452. doi: 10.1016/0031-9422(95)00507-4. [DOI] [PubMed] [Google Scholar]
- 21.Shutz B, Wright AD, Rali T, Stucher O. Prenylated flavanones from leaves of Macaranga pleiostemona. Phytochem. 1995;40:1273–1277. [Google Scholar]
- 22.Taiwo BJ, Aderogba MA, Ogundaini AO. Antioxidant lignans from the leaves of Ficus exasperata. Nigerian Journal of Natural Product and Medicine. 2006;10:111–113. [Google Scholar]
- 23.Taiwo I A, Adebesin OA, Funmilayo A F, Lawal RI, Odeiga PGC. Glycaemic activity of Ficus exasperata in fructose-induced glucose intolerance in rats. Researcher. 2010;2:80–83. [Google Scholar]
- 24.Tereschuk ML, Riera MV, Castro GR, Abdala LR. Antimicrobial activity of flavonoids from leaves of Tagetes minuta. J of Ethnopharmacol. 1997;56:227–232. doi: 10.1016/s0378-8741(97)00038-x. [DOI] [PubMed] [Google Scholar]
- 25.Waage SK, Hedin PA. Quercetin 3-O-galactosyl-(1, 4, 6)-glucoside, a compound from narrow leaf vetch with antibacterial activity. Phytochem. 1985;24:243–245. [Google Scholar]
- 26.Vaughn S F. Phytotoxic and antimicrobial activity of 5,7-dihydroxychromone from peanut shells. J of Chem Ecol. 1995;21:107–115. doi: 10.1007/BF02036645. [DOI] [PubMed] [Google Scholar]
- 27.Woode E, Poku RA, Abotsi WK. Anticonvulsant effects of leaf extract of Ficus exasperata Vahl (Moraceae) in mice. Int J Pharmacol. 2011;7:405–409. [Google Scholar]
- 28.Woode E, Poku RA, Abotsi WK. Anxiolytic-like effects of a leaf extract of Ficus exasperata Vahl (Moraceae) in Mice. West Afr J Pharm. 2011;22:75–81. [Google Scholar]