Abstract
Background
The ethyl acetate and chloroform extracts of stems, leaves and fruits of Phaleria macrocarpa were screened for their antioxidant capacity and tyrosinase inhibition properties.
Material and Method
The total phenolic content (TPC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging and ferric-ion reducing power (FRAP) were used to evaluate their antioxidant capacity. Tyrosinase inhibition effect was measured using mushroom tyrosinase inhibition assay.
Result
Ethyl acetate extract of P. macrocarpa's stem exhibited highest total phenolic content, DPPH free radical scavenging and ferric reducing power. Meanwhile, chloroform extracts of leaves and fruits demonstrated potent anti-tyrosinase activities as compared to a well-known tyrosinase inhibitor, kojic acid.
Conclusion
Since chloroform extracts of leaves and fruits have low antioxidant capacities, the tyrosinase inhibition effect observed are antioxidant independent. This study suggests direct tyrosinase inhibition by chloroform extracts of Phaleria macrocarpa.
Keywords: Phaleria macrocarpa, Antioxidant, Total phenolic content, Tyrosinase inhibition
Introduction
Cellular metabolisms generate reactive oxygen species (ROS). However, excessive production of ROS contributes significantly to oxidative stress. ROS causes oxidation of proteins, lipids, DNA and may leads to cell death (Ragu et al., 2007). It has been shown to elevate certain genes in several cells and associate with various illnesses such as cardiovascular diseases and cancer (Kunsch and Medford, 1999). The skin as an organ is quite vulnerable to oxidative stress given its continuous exposure to direct ultraviolet (UV), radiation from sunlight that can cause hyperpigmentation and pre-matured aging (Kim et al., 2008; Heo et al., 2009).
Melanogenesis of the skin involve tyrosinase enzyme which convert L-DOPA into melanin (Chang, 2009). Although melanin is a protective barrier against UV radiation from the sunlight, accumulation of an abnormal amount of melanin in different specific parts of the skin results in the development of freckles or melasma which may affect the patient's quality of life (Chang, 2009). Thus, the development of nontoxic lightening agents has become an issue of great interest. There have been attempts to the control of the metabolism of pigmentations by means of synthetic agents. However, due to safety and minimal adverse reaction, natural sources of inhibitor are preferable.
Phaleria macrocarpa (Scheff.) Boerl, commonly known as God's crown fruit which is indigenous to Indonesia and Malaysia (Altaf et al., 2013). P. macrocarpa have been used in folklore medicine, and scientific research confirmed its biological activities (Altaf et al., 2013). The extracts have been reported to have various biological properties, including anti-cancer, anti-hyperglycemic, anti-hyperlipidemic, anti-inflammatory, anti-microbial and vasorelaxant (Faried et al., 2007; Hendra et al., 2011). However, very little is known about antioxidant activities of P. macrocarpa's organs. The present study investigated the total phenolic content, free radical scavenging activities, ferric reducing antioxidant power and tyrosinase inhibition of P. macrocarpa extracts from different parts.
Materials and Methods
Plant material
Fruits, stems and leaves of Phaleria macrocarpa (voucher no. SK2248/13) were collected from Johor, Malaysia in May 2010. The fruits were dried at room temperature for 2 weeks and ground into a small pieces (cotton-like), using grinding machine.
Preparation of extracts
The dried fruits, leaves and stems (500 g), each were extracted with ethanol at room temperature for 48 hrs. The residue was extracted and filtered twice using Whatman No. 1 filter paper. The filtrate was then evaporated to dryness using vacuum distillation and rotary evaporator at 50 °C. The ethanol extract was partitioned with water-chloroform-ethyl acetate to give chloroform, ethyl acetate and aqueous extracts. Evaporation of chloroform and ethyl acetate extracts afforded chloroform (7.55 g), and ethyl acetate extracts (2.40 g).
Chemicals
1,1-diphenyl-2-picryl hydrazyl (DPPH), ascorbic acid, mushroom tyrosinase (1000 units/mL), gallic acid, 3,4-dihydroxy-L-phenylalanine (L-DOPA), Folin-Ciocalteau's reagent, trichloroacetic acid, methanol were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Total phenolic content (TPC)
The total phenolics content was quantified using Folin-Ciocalteu assay as described previously (Waterhouse 2003). A total volume of 0.1 µL extracts (1 mg/mL), or gallic acid was added with 0.9 µL distilled water and followed by the addition of 0.05 µL Folin-Ciocalteu Reagent. The mixture was then mixed and incubated for about 2 minutes. After 2 minutes, 0.5 µL of 5% sodium carbonate solution (Na2CO3), and 2.5 µL of distilled water were then added to the mixture. After 1 hour incubation in dark condition, the sample was measured at 765 nm using spectrophotometer. The graph of absorbance versus concentration was plotted. The total phenolic content was reported as Gallic acid equivalent of sample (GAE/L).
DPPH free radical-scavenging activities
The free radical scavenging activities was measured by DPPH assay with minor modifications (Dasgupta and De, 2007) as performed in 96-well microtiter plate. A total volume of 100µL of sample stock solution was diluted two fold to give final concentration of 500, 250, 125, 63, 31, 16, 8, 4 and 2 µg/mL. Ascorbic acid was used as positive control. After that, 100 µL of 0.04% (w/v), DPPH was added into each well. The mixture was mixed and incubated for 30 minutes in the dark at room temperature. The absorbance was read using microplate reader at 515 nm. Blank for test sample consists of sample in methanol only. Control well contained methanolic solution of DPPH. The final volume for each well is 200 µL. All tests were conducted in triplicates. The percentage of inhibition was calculated using the following formula:
Percentage of inhibition: (Control OD − (Sample OD/Control OD)) × 100.
Where Control OD is absorbance of negative control
Sample OD is absorbance of test sample.
Both Control OD and test sample were subtracted by blank prior to calculation
Ferric reducing antioxidant power (FRAP)
FRAP assay was performed according to Benzie and Strain (1996), in 96-well microtiter plate. A serial dilution test samples were prepared starting with 50 mg/mL to 2 µg/mL. The reaction mixture consists of 5 µL of test sample, 15 µL distilled water, and 150 µL of FRAP assay reagent. Distilled water and FRAP reagent were used in controls well as a replacement for test samples. First reading was taken at 0 min prior to incubation at 37°C. Second reading was performed after a 4 min reaction time. The absorbance reading was measured at 575 nm. FRAP assay were performed in triplicates in three independent experiments (n=9). Data was analyzed by constructing a linear regression line by plotting the FRAP values (y-axis), versus its concentrations (x-axis). The linear regression line equation was used to calculate the antioxidant capacity of samples and compared to the ascorbic acid as standard solution. From the linear regression equation, y = mx + c and (m), values indicates the antioxidant reducing capacity. The higher the slope (m) values showed the higher antioxidant reducing capacity.
Anti-tyrosinase assay
The assay was determined spectrophotometrically as described by Wong et al. (2010) with minor modification. L-DOPA was used as substrate. Each well contained 40 µL of sample (final 0.1 mg/mL) with 80 µL of phosphate buffer (0.1 M, pH 6.8), 40 µL of tyrosinase (1000 units/m), and 40 µL of L-DOPA (2.5 mM). Each sample was accompanied by a blank that had all the components except tyrosinase. Kojic acid was used in place of sample as positive control. Absorbance was measured at 515 nm with 655 nm as a reference. Percentage of inhibition was calculated using the following formula:
Percentage of tyrosinase inhibition (%): (Control OD − Sample OD/Control OD), × 100
Where Control OD is absorbance of control and Sample OD is absorbance of test sample.
Statistical analysis
All data were expressed as means ± standard errors of triplicate measurements and were subjected to statistical analysis of ANOVA (SPSS 15.0). A value of P <0.05 was considered as significant value of the data.
Result and Discussion
Total phenolic content (TPC)
Plant bioactive compounds such as phenolic acids abundantly present in fruit and vegetables have received considerable attention because of their wide range beneficial effects which includes; antioxidant, anti-cancer, and anti-inflammatory (Yang et al., 2001; Santos, 2004). The phenolic contents of chloroform extract of the fruits, leaves and stems of P. macrocarpa were found to be 74.39, 10.04 and 27.86 GAE/mg compared to 145.26, 88.74 and 277.87 GAE/mg for the ethyl acetate extracts respectively (Table 1). Ethyl acetate was found to be a better extractive solvent of the phenolic constituents of the plant than chloroform.
Table 1.
Total phenolic contents of fruits, leaves and stems of P. macrocarpa extracts.
| Samples | Gallic acid equivalent (GAE/mg) |
| FEA | 145.26 ± 0.25 |
| LEA | 88.89 ± 1.19 |
| SEA | 277.73 ± 1.82 |
| FC | 74.25 ± 0.38 |
| LC | 9.90 ± 0.77 |
| SC | 27.87 ± 0.90 |
Data represent mean of three independent experiments ± S.E.M (n=9).
DPPH free radical scavenging assay
In DPPH assay system, antioxidants will either transfer an electron or hydrogen atom to the stable DPPH radical, thus neutralizing its free radical character. The ability of an extract or any other agent to neutralize DPPH can be considered, at least as part of the antioxidant properties. Results in Table 2 showed free radical scavenging activity of all crude extracts.
Table 2.
Free radical scavenging capacities of the extracts measured by DPPH assay
| Samples | Percent inhibition (mean ± S.E.M) |
IC50 (µg/mL) |
| FEA | 94.71 ± 0.41 | 26.5 |
| LEA | 93.73 ± 0.51 | 71 |
| SEA | 94.16 ±0.46 | 27 |
| FC | 94.81 ± 0.74 | 60 |
| LC | 93.16 ± 0.85 | 120 |
| SC | 48.22 ± 2.54 | ND |
| Ascorbic acid | 84.36 ± 0.14 | 4 |
Data represent concentration that provides 50% inhibition (IC50) of three independent experiments. ND denotes non-detected
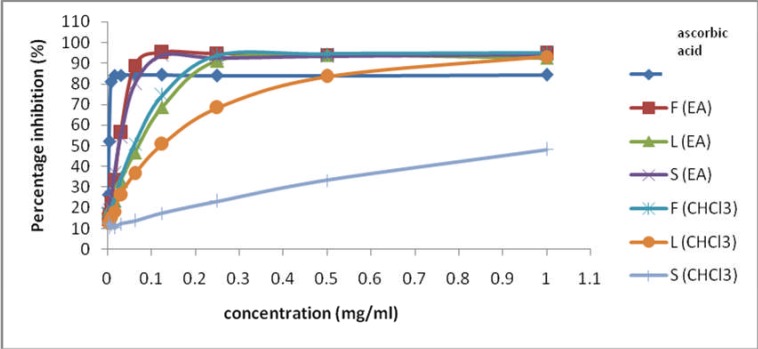
In general, all crude extracts showed effective free radical scavenging activity based on their IC50 values. P. macrocarpa extracts quenched DPPH free radical in dose-dependent manner (see Figure 1). Their activities were lower than ascorbic acid; nevertheless it still provides an overview about the ability of these extracts to scavenge the free radical. The order of activity was found as FEA > SEA > FC > LEA > LC, however, the SC extract was inactive. The result of this study is in conformity with Yosie et al. (2011) who reported that the leaves CHCl3 extract of P. macrocarpa exhibit higher activity than ethyl acetate extracts. However, Hendra et al (2011) reported that the inhibitory concentration (IC50), of the individual pericarp, mesocarp, and seed of P. macrocarpa fruits methanol extract ranged from 142–245 µg/mL against DPPH lower than the result of the present study although using semi polar solvent, ethyl acetate. This finding suggests that the whole fruits extract have stronger free radical scavenging activity than particular parts of fruits.
Figure 1.
Percentage free radical scavenging activity of test samples and ascorbic acid
Ferric reducing antioxidant power (FRAP) assay
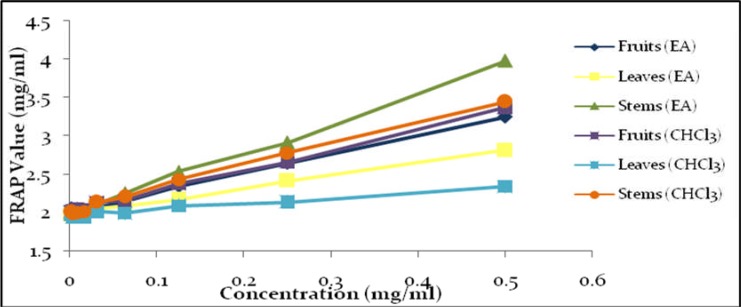
To further investigate the capability of crude extracts as an antioxidant, we investigated total reducing power using FRAP assay. The assay utilizes the reduction of Fe (TPTZ)2 (III) into Fe (TPTZ)2 (II) by antioxidant which have maximum absorption at 593 nm (Ou et al., 2002). From Table 3 and Figure 2, total reducing power activity of P. macrocarpa extracts are in order of SEA > SC > FC > FEA > LEA > LC.
Table 3.
Slope value of sample extracts
| Samples | m value | R2 value |
| FEA | 2.491 | 0.996 |
| LEA | 1.654 | 0.998 |
| SEA | 3.958 | 0.997 |
| FC | 2.685 | 0.998 |
| LC | 0.763 | 0.967 |
| SC | 2.940 | 0.994 |
Data represent mean of three independent experiments (n=9).
Figure 2.
FRAP value of extracts against concentration
From Tables 1, 2 and 3, we found that Stem (EA) have great potential as antioxidant since this extracts exhibits strong free radical scavenging and reducing power activity due to the presence of high phenolic content.
Tyrosinase inhibitory activity
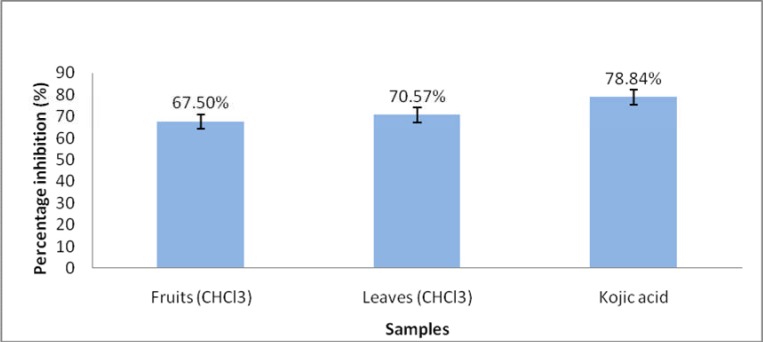
Normally, antioxidant agent poses tyrosinase inhibition activity. Hence, further investigations on tyrosinase inihibition of these extracts were conducted. In this study we investigated the direct tyrosinase inhibition mechanism. Figure 3 showed anti-tyrosinase activities of P. macrocarpa extracts. Out of six samples, only fruits and leaves chloroform extracts showed strong tyrosinase inhibition activities (percentage inhibition >50%). At concentration of 0.1 mg/mL, the tyrosinase inhibition activity by fruits and leaves were 67.50% and 70.57% respectively. The tyrosinase inhibition of extracts was comparable to a well-know powerful tyrosinase inhibitor, kojic acid (78.84%) at same concentration. The chloroform extracts of fruit and leaves are potential tyrosinase inhibitory agents (figure 3).
Figure 3.
Percentage inhibitory tyrosinase activities of leaves and fruits chloroform extracts
Several studies have reported the ability of polar substances of plant extracts to inhibit tyrosinase and it has been found that the activities are partly contributory by antioxidant potentials of the extracts. However, study by Nattapong and Omboon (2008), has shown the ability of non-polar plant extracts in inhibiting tyrosinase activity which is similar to our finding. They suggested that non-polar extracts containing betulinic acid can inhibit tyrosinase activity in Thai Mulberry extract. In our opinion, non-antioxidant tyrosinase inhibitor is more stable than antioxidant tyrosinase inhibitor as they are not easily oxidized. The true tyrosinase inhibitor can be competitive, uncompetitive inhibitors, mixed type (competitive/uncompetitive) inhibitors, and non-competitive inhibitors (Chang, 2009). This study suggested direct tyrosinase inhibition by chloroform extracts of P. macrocarpa. In conclusion, antioxidant and tyrosinase inhibition activities of P. macrocarpa are organs dependent. The antioxidant and strong tyrosinase inhibitory properties of P. macrocarpa could contribute to its potential as lightening agent.
Acknowledgement
The authors acknowledge to Research University Grant (GUP) from the Ministry of Higher Education (MOHE) for financial support under vote QJ13000.2645.09J16 and the Faculty of Biosciences and Medical Engineering, Universiti Teknologi Malaysia for facilities supports.
List of abbreviations
- DPPH
1,1-diphenyl-2-picryl-hydrazyl
- TPTZ
2,4,6-tri-(2-pyridyl)-1,3,5-triazine
- L-DOPA
3,4-dihydroxy-L-phenylalanine
- FEA
Fruit ethyl acetate
- LEA
Leave ethyl acetate
- SEA
Stem ethyl acetate
- FC
Fruit chloroform
- SC
Stem chloroform
- LC
Leave Chloroform
References
- 1.Altaf R, Asmawi M Z, Dewa A, Sadikun A, Umar M I. Phytochemistry and medicinal properties of Phaleria macrocarpa (Scheff.) Boerl. extracts. Pharmacogn Rev. 2013;7:73. doi: 10.4103/0973-7847.112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benzie IF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 3.Chan Y Y, Kim K H, Cheah S H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J Ethnopharmacol. 2011;137:1183–1188. doi: 10.1016/j.jep.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 4.Chang T S. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasgupta N, De B. Antioxidant activity of some leafy vegetables of India: A comparative study. Food chem. 2007;101:471–474. [Google Scholar]
- 6.Faried A, Kurnia D, Faried L S, Usman N, Miyazaki T, Kato H, Kuwano H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int J Oncol. 2007;30:605. doi: 10.3892/ijo.30.3.605. [DOI] [PubMed] [Google Scholar]
- 7.Hendra R, Ahmad S, Oskoueian E, Sukari A, Shukor M Y. Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff Fruit. BMC Complement Altern Med. 2011;11:110. doi: 10.1186/1472-6882-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heo S J, Ko S C, Cha S H, Kang D H, Park H S, Choi Y U, Kim D, Jung WK, Jeon Y J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol In Vitro. 2009;23:1123–1130. doi: 10.1016/j.tiv.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y J, Kang K S, Yokozawa T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem Toxicol. 2008;46:2466–2471. doi: 10.1016/j.fct.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Kunsch C, Medford R M. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 11.Nattapong S, Omboon L. A new source of whitening agent from a Thai Mulberry plant and its betulinic acid quantitation. Nat Prod Res. 2008;22:727–734. doi: 10.1080/14786410601130794. [DOI] [PubMed] [Google Scholar]
- 12.Ou B, Huang D, Hampsch-Woodill M, Flanagan J A, Deemer E K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem. 2002;50:3122–3128. doi: 10.1021/jf0116606. [DOI] [PubMed] [Google Scholar]
- 13.Ragu S, Faye G, Iraqui I, Masurel-Heneman A, Kolodner R D, Huang M E. Oxygen metabolism and reactive oxygen species cause chromosomal rearrangements and cell death. Proc Natl Acad Sci. 2007;104:9747–9752. doi: 10.1073/pnas.0703192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos A R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004;70:93–103. doi: 10.1055/s-2004-815483. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse AL. Determination of Total Phenolics, in Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Inc.; 2003. [Google Scholar]
- 16.Wong SK, Lim YY, Chan EWC. Evaluation of antioxidant, anti-tyrosinase and antibacterial activities of selected Hibiscus species. Etnobotanical Leaflets. 2010;14:781–796. [Google Scholar]
- 17.Yang C S, Landau J M, Huang M T, Newmark H L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 18.Yosie A, Effendy M A W, Sifzizul T M T, Habsah M. Antibacterial, radical-scavenging activities and cytotoxicity properties of Phaleria macrocarpa (scheff.) Boerl. Leaves in hepg2 cell lines. Int J Pharmac Sci Res. 2011;2:1700–1706. [Google Scholar]