Abstract
Background
Raphanus sativus is reported to have a variety of biological activities. This work screened the hepato-protective and antioxidant activity of ethanol (ERS), and aqueous (ARS), extracts of leaves of Raphanus sativus in Carbon tetrachloride (CCl4), model in rats.
Material and Methods
The extracts were subjected to antioxidant tests (Total reducing power and Total phenolic content), and preliminary phytochemical screening. A pilot study was done on 100 and 300 mg/kg extracts, form which 300 mg was chosen for further experiments. The albino rats (200–250 grams), were divided into 5 groups of 6 animals each (n=6). There were three control groups comprising of normal control (normal saline −1ml/kg), negative control group (CCl4 1ml/kg in olive oil in a ratio of 1:1 v/v), and positive control group (Silymarin 50mg/kg). The Test drugs were given in a dose of 300 mg/kg for both ERS and ARS extract for 7 days. Biochemical parameters (AST, ALT, Alkaline phosphatase, Total Bilirubin), histo-pathological examination of liver and in vivo antioxidant tests [CAT, GSH and MDA] were done.
Results
The phytochemical study showed the presence of flavanoids, terpenoids, alkaloids, saponins and sterols. A dose dependent increase in the oxidative potential was observed in both the extracts with total phenolic content 70.1 and 44.4 GAE/g extract for ERS and ARS respectively. ERS 300mg/kg showed a significant (p<0.001) increase in levels of AST, ALT and alkaline phosphatase as compared to negative control (percentage hepatoprotection =45.3%) while ARS 300 mg/kg (p<.01) group showed 30% hepatoprotection. The GSH (p<0.001) and CAT (p<0.05) in ERS and ARS were significantly increased while MDA levels were decreased (P< 0.01), as compared negative control. The findings were confirmed histo-pathological examination.
Conclusion
The ethanol and aqueous extract of Raphanus sativus have partial hepatoprotection against CCl4 toxicity.
Keywords: Raphanus sativus, hepatoprotection, CCl4, antioxidant
Introduction
The liver is one of the largest organs in the body that plays an important role in the metabolism of endogenous and exogenous chemicals. Hepatic injury due to various aetiologies causes decline in liver functions. As there is no definitive treatment for the majority of the liver diseases, many scientists are turning back to traditional medicinal knowledge for answers. Raphanus sativus is one of such plants which being used in Indian traditional medicine for the treatment of liver ailments. It is commonly known as radish, belonging to the family Brassicaceae and is cultivated in temperate and warm countries (Kiritikar and Baus, 1987).
Raphanus sativus is reported to have antimicrobial activity (Abdou et al., 1972; Khan et al. 1985; Rani et al., 2008; Shetty et al., 2011), anticancer (Kim et al., 2006; Hanlon et al., 2007), anti-diabetic (Taniguchi et al., 2006; Shukla et al., 2011), gastrointestinal and uterine tone modulatory (Gilani and Ghayur, 2004), anti-ulcer and cardio-modulatory activities (Ghayur & Gilani, 2006; Suh et al., 2006). The hepatoprotective activity of root was investigated by Popvic et al. (1993) using paracetamol induced hepatotoxicity model. Chaturvedi et al. (2007) and Baek et al. (2008) also showed hepatoprotective effect of root extract on paracetamol and CCl4 induced heptotoxicity in experimental animals, respectively.
To our knowledge there is no study of hepatoprotective and anti-oxidant properties of aqueous and ethanol extract of R. sativus on rats, so in this study we intend to find out the same.
Material and method
Plant material
The leaves of R. sativus (voucher No. SC-0129/11) were purchased from the local market in Aligarh, India. The leaves were authenticated by Prof. S. H. Afaq, Section of Pharmacognosy, Ilmul Advia, A.K. Tibbya College, A.M.U. and voucher specimen was submitted (SC-0129/11). The plant material was shade dried and two extracts, ethanol (ERS), & aqueous (ARS), were prepared using soxhlet's extractor.
Preliminary Phyotchemical Study
Phytochemical analysis was done by the methods described by Harborne (1996). A solution of extract in water in a concentration of 1 g/ml was prepared and used for the analysis of flavanoids, anthraquinones, terpenoids, alkaloids, glycosides, saponins, and sterols.
In vitro antioxidant tests
For performing in vitro tests, the test compounds were serially diluted to make 8 different samples of concentration 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 1.75 and 2.00 mg/ml.
Total Reducing Power: The test was performed by the method described by Oyaizu et al (1996). 1 ml of the extract was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% potassium ferrocyanide. It was incubated at 50°C for 30 mins. To this solution 2.5 ml of trichloroacetic acid (10%), was added and the whole sample was centrifuged at 3000 rpm for 10 minutes. 2.5 ml of the supernatant was then mixed with 2.5 ml of distilled water and 0.5 ml of FeCl3 (0.1%). Ascorbic acid was taken as control. Absorbance was measured at 700nm using spectrophotometer.
Total Phenolic content: The test was done as described by Saucier et al. (1999). 20 µl of extract or standard (Gallic acid) was mixed with 1.58 ml of distilled water. To this solution 100 µl of Folin ciocalteau's (FC), reagent was added. After 30 sec and before 8 mins, 300 µl of 20% of sodium bicarbonate solution was added and shaken. The solution was incubated for 2 hours at room temperature. Gallic acid solution at concentration of 0, 50, 100, 150, 250 and 500 mg/l was used to make standard curve. Absorbance was measured at 765 nm using spectrophotometer and expressed as Gallic acid equivalent (GAE/gram of extract).
Experimental animals
The ethical clearance was taken from the Institutional Animal Ethics Committee (401/CPCSEA, dated 23/03/2011), and all the experiments were carried out as per CPCSEA guidelines. Adult albino rats of either sex weighing 200–250g of Charles foster strain were divided into 5 groups of six animals each (n=6). They were kept in standard environmental conditions and given standard pellet diet & water ad libitum throughout the study.
Acute toxicity study
Acute toxicity testing was done to find out the LD50 of the extracts. The testing was done according to the OECD guidelines. For performing the study, 3 non pregnant female rats of 200–250g were chosen. ARS and ERS extracts were given in a dose of 2 g/kg b.w. The animals were observed daily for a total period of 14 days. The findings were confirmed by repeating the above mentioned experiment on same dose and observing for another 14 days. As no lethal toxicity was observed at a dose of 2 g/kg so, a lower dose of 100, and 300mg/kg for both ARS and ERS was chosen for the experiment.
Experimental Design
A pilot study was done on 100 and 300 mg/kg extracts, based on higher hepatoprotection, dose of 300 mg was chosen for further experiments. The hepatotoxicity was induced by administering carbon tetrachloride (CCl4) p.o. (Anandan et al., 2009). Out of 6 groups, the first group was the baseline control group given only distilled water per orally (1ml/kg), second group was given CCl4 in a dose of 1 ml/kg mixed with olive oil in a ratio of 1:1 v/v and served as the negative control group; the third group was positive control (silymarin 50mg/kg). There were two test groups, which were given R.sativus ethanol (ERS), extract and aqueous extract (ARS), in a dose of 300 mg/kg b.w. All the drugs were given orally every day for a period of 7 days. The animals were sacrificed on the 8th day. The blood and liver were collected for liver function test (AST, ALT, Alkaline Phosphatase, Total bilirubin) (Reitman & Frankel, 1957; Marsh et al. 1959; Jendrassik-Gróf, 1938) and in vivo antioxidant tests [Catalase (CAT), Reduced glutathione (GSH) and malonylaldehyde (MDA)]. A part of liver tissue was also used for histopathological examination.
In vivo antioxidant tests
The liver was homogenised in 10 % w/v of phosphate buffer (0.2M, pH-6.6) such that, 1 gram of liver was homogenised with 10 ml of buffer. The homogenate was used as such for employing GSH assay. It was further centrifuged at 6000 rpm for 10 minutes and supernatant was used for performing MDA and Catalase assay. GSH assay was performed by Ellman (1959) technique. In short, 400 µl of tissue homogenate was added to 80 µl of 50 % w/v of trichloroacetic acid and 320 µl of distilled water and was centrifuged at 1800g for 15 minutes. 400 µl of this supernatant was separated in a glass test tube and mixed with 2200 µl of tris buffer (0.4 M, PH 8.9), and 400 µl of 5,5 DITHIO BIS (0.01 M). The solution was mixed by vigorous shaking. All the samples were tested in triplicate. The absorbance of test and control samples was measured at 412 nm within 5 minutes and expressed as µmol/ mg of protein using standard curve. For performing Catalase (Beers & Sizer, 1952), a solution was made by mixing 2 ml of phosphate buffer (0.2M, pH-6.6), with 0.5 ml of 30 mM H2O2. The reaction was started by adding 20 µl of tissue extract to the solution. Optical density of test and standard was measured at 0 and 60 seconds at wavelength of 240 nm using spectrophotometer and expressed as Units of H2O2 consumed/ min/ mg of protein. The MDA assay was done according to the method described by Niehans and Samuelsson (1968). For performing the assay 0.2 ml of tissue homogenate was added to a solution of 0.2 ml of 8.1 % of sodium dodecyl sulphate, 1.5 ml of 20 % of acetic acid and 1.5 ml of 0.8 % of thiobarbituric acid. The total volume of the resultant solution was made 4 ml by adding distilled water. The sample was heated at 95°C for 60 minutes in a water bath and allowed to cool down. This solution was gently mixed with 5 ml mixture of n-butanol and pyridine (15:1) and centrifuge at 4000 rpm for 10 minutes. The organic layer (upper layer) was separated and its absorbance was measured at 532 nm and expressed as µmol/mg of tissue.
Percentage of Hepatoprotection
Hepatoprotection was calculated as percentage by using the following formula (Dasgupta et al., 2008):
P = 1 − ((T − C)/(N − C) × 100
Where P = Percentage of Hepatoprotection, T = Mean value of test group, N = Mean value of negative control group and C = Mean value for baseline control group animals.
Statistical analysis
The results were presented as Mean ± Standard Error of Mean (SEM). The groups were compared by One way analysis of variance (ANOVA), followed by post hoc “Dunnett's Multiple comparison test” to analyse statistical significance. P value of less than 0.05 was considered to be significant. ANOVA and Dunnett's multiple comparison tests were employed by using software SPSS 17.
Result and Discussion
The yield of extract after extraction was 4.0% and 5.6% for aqueous (ARS), and ethanol (ERS), extract, respectively. Phenolic content of an extract is directly proportional to the antioxidant activity present in that extract. The antioxidant property of phenols is due to the presence of benzene ring which is characterized by the ability to donate π-electrons. The phenolic content of the extracts was measured as Gallic acid equivalents (GAE) per gram of the extract. The total phenolic content in ERS and ARS were found to be 70.1 and 44.4 GAE/g of extract respectively.
Flavanoids, terpenoids and glycosides were found to be present in ARS while ERS was shown to have flavanoids, terpenoids, alkaloids, saponins and sterols.
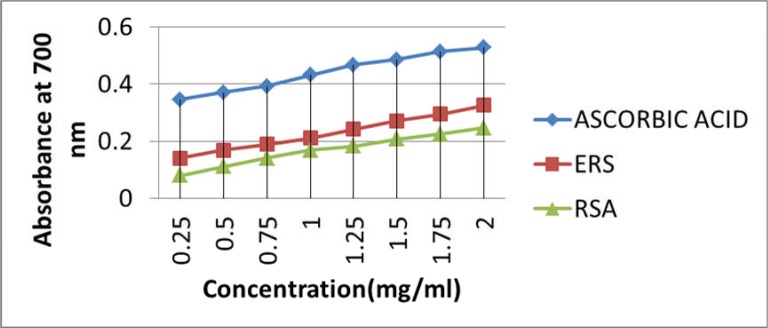
The in vitro antioxidant tests were employed for preliminary screening of the free radical scavenging activity of the extracts. Ethanol extract fared better in both in vitro antioxidant tests. In total reducing power test, both ERS and ARS showed dose dependent increase in the free radical neutralizing potential as shown in Figure 1.
Figure 1.
Total reducing power of Ethanolic and Aqueous extracts of Raphanus sativus
(Negative control group was compared with Normal control group and all other groups were compared with Negative control group, * p< 0.05, **p<0.01 and ***p<0.001 were considered significant)
CCl4 induced hepatic injury is the most common model used to study for liver damage. CCl4 is a clear, colourless, non-flammable, volatile liquid with a characteristic sweet odour. It is well absorbed form gastrointestinal tract and distributed throughout the whole body, with highest concentrations in liver, brain, kidney, muscle and fat (CCl4, Environment health criteria, 2004). CCl4 is metabolised by cytochrome P450-mediated transfer of an electron to the C-Cl bond, forming an anion radical, trichloromethyl radical (CCl3*) and further metabolised to a more reactive intermediate trichloromethylperoxyl radicals (CCl3OOC*) (Gruebele et al., 1996; Raucy et al., 1993). These free radicals donate their electron to the organic components of the hepatocytes thus oxidising them and causing severe intracellular damage. The damage to the cell membrane by free radical mediated lipid per oxidation causes leakage of intracellular hepatic enzymes which is reflected by the increased in Transaminases (AST and ALT) and alkaline phosphatases in negative control group. The levels of hepatic transaminases were significantly lower in the silymarin control group (p< 0.001) as well as in test groups (ERS & ARS) (p< 0.001) implying their hepatoprotective action (Table 1). The percentage hepatoprotection as a function of ALT was 45.3% and 30.3% for ERS and ARS respectively. The levels of alkaline phosphatase were also found to be significantly increased in ERS (p<0.01) and ARS (p<0.01) as compared to negative control group. While the levels of total bilirubin showed no significant changes in both control and test groups, which could be due to the short duration of the study.
Table 1.
Results of liver function test and percentage hepatoprotection after CCl4 induced liver toxicity
| Biochemical Test (Mean ± SEM) | Groups (Percentage Hepatoprotection %) |
||||
| NORMAL CONTORL (Water) |
NEGATIVE CONTROL (CCl4) |
POSITIVE CONTROL (SILYMARIN) |
R. sativus Ethanolic (300mg/kg) |
R. sativus Aquoues (300mg/kg) |
|
| AST (IU/ml) | 39.1 ± 1.4 | 154.0 ± 6.1*** | 60.5± 6.4*** (81.3 %) |
95.1 ± 3.8*** (51.2%) |
122.8 ± 3.7** (27.1%) |
| ALT (IU/ml) | 38.8 ± 1.2 | 161.3 ± 2.2*** | 55 ± 2.6*** (86.7%) |
105.7 ± 4.5*** (45.3%) |
124.0 ± 4.0*** (30.3%) |
| ALP (KAU/dl) | 42.8 ± 2.3 | 78.6 ± 4.2*** | 50.2 ± 4.7** | 55.5 ± 5.1** | 57.7 ± 5.0** |
| BILIRUBIN (mg/ml) | 0.7 ± 0.026 | 0.83 ± 0.049 | 0.7 ± 0.026 | 0.76 ± 0.033 | 0.78 ± 0.033 |
The In vivo antioxidant properties of the drug were also measured by levels of the antioxidant enzymes in the cell. Free radicals that are generated due to CCl4 biotransformation are neutralized by the scavenging enzymes (Raucy et al., 1993). So, a decreased level of enzyme are seen in negative control group as excess amount of antioxidant enzymes are being used to neutralize the free radical. In test groups (ERS & ARS), there is an increased level of Catalase (p< 0.05), and GSH (p< 0.001), enzymes implying either decreased reactive species generation or increased free radical scavenging by the test drug (Table 2). The results were confirmed by lipid peroxidation studies. Active species starts a cascade of membrane lipid peroxidation producing MDA as one of the by product. MDA served as a biomarker for membrane lipid peroxidation, whose levels are raised in the negative control group (administered only CCl4). The levels of MDA were decreased in both test groups (ERS p< 0.001 & ARS p< 0.01) which were comparable to that of silymarin positive control group (p< 0.001). In general, the ethanol extract has shown better results than aqueous extract.
Table 2.
Results of in vivo antioxidant tests after CCl4 induced liver toxicity
| Biochemical Test (Mean ± SEM) | Groups (Percentage Hepatoprotection %) |
||||
| NORMAL CONTORL (Water) |
NEGATIVE CONTROL (CCl4) |
POSITIVE CONTROL (SILYMARIN) |
R.sativus Ethanolic (300mg/kg) |
R.sativus Aquoues (300mg/kg) |
|
|
Catalase (U/ min/mg) |
82.2 ± 3.7 | 47.4 ± 1.6*** | 67.7 ± 1.0*** | 57.2 ± 3.6* | 55.3 ± 2.7 |
|
GSH (µmol /mg) |
5.25 ± .21 | 2.15 ± 0.25*** | 4.44 ± 0.11*** | 3.64 ± 0.08*** | 3.33 ± 0.20*** |
|
MDA (nmol/mg) |
203.9 ± 8.9 | 445.9 ± 17.8*** | 255.8 ± 7.7*** | 330.7 ± 26.3*** | 353.8 ± 26.2** |
(Negative control group was compared with Normal control group and all other groups were compared with Negative control group, * p< 0.05, **p<0.01 and ***p<0.001 were considered significant)
The baseline control group was marked by normal contour of the hepatocytes and cords. Negative control, on the hand, showed completely destroyed architecture of the tissue, marked inflammation and necrosis (Fig 2). The effects of CCl4, to a large extent, were neutralized by positive control group which demonstrated normal looking hepatocytes with no inflammatory infiltrates. The ERS group showed well preserved architecture and less conspicuous inflammatory infiltrates while a few areas of necrosis and inflammatory infiltrates were visualized in the ARS slide (Figure 3).
Figure 2.
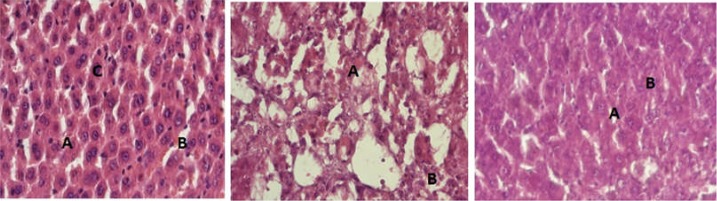
Histology of Control Group
(Figure 2A: Photomicrograph of rat liver from Normal Control Group showing normal liver microstructure with intact hepatic cords (A) and sinusoids (B). Hepatocytes (C) show normal contour. There is neither obvious congestion nor inflammatory cell infiltration. X400. H & E stain
Figure 2B: Photomicrograph of rat liver from Negative control group shows almost complete disorganization of hepatic microstructure. Most of the hepatocytes show either lytic or coagulate changes (A) and there is inflammatory cell infiltration (B).
Figure 2C: Photomicrograph of rat liver from Positive control group showing maintained hepatic microstructure. The hepatic cords (A) are intact and the contour of hepatocytes appear very akin to normal (B). There is no obvious inflammatory cell infiltration.)
Figure 3.
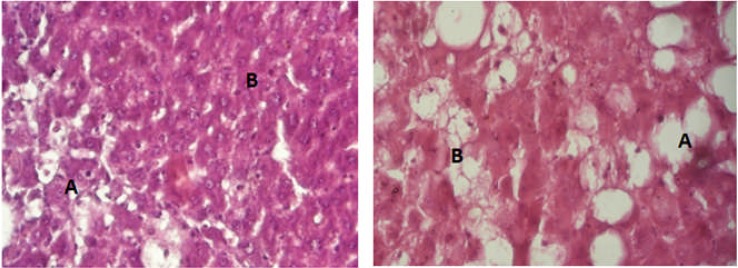
Histology of Test Group
(Figure 3A: Photomicrograph of rat liver from Test group ERS 300 mg/kg showing degenerative changes in the left lower field (A) while the remaining part is occupied by spared hepatocytes having normal appearance(B). There is no sign of congestion and undue inflammatory cell infiltration. X400. H & E stain.
Figure 3B: Photomicrograph of rat liver from Test group ARS 300 mg/kg showing marked alteration in the hepatic microstructure. There is loss of hepatic cord and sinusoids. Quite a large number of cells show ballooning (A) while others show moderate coagulate changes (B). Inflammatory cells are not too obvious. X400. H & E stain.)
The ethanol and aqueous extracts of leaves of R. sativus in a dose of 300mg/kg showed a partial protective effect against the liver damage caused by CCl4. The hepatoprotection may be due to the antioxidant principles in present in the extracts as shown in our study. The phytochemicals like flavanoids, terpenoids and polyphenols present in the extract have shown to possess antioxidant potential in various studies. R. sativus is also shown to possess the antioxidant activity as identified by Salah-Abbes et al. (2008), Chaturvedi (2008), Lugasi et al. (2005), Souri et al. (2004) which may be the basis of its hepatoprotective, anticancer and anti-inflammatory effect. Beevi et al. (2010) also reported that the methanolic and acetone extracts of R. sativus leaves has total polyphenolic content of 86.16 and 78.77 mg/g dry extract giving a rough estimated of its free radical scavenging activity. Takaya et al. (2003) showed that the polyphenols like sinapic acid and ferulic acid & flavanoids like kaempferol are present in the R. sativus have antioxidant properties. Beevi et al. (2010) used HPLC to identifiy polyphenols like catechin, protocatechuic acid, syringic acid, vanillic acid, ferulic acid, sinapic acid, o-coumaric acid, myricetin, and quercetin in leaves and stem extract of R. sativus. Hepatoprotective activity of roots of R. sativus has been demonstrated in many studies. To the best of our knowledge, we have for the first time demonstrated the hepato-protective, and in vivo anti-oxidant activity of aqueous extract of R. sativus in rats. So, we infer form this study that the ethanol and aqueous extracts of leaves of R. sativus in a dose of 300 mg/kg offer partial protection against hepatotoxicity induced by carbon tetrachloride.
Acknowledgements
We thank JNMC, AMU for financial support provided for the study. We also thank Dr. Onkar Singh and Mr. Shabbir Khan for technical assistance.
Competing interests
The author(s) declare that they have no competing interests
References
- 1.Kiritikar RK, Bausm BD. Indian medical plants. Ed 2nd. I. India: International Book Distributors; 1987. 9/B, Rajpur Road, Dehradun. [Google Scholar]
- 2.Abdou IA, Abou-Zeid AA, El-Sherbeeny MR, Abou-El-Gheat ZH. Antimicrobial activities of Allium sativum, Allium cepa, Raphanus sativus, Capsicum frutescens, Eruca sativa, Allium kurrat on bacteria. Qualitas Plantarum. 1972;1:29–35. [Google Scholar]
- 3.Khan KI, Khan FJ, Shahida K. The antimicrobial activity of Allium sativum (garlic) Allium cepa (onion) and Raphanus sativus (radish) Journal of Pharmacy. 1985;6:59–72. [Google Scholar]
- 4.Rani I, Akhund S, Abro H. Antimicrobial potential of seed extracts of Raphanus sativus. Pakistan Journal of Botany. 2008;40:1793–1798. [Google Scholar]
- 5.Shetty D, Kamath R, Bhat P, Hegde K, Shabaraya AR. Athelmitic activity of root extract of Raphaus sativus. Pharmacology online. 2011;1:675–679. [Google Scholar]
- 6.Kim SJ, Kim BS, Kyung TW, Lee SOC, Rho CW, Choi KR, Hwang HJ, Choi HS. Suppressive effects of young radish cultivated with sulfur on growth and metastasis of B16-F10 melanoma cells. Archives of Pharmacology Research. 2006;29:235–240. doi: 10.1007/BF02969399. [DOI] [PubMed] [Google Scholar]
- 7.Hanlon PR, Webber DM, Barnes DM. Aqueous extract from Spanish black radish (Raphanus sativus L. Var. niger) induces detoxification enzymes in the HepG2 human hepatoma cell line. Journal of agricultural and food chemistry. 2007;55:6439–6446. doi: 10.1021/jf070530f. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi H, Kobayashi-Hattori K, Tenmyo C, Kamei T, Uda Y, Sugita-Konishi Y, Oishi Y, Takita T. Effect of Japanese radish (Raphanus sativus) sprout (Kaiwaredaikon) on carbohydrate and lipid metabolisms in normal and streptozotocin-induced diabetic rats. Phytotherapy Research. 2006;20:274–278. doi: 10.1002/ptr.1851. [DOI] [PubMed] [Google Scholar]
- 9.Shukla S, Chatterji S, Mehta S, Rai PK, Singh RK, Yadav DK, Watal G. Antidiabetic effect of Raphanus sativus root juice. Pharmaceutical Biology. 2011;49(1):32–37. doi: 10.3109/13880209.2010.493178. [DOI] [PubMed] [Google Scholar]
- 10.Gilani AH, Ghayur MN. Pharmacological basis for the gut stimulatory activity of Raphanus sativus leaves. Journal of Ethnopharmacology. 2004;95:169–172. doi: 10.1016/j.jep.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Ghayur MN, Gilani AH. Radish seed extract mediates its cardiovascular inhibitory effects via muscarinic receptor activation. Fundamental and clinical pharmacology. 2006;20:57–63. doi: 10.1111/j.1472-8206.2005.00382.x. [DOI] [PubMed] [Google Scholar]
- 12.Suh SJ, Moon SK, Kim CH. Raphanus sativus and its isothiocyanates inhibit vascular smooth muscle cells proliferation and induce G1 cell cycle arrest. International Immunopharmacology. 2006;6:854–861. doi: 10.1016/j.intimp.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Popovic M, Lukie V, Jakovljevie V, Mikov M. The effect of the radish juice on liver function. fitoterapia. 1993;64:229–231. [Google Scholar]
- 14.Chaturvedi P, George S, Machacha CN. Protective role of Raphanus sativus root extract on paracetamol-induced hepatotoxicity in albino rats. International Journal for Vitamin and Nutrition Research. 2007;77:41–45. doi: 10.1024/0300-9831.77.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Baek SH, Park M, Suh JH, Choi HS. Protective effects of an extract of young radish (Raphanus sativus L.) cultivated with sulfur (sulfur-radish extract) and of sulforaphane on carbon tetrachloride-induced hepatotoxicity. Bioscience Biotechnology Biochemistry. 2008;72:1176–1182. doi: 10.1271/bbb.70545. [DOI] [PubMed] [Google Scholar]
- 16.Harborne JB. Phytochemical Methods. Vol. 1. Chapman and Hall; 1996. pp. 52–105. [Google Scholar]
- 17.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Japanese Journal of Nutrition, write journal name in full. 1986;44:307–315. [Google Scholar]
- 18.Saucier CT, Waterhouse AL. Synergetic Activity of Catechin and other antioxidant. Journal of Agriculture and Food Chemistry. 1999;47:4491–4494. doi: 10.1021/jf990352t. [DOI] [PubMed] [Google Scholar]
- 19.Anandan R, Jayakar B, Karar B, Babuji S, Manavalan R, Kumar RS. Effect of ethanol extract of flowers of Vitex trifolia Linn. On CCl4 induced hepatic injury in rats. Pakistan Journal of Pharmaceutocal Science. 2009;22(4):391–394. [PubMed] [Google Scholar]
- 20.Reitman S, Frankel S. Acolorimetric methods for the determination of serum levels of glutamic oxaloacetic acid and pyruvic acid transaminases. American Journal of Clinical Pathology. 1957;10:394–399. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Marsh WH, Fingerhut B, Kirsch E. Adaption of alkaline phosphatise method for automatic colorimetric analysis. Clinical Chemistry. 1959;5:119–126. [PubMed] [Google Scholar]
- 22.Jendrassik-Gróf Method on photometric systems for in vitro determination of direct and total bilirubin. Biochem Zeitschrift. 1938;297:82–89. [Google Scholar]
- 23.Ellman GOC. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biological Chemistry. 1952;195(1):133–140. [PubMed] [Google Scholar]
- 25.Niehans WG, Samuelsson D. Formation of malondialdehyde from phospholipids arrachidonate during microsomal lipid peroxidation. European Journal of Biochemistry. 1968;6(1):126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 26.Dsagupta B, Kalita JOC, Chowdury A. Hepatoprotective activity of Nelsonia canescens (Lam.) Spreng on acute hepatotoxicity induced by paracetamol. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(1):107–112. [Google Scholar]
- 27.Carbon Tetrachloride, Environmental Health Criteria 208. Geneva: World Health Organization; 2004. [Google Scholar]
- 28.Gruebele A, Zawaski K, Kapalan D, Novak RF. Cytochrome P4502E1-and cytochrome P4502B1/2B2-catalysed carbon tetrachloride metabolism. Drug Metabolism and Disposition. 1996;24:15–22. [PubMed] [Google Scholar]
- 29.Raucy JL, Kraner JOC, Lasker JM. Bioactivation of halogenated hydrocarbons by cytochrome P4502E1. Critical Reviews in Toxicology. 1993;23:1–20. doi: 10.3109/10408449309104072. [DOI] [PubMed] [Google Scholar]
- 30.Salah-Abbes BJ, Abbes S, Ouanes Z, Houas Z, Abdel-Wahhab MA, Bacha H, Oueslati R. Tunisian radish extract (Raphanus sativus) enhances the antioxidant status and protects against oxidative stress induced by zearalenone in Balb/c mice. Journal of Applied Toxicology. 2008;28:6–14. doi: 10.1002/jat.1240. [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi P. Inhibitory response of Raphanus sativus on lipid peroxidation in albino rats. eCAM. 2008;5:55–59. doi: 10.1093/ecam/nel077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lugasi A, Blazovics A, Hagymasi K, Kocsis I, Kery A. Antioxidant effect of squeezed juice from black radish (Raphanus sativus L. var niger) in alimentary hyperlipidaemia in rats. Phytotherapy Research. 2005;19:587–591. doi: 10.1002/ptr.1655. [DOI] [PubMed] [Google Scholar]
- 33.Souri GE, Farsam AH, Andaji S. The antioxidant activity of some commonly used vegetables in Iranian diet. Fitoterapia. 2004;75:585–588. doi: 10.1016/j.fitote.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Souri GE, Farsam AH, Andaji S. The antioxidant activity of some commonly used vegetables in Iranian diet. Fitoterapia. 2004;75:585–588. doi: 10.1016/j.fitote.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Takaya Y, Kondo Y, Furukawa T, Niwa M. Antioxidant Constituents of Radish Sprout (Kaiware-daikon), Raphanus sativus L. Journal of Agricultural and Food Chemistry. 2003;51:8061–8066. doi: 10.1021/jf0346206. [DOI] [PubMed] [Google Scholar]