Abstract
Background
Carvacrol (2-methyl-5-(1-methylethyl)-phenol) is a predominant monoterpenic phenol which occurs in many essential oils of the family Labiatae including Origanum, Satureja, Thymbra, Thymus, and Corydothymus species. It is well known for its anti-inflammatory, antioxidant and antitumor activities. The present study investigates the influence of carvacrol on CYP2E1 and PPAR-α on D-Galactosamine (D-GalN)-induced hepatotoxic rats.
Materials and Methods
The mRNA and protein expression levels of CYP2E1 and PPAR-α have been assayed by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and western blot analysis.
Result
The result demonstrated that the mRNA and protein expressions of CYP2E1(p=0.012; p=0.015) significantly up-regulated while the mRNA and protein expressions of PPAR-α (p=0.026; p=0.03) significantly down-regulated on D-galactosamine induced hepatotoxic rats and treatment with carvacrol significantly suppressed the mRNA and protein (CYP2E1, p=0.010; p=0.011) (PPAR-α, p=0.033; p=0.037) expressions of these genes.
Conclusion
Thus, the present results have shown that carvacrol has the hepatoprotective effect and also alleviates liver damage associated with GalN induced hepatotoxic rats by down-regulating the CYP2E1 and up-regulating the PPAR-α expression.
Keywords: D-GalN, hepatotoxic rats, Carvacrol, CYP2E1, PPAR-α
Introduction
Liver injury can be caused by different agents, such as chemicals and viruses etc. D-galactosamine is a well estabilished hepatoxicant, it induces liver injury closely resembling human viral hepatitis. Cytochrome (CYP) P450 enzymes are a superfamily of heme proteins responsible for the metabolic activation or inactivation of most clinically used drugs and many toxins. CYP isoforms can be found in most tissues, but the largest concentrations of P-450 are found in the liver. The expression of P-450 enzymes is regulated by a variety of factors such as genetic polymorphism, drugs, hormones, development, and diet (Morgan, 2001). It is known that the mediators involved in inflammation and infection can cause changes in the activities and expression of the P-450 enzyme system. A large number of reports have shown that CYP isoforms and their activities are, for the most part, suppressed in animal models of endotoxemia as well as in cultured hepatocyte stimulated by endotoxin (Morgan, 2001; Shedlofsky et al., 1994). A recent study showed that hepatic CYP2E1, the most predominant CYP isoform in the rat liver (Spatzenegger et al., 2000), is down-regulated during D-GalN induced hepatotoxic rats (Crawford et al., 2004).
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor superfamily of ligand-activated transcription factors that are related to retinoid, steroid and thyroid hormone receptors. The PPAR-y receptor subtype appears to play a pivotal role in the regulation of cellular proliferation and inflammation (Cuzzocrea et al., 2006). Rosiglitazone, a PPAR-y receptor subtype ligand was demonstrated to exert anti-inflammatory effects in the rat paw oedema model or in the carrageenan-induced pleurisy (Cuzzocrea et al., 2004). It was also reported to reduce pulmonary inflammation response in a rat model of endotoxemia (Liu et al., 2005), to reduce chronic colonic inflammation in rats (Sanches-Hidalgo et al., 2005), and to have beneficial effects in ischemia-perfusion injury, inflammation and shock (Abdelrahman et al., 2005; Villegas et al., 2004). PPARs have also been claimed to have immunoregulatory roles [Wang et al., 2001]. Moreover, a recent study indicated that the endogenous ligand for PPAR-α, 15-deoxy-Δ12, 14-prostaglandin J2 (15d-PGJ2), contributed to the observed protection of the liver in hemorrhagic shock in rat model via activation of PPAR-α (Abdelrahman et al., 2004).
Different pharmacological compounds have been tested to reduce the acute or chronic inflammation when the liver gets injury. Compound those which increase the levels of PPAR-α and, reduces the inflammation are the good hepatoprotective agent which has been already reported (Ariyoshi, 2001; Ekins, 1999; Stiborova, 2002). Carvacrol, (2-methyl-5-(1-methylethyl)-phenol), is a major component of the essential oils of oregano and thyme (Kisko and Roller, 2005; Lampronti et al., 2006). Carvacrol possesses strong antioxidant properties equivalent to those of ascorbic acid, butyl hydroxyl toluene (BHT) and vitamin E (Aeschbach et al., 1994; Mastelic et al., 2008). Although the anti-proliferative properties of carvacrol on non-small lung cancer cells, A549, chronic myeloid leukemia cells, K562, murine B16 melanoma cells have been shown (He et al., 1997; Horvathova et al., 2007; Karkabounas et al., 2006; Koparal and Zeytinoglu, 2003; Lampronti et al., 2006) the molecular mechanisms involved in its action remains elusive. We have recently demonstrated that, carvacrol have possess hepatoprotective action against D-galactosamine induction. In this study, we aimed to evaluate whether this compound is having anti-inflammatory effect mediating through PPAR-α and cytochrome P450. We found that carvacrol is a major suppressor of Cyt p450 and an activator of PPARα associated with hepatoprotective activity.
Materials and Methods
Chemicals
D-galactosamine, carvacrol, primary and secondary antibodies for TNF-α, IL-6, iNOS, COX-2 and NF-κB were purchased from Sigma-Aldrich Co. (St. Louis, Missouri, USA). All other chemicals used were of analytical grade obtained from E. Merck or HIMEDIA, Mumbai, India.
Animals
Male albino Wistar rats (weighing 160–180 g) were procured from the Central Animal House, Department of Experimental Medicine, Rajah Muthiah Medical College and Hospital, Annamalai University and maintained in an air-conditioned room (25 ± 1 °C) with a 12 h light/12 h dark cycle. Feed and water were provided ad libitum to all the animals. The study was approved by the Institutional Animal Ethics Committee of Rajah Muthiah Medical College and Hospital (Reg no.160/1999/CPCSEA, Proposal number: 427), Annamalai University, Annamalainagar.
Experimental induction of hepatotoxicity
Hepatotoxicity was induced in animals by an intraperitoneal injection of D-galactosamine (400 mg/kg body weight) in a freshly prepared physiological saline as a single dose on the first day.
Experimental design
The animals were divided into five groups of six animals each as given below. Carvacrol and silymarin (Sigma Aldrich, USA) were administered orally once in a day in the morning for 6 days. The compound was suspended in a 0.5% DMSO vehicle solution and fed by intubations.
Group I: Normal rats received 0.5% DMSO.
Group II: Normal + carvacrol (20 mg/kg BW).
Group III: D-GalN control (400 mg/kg BW) in saline.
Group IV: D-GalN + carvacrol (20 mg/kg BW).
Group V: D-GalN + silymarin (25 mg/kg BW).
On 8th day morning the animals were anesthetized by an intramuscular injection of ketamine (25 mg/kg BW) and sacrificed by cervical dislocation. Liver was removed, cleared off blood and immediately transferred to ice-cold containers containing saline and used for the determination of various inflammatory markers.
Reverse transcription polymerase chain reaction (RT-PCR)
For total RNA, the liver tissue samples were minced and homogenized (100 mg/ml) in RNA isolation buffer. The RNA was precipitated at 12,000 rpm for 15 min and washed with 80% ethanol. The pellet was dried briefly in vacuum and dissolved in minimal volume of sterile diethylpyrocarbonate DEPC treated water. The amount of RNA was quantified spectrophotometrically. The RNA was quantified by UV-absorbance spectrophotometry. Total RNA (2 µg) was reverse transcribed and 4 µl cDNA obtained was used for polymerase chain reaction (PCR) amplification to estimate the expression of CYP2E1 and PPAR-α. GAPDH was used as an internal standard. Primer sequences and the resultant PCR products (Gene expressed) are listed in table 1. After amplification, the PCR samples were electrophoresed in 1.2% agarose gel stained with ethidium-bromide. The bands were compared densitometrically. Densitometry was done using ‘Image J’ analysis software.
Table 1.
Primers used for SQRT-PCR studies in rats.
| Gene | Primer Sequence |
| CYP2E1 | F: 5′-GGGTGGACTTTGCTGGCCGA -3′ |
| R: 5′-TCGTGGAGCTGCCCTGGGTC -3′ | |
| F: 5′-TGGCGTACGACAAGTGTGTGAT -3′ | |
| PPAR-α | R: 5′-GTTTGCAAAGCCTGGGATAG -3′ |
| GAPDH | F: 5′-TCGAGTCTACTGGCGTCTT -3′ |
| R: 5′-ATGAGCCCTTCCACGAT -3′ |
Western blot analysis
Cells were collected by centrifugation and washed once with phosphate buffered saline (PBS). The washed cell pellets were resuspended in lysis buffer (50 mM HEPES, pH 7.0, 250 mM NaCl, 5 mM EDTA, 0.1% NP-40, 1 mM PMSF, 0.5 mM DTT, 5 mM sodium fluoride (NaF), 0.5 mM sodium orthovanadate containing 5µg/ml each of leupeptin and aprotinin and incubated for 30min at 4 °C. Cell debris was removed by centrifugation followed by quick freezing of the supernatants. The protein concentration was determined by Bradford reagent. 60µg of protein from treated and untreated extracts were electro blotted on to a nitrocellulose (Biotrace NT; Pall Gelman) membrane after separation on 10% SDS-polyacrylamide gel electrophoresis. The blot was incubated for 1 h with blocking solution (5% skim milk) at room temperature after incubation for 4 h with a 1:1000 dilution of monoclonal anti-CYP2E1, PPAR-α and β-actin antibodies (Santa Cruz Biotechnology Inc.). Blots were washed two times with tween 20/Tris buffered saline (TTBS) and incubated with a 1:2000 dilution of alkaline phosphatase conjugated goat antimouse IgG secondary antibody for 2 h at room temperature. Blots were again washed 3 times with TTBS and then developed by 3, 3'-diaminobenzidine tetrahydrochloride. Densitometry was done using ‘Image J’ analysis software.
Statistical analysis
Statistical evaluation was performed using one-way ANOVA followed by Duncan's multiple range test (DMRT) using statistical package of social science (SPSS Inc., Chicago, IL, USA) 10.0 for Windows. Significance level was set at P < 0.05.
Results
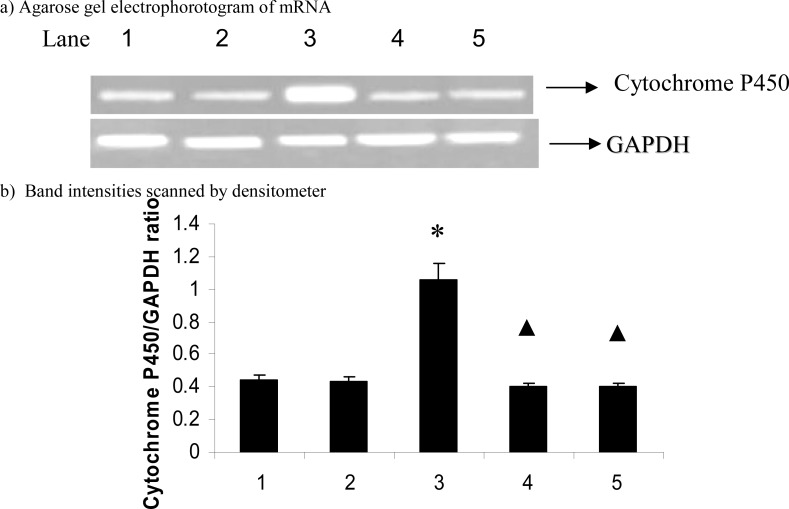
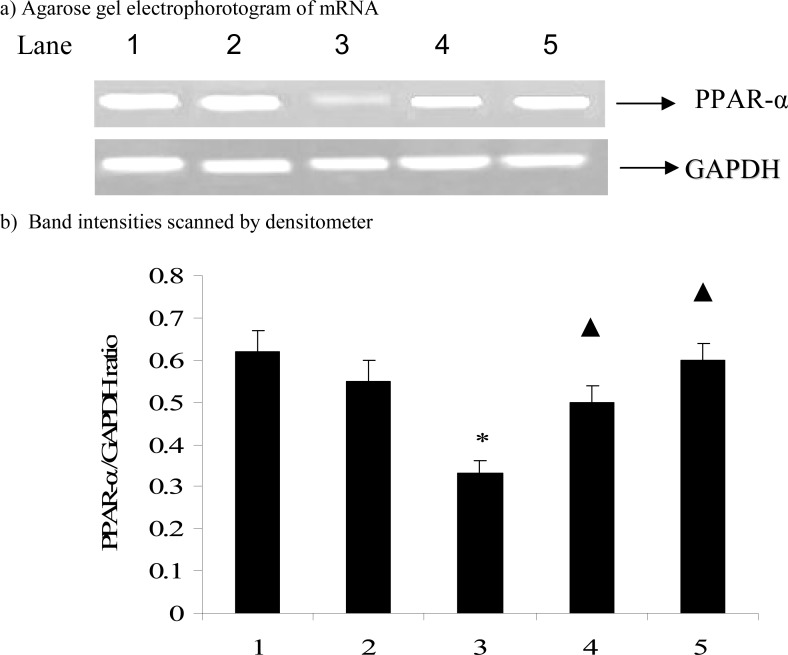
Figures 1 and 2 show the effect of carvacrol in CYP2E1 and PPAR-α mRNA expression in D-galactosamine induced hepatotoxic rats. The mRNA expression of CYP2E1(p=0.012) significantly up-regulated and PPAR-α (p=0.026) significantly down-regulated on D-galactosamine induced hepatotoxicity rats while treatment with carvacrol significantly(CYP2E1, p=0.010; PPAR-α, p=0.033) inhibited the mRNA expression of CYP2E1 and PPAR-α.
Figure 1.
Effect of carvacrol on cytochrome P450 mRNA expression in the liver of D-GalN rats.
The data are expressed as ratio of cytochrome P450/GAPDH and given as means ± S.D. for three experiments. * P ≤ 0.05 compared with control rats; ▲ P ≤ 0.05 compared with hepatotoxic control rats. Lanes: 1. Control; 2. Control + Carvacrol; 3. Hepatotoxic control; 4. Hepatotoxic + Carvacrol; 5. Hepatotoxic + Silymarin. GAPDH-glyceraldehyde 3-phosphate dehydrogenase (internal standard).
Figure 2.
Effect of carvacrol on PPAR-α mRNA expression in the liver of D-GalN rats.
The data are expressed as ratio of PPAR-α /GAPDH and given as means ± S.D. for three experiments. * P ≤ 0.05 compared with control rats; ▲ P ≤ 0.05 compared with hepatotoxic control rats. Lanes: 1. Control; 2. Control + Carvacrol; 3. Hepatotoxic control; 4. Hepatotoxic + Carvacrol; 5. Hepatotoxic + Silymarin. GAPDH-glyceraldehyde 3-phosphate dehydrogenase (internal standard).
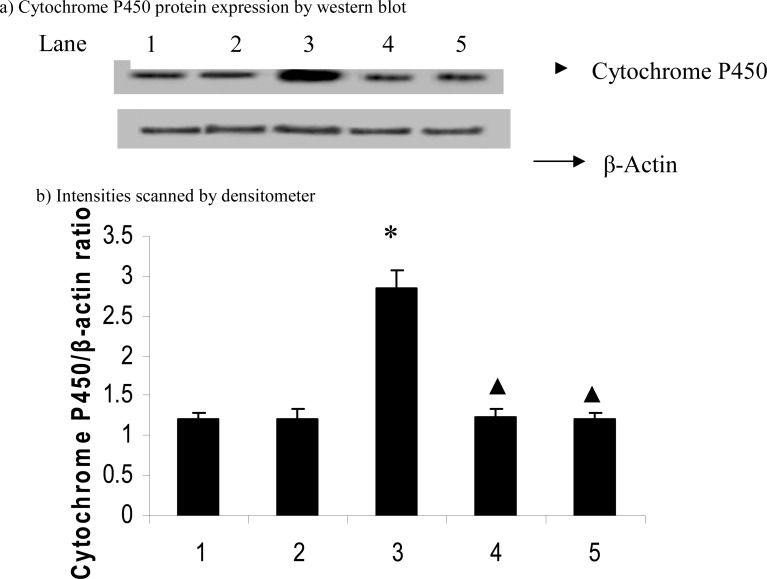
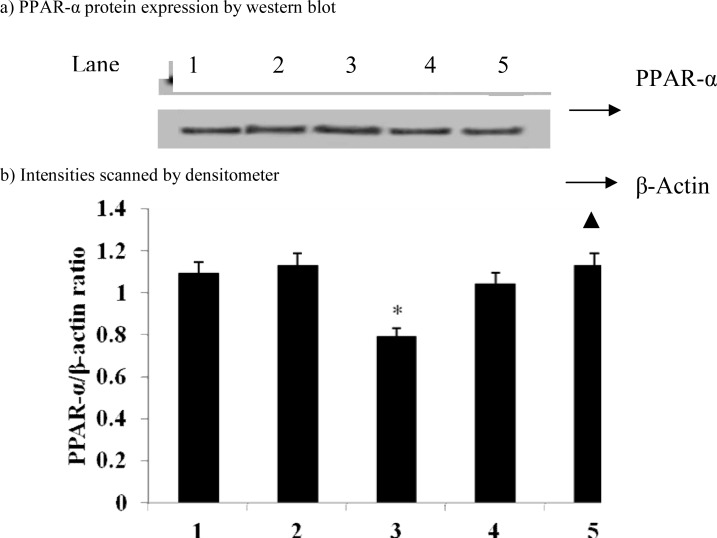
Figures 3 and 4 show the effect of carvacrol on CYP2E1 and PPAR-α protein expression in D-galactosamine induced hepatotoxicity rats. We found that the protein expression of CYP2E1 (p=0.015) significantly up-regulated and PPAR-α (p=0.03) down-regulated in D-galactosamine induced hepatotoxic rats and treatment with carvacrol significantly (CYP2E1, p=0.011; PPAR-α, p=0.037) inhibited the protein expression of these genes.
Figure 3.
Effect of carvacrol on cytochrome P450 protein expression in the liver of D-GalN-rats
Band intensities were expressed as ratio of cytochrome P450/β-actin and given as means ± S.D of three experiments. * P ≤ 0.05 compared with control rats; ▲ P ≤ 0.05 compared with hepatotoxic control rats. Lane: 1. Control; 2. Control + Carvacrol; 3. Hepatotoxic control; 4. Hepatotoxic + Carvacrol; 5. Hepatotoxic + Silymarin.
Figure 4.
Effect of carvacrol on PPAR-α protein expression in the liver of D-GalN-rats
Band intensities were expressed as ratio of PPAR-α/β-actin and given as means ± S.D of three experiments. * P ≤ 0.05 compared with control rats; ▲ P ≤ 0.05 compared with hepatotoxic control rats. Lane: 1. Control; 2. Control + Carvacrol; 3. Hepatotoxic control; 4. Hepatotoxic + Carvacrol; 5.
Discussion
In our body, liver is the major organ responsible for the metabolism of drugs and toxic chemicals and thus it is the primary target organ for nearly all toxic chemicals. Various pharmacological or chemical substances (such as acetaminophen, galactosamine, chloroform, dimethylnitrosamine, etc.) are known to cause hepatic injuries (Weber, 2003). Excessive exposure to these chemicals may cause acute liver injury characterized by abnormality of hepatic function, and degeneration, necrosis or apoptosis of hepatocytes, and so forth. In modern civilization drugs or chemicals induced liver injury has become a serious clinical problem.
It has been established that chemical-induced hepatotoxicity depends on its reductive dehalogenation, catalyzed by the cytochrome P450 enzymes, in the endoplasmic reticulum of hepatic cells, leading to the generation of an unstable complex trichloromethyl radical. The trichloromethyl radical may bind either at the heme group of cytochrome P450 or at the active site of the enzyme near the heme group, leading to the inactivation P450 pathways (Fernandez, 1982). Among the different cytochrome P450 enzymes CYP2E1-mediated metabolism of galcatosamine may generated reactive free radicals and CYP2E1 protein might be more susceptible to chemical toxicity than other CYP isozymes. Such biotransformation enzymes as cytochrome P450 isozymes are essential for the detoxication of xenobiotics (Halliwel and Gutteridge, 1999). A significant increase in hepatic CYP2E1 mRNA and protein expression was found in the GalN-intoxicated rats. In contrast, carvacrol supplementation suppressed this mRNA and protein expression on GalN induced rats and almost restored to the normal level of CYP2E1 mRNA and protein expression. Although it is not known how carvacrol affected the biosynthetic mechanism of CYP2E1, the restoration of CYP2E1 by carvacrol supplementation implies that carvacrol could have a remarkable hepatoprotective effect which led to rapid recovery from GalN induced liver injury.
PPARs belong to a super family of nuclear, ligand activated transcription factors, which play an important role in the transcriptional regulation of genes responsible for the control of lipid utilization and storage, and modulation of lipoprotein metabolism, adipocyte differentiation and insulin action (Torra et al., 2001). PPAR-α agonists have been used for over 40 years in the treatment of dyslipidemia, mainly due to their hypolipidemic actions, such as lowering TG and raising HDL levels. PPAR-α is expressed at high levels in tissues with high rates of FA oxidation, such as brown fat, liver and heart (Braissant and Wahli, 1998). PPAR-α-mediated responses have been traditionally studied in the liver. It has been reported that Wy14,643, a PPAR-α agonist, normalized fatty livers in fat-fed rats (Ye et al., 2003) and markedly improved ethanol-induced hepatic steatosis and TG accumulation in the liver of rats (Fischer et al., 2003). In Otsuka Long-Evans Tokushima fatty rats, fenofibrate (a well-characterized PPAR-α agonist), markedly reduced hepatic TG content, accompanied by a decrease in plasma TG levels (Lee et al., 2004). PPAR-activation mediates expression of genes regulating lipid oxidation (Kersten et al., 2000). PPAR genes are involved in the energy balance and its action depends on the tissue; PPAR-gis expressed in several cells, including endothelial cells, vascular smooth muscle cells, monocytes, macrophages, fat tissue and hepatic cells (Caballero, 2005; Fasshauer and Paschke, 2003). Other studies have shown that PPAR-α produces an anti-inflammatory effect in endometriotic stromal cells by reducing levels of TNF-α (Ohama et al., 2008) and this effect may be a consequence of repression of these transcription factors due to activation of the kappa B (NF-kB) nuclear factor, a receptor of the Rel/Nf-kB family that regulates genes of pro-inflammatory cytokines (Matilla et al., 2002). In this study, we have focused on PPARs as possible molecular targets of carvacrol in preventing lifestyle-related diseases. It has been already reported that carvacrol acts as PPAR-α activators and COX-2 suppressors in U937 and BAEC cells treated 12-O-tetradecanoylphorbol-13-acetate (TPA) in vitro (Mariko Hotta et al., 2010). In this study, we found that carvacrol suppressed the activate PPAR-α mRNA and protein expression in galactosamine induced toxicity rats. Fatty acids are considered to be intrinsic ligands for PPARs at higher concentrations compared with synthetic ligands. In this case, the presence of fatty acid-binding molecules, which include specific binding proteins and nonspecific interacting lipids, both inside and outside of the cells appears to modulate PPAR activation. A similar mechanism may be at work in the activation of PPARs by carvacrol and other chemical components in the essential oils.
Conclusion
In conclusion, we identified carvacrol, a chemical component of thyme oil, as a suppressor of CYP2E1 and an activator of PPAR-α. Thus, the present observations have shown that carvacrol has the heaptoprotective effect and also alleviates liver damage associated with GalN induced hepatotoxic rats by down regulating CYP2E1 and up-regulating c PPAR-α expression.
Acknowledgment
The authors would like to extend his sincere appreciation to the deanship of scientific research at King Saud University for its funding of this research through the research group project no: RGP-VPP-266.
References
- 1.Abdelrahman M, Collin M, Thiemermann C. The peroxisome proliferator-activated receptor-gamma ligand 15-deoxyDelta12, 14 prostaglandin J2 reduces the organ injury in hemorrhagic shock. Shock. 2004;22:555. doi: 10.1097/01.shk.0000144132.13900.24. [DOI] [PubMed] [Google Scholar]
- 2.Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65(4):772–781. doi: 10.1016/j.cardiores.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Ariyoshi N, Miyazaki M, Toide K, Sawamura Y, Kamataki T. A single nucleotide polymorphism of CYP2B6 found in Japanese enhances catalytic activity by autoactivation. Biochem Biophys Res Commun. 2001;281:1256–1260. doi: 10.1006/bbrc.2001.4524. [DOI] [PubMed] [Google Scholar]
- 4.Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-a, -h, and -g during rat embryonic development. Endocrinology (Baltimore) 1998;139:2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- 5.Caballero AE. Metabolic and vascular abnormalities in subjects at risk for type2 diabetes the early start of a dangerous situation. Arch Inv Med. 2005;36:241–249. doi: 10.1016/j.arcmed.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Crawford JH, Yang S, Zhou M, Simms HH, Wang P. Down-regulation of hepatic CYP1A2 plays an important role in inflammatory responses in sepsis. Crit Care Med. 2004;32:502. doi: 10.1097/01.CCM.0000109453.57709.E2. [DOI] [PubMed] [Google Scholar]
- 7.Cuzzocrea S, Mazzon E, Di Paola R, Peli A, Bonato A, Britti D, Genovese T, Muià C, Crisafulli C, Caputi AP. The role of the peroxisome proliferators-activated receptor alpha (PPAR-alpha) in the regulation of acute inflammation. J Leukoc Biol. 2006;79:999–1010. doi: 10.1189/jlb.0605341. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, Di Paola R, Ialenti A, Genovese T, Chatterjee PK, Di Rosa M, Caputi AP, Thiemermann C. Rosiglitazone, a ligand of the peroxisome proliferatoractivated receptor-g reduces acute inflammation. Eur J Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 9.Ekins S, Bravi G, Ring BJ, Gillespie TA. Three-dimensional quantitative structure-activity relationship analyses of substrates for CYP2B6. J Pharmacol Exp Ther. 1999;288:21–29. [PubMed] [Google Scholar]
- 10.Fasshauer M, Paschke R. Regulation of adipokines and insulin resistance. Diabetologia. 2003;46:594–603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez G, Villarruel MC, de Toranzo EG, Castro JA. “Covalent binding of carbon tetrachloride metabolites to the heme moiety of cytochrome P-450 and its degradationproducts”. Res Commun Chem Pathol Pharmacol. 1982;35:283–290. [PubMed] [Google Scholar]
- 12.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPAR-alpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 13.Halliwel B, Gutteridge JMC. “Free Radicals in Biology and Medicine”. 3rd ed. New York: Oxford University Press; 1999. pp. 14–17. [Google Scholar]
- 14.Ire O, Mangeney M, Montagne J, Nordmann R, Nordmann J. Carnitine palmitoyltransferase I inhibition by D-galactosamine and role of phospholipids. Eur J Biochem. 1983;136:371–375. doi: 10.1111/j.1432-1033.1983.tb07751.x. [DOI] [PubMed] [Google Scholar]
- 15.Keppler D, Decker K. Studies on the mechanism of galactosamine hepatitis: accumulation of galactosamine-1-phosphate and its inhibition of UDP glucose pyrophosphorylase. Eur J Biochem. 1969;10:219–225. doi: 10.1111/j.1432-1033.1969.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 16.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 17.Lee GY, Kim NH, Zhao ZS, Cha BS, Kim YS. Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene: a key regulation of malonyl-CoA level. Biochem J. 2004;378:983–990. doi: 10.1042/BJ20031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu D, Zeng BX, Zhang SH, Yao SL. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflamm Res. 2005;54:464–470. doi: 10.1007/s00011-005-1379-0. [DOI] [PubMed] [Google Scholar]
- 19.Mangeney-Andreani M, Sire O, Montagne-Clavel J, Nordmann R, Nordmann J. Inhibitory effect of D-galactosamine administration on fatty acid oxidation in rat hepatocytes. FEBS Lett. 1982;23:267–270. doi: 10.1016/0014-5793(82)80180-4. [DOI] [PubMed] [Google Scholar]
- 20.Mariko H, Rieko N, Michiko K, Kazuyuki H, Saori T, Hiroyasu I. Carvacrol, a component of thyme oil, activates PPAR-α and γ and suppresses COX-2 expression. J Lipid Res. 2010;51:132–139. doi: 10.1194/jlr.M900255-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matilla B, Matriz JL, Culebras JM, Gallego J, Gonzalez P. Glycine: a cell-protecting anti-oxidant nutrient. Nutr Hosp. 2002;1:2–9. [PubMed] [Google Scholar]
- 22.Morgan ET. Regulation of cytochrome p450 by inflammatory mediators: why and how? Drug Metab Dispos. 2001;29:207. [PubMed] [Google Scholar]
- 23.Ohama Y, Harada T, Iwabe T, Taniguchi F, Takenaka Y, Terakawa N. Peroxisome proliferator-activated receptor-gamma ligand reduced tumor necrosis factoralpha-induced interleukin-8 production and growth in endometriotic stromal cells. Fertil Steril. 2008;89:311–317. doi: 10.1016/j.fertnstert.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 24.Sanches-Hidalgo M, Martin AR, Villegas I, Alarcon De La Lastra C. Rosiglitazone, an agonist of peroxisome proliferatoractivated receptor gamma, reduces chronic colonic inflammation in rats. Biochem Pharmacol. 2005;69:733–744. doi: 10.1016/j.bcp.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki S, Koide N, Shinji T, Tsuji T. Immunohistochemical study of proteoglycans in D-galactosamine-induced acute liver injury in rats. J Gastroenterol. 1996;31(1):46–54. doi: 10.1007/BF01211186. [DOI] [PubMed] [Google Scholar]
- 26.Shedlofsky SI, Israel BC, McClain CJ, Hill DB, Blouin RA. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J Clin Invest. 1994;94(6):2209–2214. doi: 10.1172/JCI117582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spatzenegger M, Horsmans Y, Verbeeck RK. Differential activities of CYP1A isozymes in hepatic and intestinal microsomes of control and 3-methylcholanthrene-induced rats. Pharmacol Toxicol. 2000;86:71–77. doi: 10.1034/j.1600-0773.2000.d01-14.x. [DOI] [PubMed] [Google Scholar]
- 28.Stiborova M, Borek-Dohalska L, Hodek P, Mraz J, Frei E. New selective inhibitors of cytochromes P450 2B and their application to antimutagenesis of tamoxifen. Arch Biochem Biophys. 2002;403:41–49. doi: 10.1016/S0003-9861(02)00259-X. [DOI] [PubMed] [Google Scholar]
- 29.Torra IP, Chinetti G, Duval C, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors: from transcriptional control to clinical practice. Curr Opin Lipidol. 2001;12:245–254. doi: 10.1097/00041433-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Villegas I, Martin AR, Toma W, de la Lastra CA. Rosiglitazone, an agonist of peroxisome proliferatoractivated receptor gamma, protects against gastric ischemia-reperfusion damage in rats: role of oxygen free radicals generation. Eur J Pharmacol. 2004;28:195–203. doi: 10.1016/j.ejphar.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Wang AC, Dui X, Luu B, Conrad DJ. Peroxisome proliferatoractivated receptor gammaregulates airway epithelial cell activation. Am J Respir Cell Mol Biol. 2001;24:688–693. doi: 10.1165/ajrcmb.24.6.4376. [DOI] [PubMed] [Google Scholar]
- 32.Weber LWD, Boll M, Stampfl A. “Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model”. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 33.Ye JM, Iglesias MA, Watson DG, Ellis B, Wood L, Jensen PB, Sorensen RV, Larsen PJ, Cooney GJ, Wassermann K, Kraegen EW. PPARalpha/gamma ragaglitazar eliminates fatty liver and enhances insulin action in fat-fed rats in the absence of hepatomegaly. Am J Physiol Endocrinol Metab. 2003;284:E531–E540. doi: 10.1152/ajpendo.00299.2002. [DOI] [PubMed] [Google Scholar]