Abstract
Background
There is a great need for novel strategies to overcome the high mortality associated with invasive pulmonary aspergillosis (IPA) in immunocompromised patients. To evaluate the antifungal and antihepatotoxic potentials of Sepia ink extract, its effect on liver oxidative stress levels was analyzed against IPA in neutropenic mice using amphotercin B as a reference drug.
Materials and Methods
Eighty neutropenic infected mice were randomly assigned into four main groups. The 1st group was treated with saline, neutropenic infected (NI), the 2nd group was treated with ink extract (200 mg/kg) (IE) and the 3rd group was treated with amphotericin B (150 mg/kg) (AMB) and 4th group was treated with IE plus AMB. Treatment was started at 24 h after fungal inoculation (1×109 conidia/ml).
Results
The present study revealed good in vitro and in vivo antifungal activity of IE against A. fumigatus. IE significantly reduced hepatic fungal burden and returns liver function and histology to normal levels. Compared with the untreated infected group, mice in the IE, AMB, and IE+ AMB groups had increased glutathione reduced (GSH) and superoxide dismutase (SOD) and significantly reduced malondialdehyde (MDA) levels at 24 and 72 h after inoculation with A. fumigatus conidia.
Conclusion
It is then concluded that in combination with antifungal therapy (AMB), IE treatment can reduce hepatic fungal burden, alleviate hepatic granulomatous lesions and oxidative stress associated with IPA in neutropenic mice
Keywords: Neutropenia, Invasive pulmonary aspergillosis, Amphotericin B, Antifungal, Sepia ink extract, Oxidative stress
Introduction
Aspergillus fumigatus is the most ubiquitous of the airborne saprophytic fungal pathogens in immunocompromised patient in developed countries (Latgé, 2001). Aspergillus fumigatus is a mold that causes severe pulmonary infections in humans, such as invasive pulmonary Aspergillosis (IPA) (Obar et al., 2013). Invasive pulmonary aspergillosis (IPA) is a major cause of morbidity and mortality in immunocompromised patients, particularly in those with hematological malignancies (Zhao et al., 2010; Russo et al., 2011). IPA is particularly prevalent in patients with neutropenia, the most common and best-characterized risk factor for IPA (Becker et al., 2003). Aspergillus fumigatus is the major cause of IPA (Marr et al., 2002). A. fumigatus are opportunistic pathogens of humans that can cause primary invasive lung infections and disseminate to other organs (Vadlapudi, 2011). A. fumigatus has angioinvasive properties and frequently disseminate from the primary lesions, usually the lung, to a variety of organs including the liver via hematogenous spread (Bai et al., 2012). Liver failure was reported to occur in severely immunocompromised patients with IPA ( Russo et al., 2011 ; Wu et al., 2012).
Part of the virulence of A. fumigatus was with its ability to produce the well-known secondary metabolite gliotoxin (GT), a mycotoxin readily detected in invasive aspergillosis, which was proven to has immunosuppressive and apoptotic activities in the host (Pahl et al., 1996; Lewis et al., 2005; Stanzani et al., 2005). Accordingly, GT was shown to be produced during the infection process and was detected in lungs and sera of mice and also humans infected with A. fumigatus (Kupfahl et al., 2006). Theoretically, the release of GT by Aspergillus during growth in tissues could aid the evasion of the fungus via the innate and professional effector immune cells (Lewis et al., 2005).
Oxidative stress represents one of the key pathogenetic mechanisms in the development of IPA (Morcillo et al., 1999). Reactive oxygen species (ROS) are essential components of the defensive mechanism against fungus infection (Ibrahim-Granet et al., 2003; Xu et al., 2009). Aspergillus fumigatus conidia are continuously inhaled and small enough to reach the lower parts of the respiratory tract. In the immunocompetent host such spores are supposed to be rapidly engulfed and eliminated by macrophages which reside within the alveoli (Latgé, 1999). Establishment of IPA occurs in immunocompromised patients as the fungus escapes from the alveolar macrophages and invades tissues (Latgé, 1999). Under conditions in which A. fumigatus conidia escape from phagocytosis, they germinate to form hyphae and are then phagocytosed by the second line of defense, the neutrophils (Dagenais and Keller, 2009). Both macrophages and neutrophils mediate powerful fungicidal effects on A. fumigatus by producing reactive oxygen species (ROS), such as H2O2, hydroxyl radical (HO−), and superoxide anions (O−) (Chauhan et al., 2006). High levels of ROS in macrophages can result in cell death by causing DNA damage, lipids peroxidation, and protein inactivation which play a significant role in the host response to A. fumigatus (Chauhan et al., 2006).
Over the past decade, the incidence of serious infections caused by opportunistic fungal pathogens has increased dramatically due to alterations in the immune status of patients. Therapeutic options for aspergillosis are limited, particularly so for oral formulations, with azole drugs forming the backbone of therapy (Walsh et al., 2008). Many patients that develop resistant infections fail treatment, so resistance is an important factor in the outcome of these cases (Howard et al., 2009). Azole resistance has predominantly been reported for A. fumigatus. Unfortunately, the widespread use of triazole antifungal agents to combat these infections has led to the emergence of clinically significant drug resistance (Vadlapudi, 2011). Although amphotericin B remains the standard therapy, a successful outcome is achieved in only 34% of patients (Denning, 1996). The success of treatment depends on many factors, most importantly on the response of the underlying disease to therapy (Denning, 1996). The majorities of patients with invasive aspergillosis who die during treatment with amphotericin B are persistently granulocytopenic due to refractory underlying disease or receive a suboptimal dose due to amphotericin B-related nephrotoxicity (Gerson et al., 1984).
Marine environment is an abundant source of undiscovered compounds leading to the development of new bioactive molecules with different properties. Several marine products (animal, algae, fungi and bacteria) were screened for their bio-activities such as antibacterial, antifungal, anticoagulant, antiviral and anti-enzyme activities (Lindequist and Schweder, 2001; Mayer and Hamann, 2004; Neifar et al., 2009; Neifar et al., 2012). In nature, animals are provided with their own protective response against their predators, likewise marine cephalopod mollusks (squid, octopus and cuttlefish), which have a striking defensive behavior-releasing ink when attacked. The cuttlefish Sepia officinalis relies for defense on the ejection of a dark ink which consists of a suspension of melanin granules in a viscous colorless medium. Mollusks ink not only exhibit the anti-microbial activity, it constitutes many classes of bioactive compounds which includes antitumor, antileukemic and antiviral activities have been reported world-wide (Rajaganapathy et al., 2000). Caldwell (2005) proposed that the ink of cephalopods contain compounds that are capable of disrupting predator's chemical senses. Background research has shown that squid ink possesses a wide range of biological roles as anti-tumor effects (Xie and He, 2002; Fahmy and Soliman, 2013), leukocyte-number elevating (Sasaki et al., 1997), anti-oxidant (Lei et al., 2007 ; Fahmy and Soliman, 2013), anti-radiation (Lei et al., 2007), anti-retrovirus (Rajaganapathy et al., 2000), and anti-bacterial properties (Funatsu et al., 2005). It has been reported that, sepia ink extract at the suitable concentration is able to alleviate the in vivo immunosuppression induced by cyclophosphamide in mice (Guang et al., 2009). Also, Zhong et al. (2009) studied the protective effects of squid ink extract towards hemopoietic injuries induced by cyclophosphamine.
The current study was based on the hypothesis that combining antioxidants with classical anti-aspergillosis treatment could alleviate ROS-induced tissue damage and thus improve the extract's therapeutic effects. So, the present study aim to evaluate the antifungal and antihepatotoxic effects of Sepia ink extract either alone or in combination with AMB against invasive pulmonary aspergillosis in mice.
Materials and methods
Chemicals
Amphotericin B was purchased as Fungizone (E. R. Squibb & Sons, Princeton, N.J.). Kits for aspartate aminotrasaminase (AST), alanine aminotransaminase (ALT), malondialdehyde (MDA), glutathione reduced (GSH) and superoxide dismutase (SOD) estimations were purchased from Biodiagnostic Company (Dokki, Giza, Egypt).
Preparation of cuttlefish ink extract (IE)
Fresh cuttlefish (Sepia officinalis) were purchased directly from a fishmonger and rapidly transferred to the laboratory where, they were dissected and the ink was collected and diluted immediately with an equal volume of distilled water. The admixture collected immediately, concentrated and lyophilized to a black residue using (LABCONCO lyophilizer, shell freeze system, USA).
Test organism and growth conditions
A strain of A. fumigatus was isolated from an immunocompromised patient with IPA served as the parental strain in this investigation. A working culture of this strain was maintained on peptone yeast extract glucose (PYG: peptone 1 g; yeast extract 1 g; glucose 3 g; per liter of distilled water) agar slants at room temperature. For the preparation of conidial suspension, a culture of A. fumigatus was grown on PYG agar for 6 days at 35°C, and the conidia were collected as described previously (Manavathu et al., 1999).
Preparation of fungal spores and evaluation of spore germination
For the germination assay, fresh conidia were re-suspended in peptone-yeast extract-glucose medium (106 conidia/ml) and incubated at 35°C with gentle agitation (160 rpm) on a gyratory shaker. At various time intervals, 10 ml aliquots were removed and the numbers of germinated conidia were assessed by hemocytometer counting. Percent germination was calculated and graphed against time of incubation in PYG broth. Conidia were counted at a magnification of 400, and a total of 200 conidia per field were counted. The counting was done three times, and the mean value of these three independent counts was used to obtain the percent germination (Manavathu et al., 1999).
To determine effect of ink extract, amphotericin B, and their combination on spore germination, conidia were allowed to germinate for 8 hours as described previously. Antifungal activity of different antifungal agents can be evaluated by spore germination assay using the slide technique (Nair et al., 1991). Antifungal agent of desired concentration and volume are added to the surface of dried slides as a film or in a cavity of a cavity slide. 106 conidia ml−1 of different concentrations of spore suspension (10–640 µg ml−1) of A. fumigatus are spread over the film whereas in controlled treatment, distilled water is added in place of spore suspension. Slides are then placed on a glass rod in Petri dish under moistened conditions and incubated at 35°C. After incubation, slides are fixed in lacto phenol cotton blue and observed microscopically for spore germination. Percentage spore germination is calculated according to the following formula.
% Spore Germination = Germinated spores (No.) /Total Spores (No.) X 100
The effect of ink extract and amphotericin B combination (1:1) was assayed with A. fumigatus, according to the checkerboard method (Scott et al., 1995). Hypothetical interactions occurring between the two components were determined following the calculation of the fractional inhibitory concentration (FIC) and the fractional inhibitory index (FIX). Synergistic, indifferent and antagonistic interactions were defined, respectively, by an FIX of < 0.5, 0.5–4.0 or > 4.0 (Scott et al., 1995).
Animals
Healthy male Mus musculus mice (weighing 20±2 g), obtained from a closed random-bred colony at the animal's house, National Research Center). Animals were housed in polycarbonate boxes with steel-wire tops (not more than five animals per cage) and bedded with wood shavings. Ambient temperature was controlled at 22±3 °C with a relative humidity of 50±15% and a 12-h light/dark photoperiod. Food and water were provided ad libitum.
Toxicity study (OECD 420)
Mus musculus mice weighing (20–25 g) were used for acute toxicity studies. The animals were divided into control and test groups containing six animals each. Mice were administered orally with cuttlefish ink extract (IE) at dose levels of 5 g/kg (high dose) and 2 g/kg (low dose). Normal control mice received the same amount of vehicle (distilled water) only. Animals were observed carefully for 24 hours after extract administration and then for the next 14 days. At the end of this experimental period, animals were observed for signs of toxicity, morphological behavior, and mortality. Acute toxicity was evaluated based on the number of deaths (if any). Acute toxicity was calculated as per OECD guidelines 420 (Fixed dose method) (Vanden Heuvel et al., 1990; Whitehead and Curnow, 1992).
Immunosuppression
Neutropenia was induced by intraperitoneal (i.p) administration of 150 mg·kg−1·d−1 cyclophosphamide (Sbaraglia et al., 1984), 72 hours before fungal inoculation. Animals of control (20 mice) (injected by saline, i.p, for 72 hs) and neuropenic groups (80 mice) were kept under strict hygienic conditions and were observed daily until the end of the study. At this time point, the mice were at an immunocompromised state, as determined by decrease in the number of white blood cells (WBC) and reduction in the body weight. Protocol was approved by the Cairo University, faculty of Science Animal Care and Use Committee (IACUC) (Egypt), and all the experimental procedures were carried out in accordance with international guidelines for care and use of laboratory animals.
Murine model of invasive pulmonary aspergillosis (IPA)
A strain of A. fumigatus was isolated from an immunocompromised patient with IPA. The fungus was cultured on Sabouraud agar and conidia were harvested in sterile saline. Preparation of a microscopically confirmed, hyphae-free conidial suspension was performed as previously described (Leenders et al., 1996). On day 0, all neutropenic mice were infected with conidia of A. fumigatus, a suspension of 1×109 conidia/ml was then prepared prior to inoculation. Each inoculums consisted of 0.025 ml of suspension (106 conidia) administered by intranasal instillation, using a 1 cc insulin Syring, under light isofurane anesthesia.
Design of the experiment
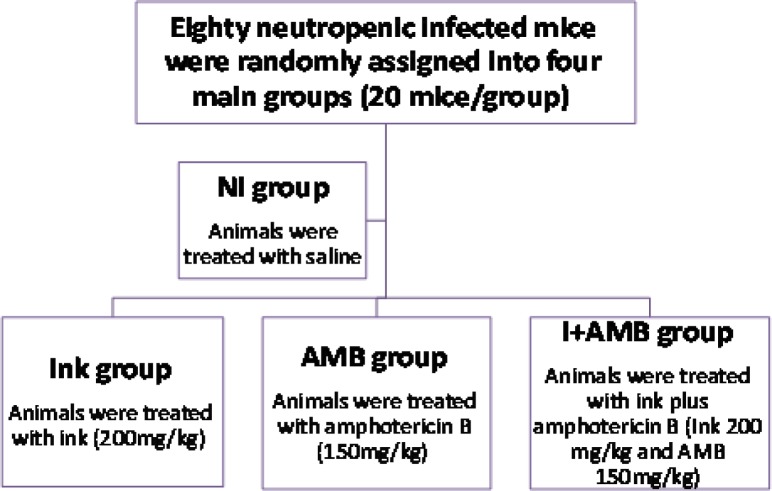
Eighty neutropenic infected mice were randomly assigned into four main groups (20 mice/group). The 1st group was treated with saline, neutropenic infected (NI group), the 2nd group was treated with ink extract (200 mg/kg) (IE) and the 3rd group was treated with amphotericin B (150 mg/kg) (AMB group) and 4th group was treated with ink plus amphotericin B (IE, 200 mg/kg and AMB,150 mg/kg) (IE+AMB group) (Fig. 1). Treatment was started at 24 h after fungal inoculation.
Fig. 1.
Experimental Design.
Animals handling
Animals were euthanized under isofurane after 24 h and 72 h post-inoculation and after being fasted over night; blood was collected in EDTA and centrifuge tubes for hematological and biochemical parameters. Liver was removed and immediately blotted using filter paper to remove traces of blood and then divided into two parts, the first part stored at −80°C. While, the second part was suspended in 10% formal saline for fixation preparatory to histological processing. Part of the frozen liver tissues were shipped to micro analytical centre, Faculty of science, Cairo University in frozen state. Upon receipt, the samples were kept frozen (−20°C) until used for analysis of gliotoxin.
White blood cells count
White blood cells count (WBCs) was measured by HEMAVET multispecies hematology system-HV950FS (Drew Scientific, Dallas, TX) per the manufacturer's instructions.
Sample Preparation
Serum Preparation
Blood samples collected in centrifuge tubes were centrifuged at 3000 rpm for 20 minutes. Serum was stored at −20°C until used for biochemical assays.
Liver Tissue homogenate Preparation
Liver was homogenized (10% w/v) in ice-cold 0.1 M phosphate buffer (pH 7.6). The homogenate was centrifuged at 3000 rpm for 15 min at 4° C and the resultant supernatant was used for different oxidative stress markers. The sediment of the homogenates was then cultured on potato dextrose agar plates .
Histopathological Preparation
The fixed liver tissues were sectioned (5-micron thickness) and sections firstly stained with basic dyes, hematoxylin and Eosin (H&E) according to Conn (1946) procedure and photomicrographs developed (× 400) as shown below.
Assessment of fungal load in liver tissue
For tissue burdens, aliquots of tissue were semi-quantitatively cultured on potato dextrose agar plates using serial 10-fold colony count dilutions. Plates were placed in an incubator at 37°C until colonies could be counted. The undiluted homogenate was saved at 4°C until initial cultures were counted. When the undiluted homogenate had no counts, the entire organ homogenate was cultured. Counts were expressed as cfu/organ.
Assessment of gliotoxin in liver tissue
Extraction of glotoxin from infected liver tissue was performed according to method described by Richard and DeBey (1995). Briefly, liver sample was macerated in a plastic bag as much as possible with mallet then was transferred with 5 ml of distilled water to TenBroek tissue homogenizer. The sample was homogenized and 10 ml 6N HCl was added to homogenate on a shaker for 30 min. The mixture was extracted with 200 ml of chloroform passed through 5 g sodium sulfate and filtered through No. 580 glass filter. The elute was transferred to round bottom flask and dried on rotavap at 30 °C. 10 ml syringe was attached to silica column then column was rinsed with 4 ml hexane using vacuum and 8 ml hexane were added to reservoir. round bottom was rinsed, from above, 4X with 1ml each of CHCl3 and hexane was added in column reservoir. Sample was drown through column and rinsed with 12 ml hexane and 12 ml hexane: diethyl ether (1:1, v/v). Gliotoxin was eluted with 15 ml ether: acet24 h (95:5, v/v) in 25 ml beaker and evaporated under stream of nitrogen at 30 °C. Sample was quantitively transferred to 0.5 dram vial using ether: acetone 24 h (95:5, v/v) and the solution in the vial was dried. Samples were then analyzed by TLC.
Assessment of biochemical parameters
The appropriate kits (Bio-Diagnostic, Dokki, Giza, Egypt) were used for the determination of serum aminotransferase enzymes activities (AST and ALT) according to (Reitman and Frankel, 1957).
Assessment of oxidative stress markers
Oxidative stress markers were detected in the resultant supernatant of liver homogenate. The appropriate kits (Biodiagnostic kits, Biodiagnostic Dokki, Giza, Egypt) was used for the determination of malondialdehyde (MDA) (Ohkawa et al., 1979), glutathione reduced (GSH) (Aykaç et al., 1985) and superoxide dismutase (SOD) (Nishikimi et al., 1972).
Statistical method
All data are expressed as means ± SEM. In general, the data were analyzed by two-way ANOVA followed by the Bonferroni test and Duncan's multiple range test. Student's t test was used when only two data groups were compared with each other. P-value of < 0.05 was considered as statistically significant. All calculations were performed using GraphPad Prism software 5.01 (La Jolla, CA, USA).
Results
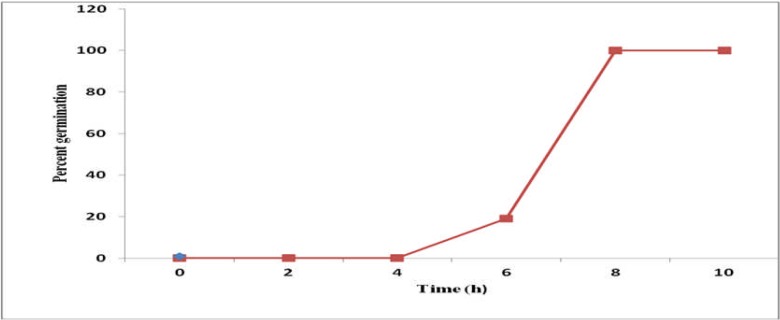
Table 1 summarizes the minimum inhibitory concentrations (MICs) of ink extract, amphotericin B, and their combination. The data revealed that A. fumigatus isolate used in this study was susceptible to antifungal drugs. The antifungal potency of all treatments varies significantly. Amphotericin B seemed to be the most effective antifungal drug with the lowest MIC value 80 µg ml−1 followed by ink extract (MIC 320 µg ml−1) whereas their combination was relatively weak antifungal drug to A. fumigatus growth (MIC 1280 µg ml−1). The interaction between amphotericin B and ink extract was antagonistic for A. fumigatus isolate. For the antagonistic interactions the concentrations of ink extract and amphotericin B in the combination were 1:1. As shown in Figure 2, the germination of A. fumigatus conidia at 35°C in PYG broth was a multistep process. During the initial 4 h of incubation, the conidia underwent a significant increase in volume till reach 100% germination after 8 hours. The fungicidal activities of ink extract (IE) and / or amphotericin B(AMB) against germinated conidia of A. fumigatus are shown in Table 1.
Table 1.
(Antimicrobial activities of ink extract (IE) and / or amphotericin B (AMB) against Aspergillus fumigatus:)
| Concentration gradient (µg ml−1) | Spore germination (%) | |||
| A | B | C | Effect | |
| 0 (Control) | 100a±0.3 | 100a±0.8 | 100a±0.6 | - |
| 10 | 96b±0.6 | 87c±0.4 | 98a±1.2 | Antagonistic |
| 20 | 85b±0.1 | 73c±1.0 | 89a±1.0 | Antagonistic |
| 40 | 79b±0.5 | 62c±0.9 | 82a±0.9 | Antagonistic |
| 80 | 60b±0.2 | 36c±0.6 | 66a±0.5 | Antagonistic |
| 160 | 45b±0.1 | 0c±0.0 | 50a±1.0 | Antagonistic |
| 320 | 33b±0.4 | 0c±0.0 | 37a±0.2 | Antagonistic |
| 640 | 0b±0.0 | 0b±0.0 | 32a±0.1 | Antagonistic |
| 1280 | 0a±0.0 | 0a±0.0 | 0a±0.0 | indifferent |
A: Spore germination percentage that occurred of IE. B: Spore germination percentage that occurred of AMB. C: Spore germination percentage that occurred when IE and AMB are used together (1:1).Data are presented as mean × SEM (n=3 in each group).The means followed by same letter in the same raw are not significantly different according to ANOVA and Duncan's multiple range tests.
Figure 2.
kinetics of germinating conidia of A. fumigatus in PYG broth at 35°C.
Induction of IPA in neutropenic mice
A neutropenic mice model was successfully established, as evidenced by a median white blood cell count of (6.02±0.92)×108/L, as compared with 2.3±1.2×109 in normal mice. Liver sections of untreated showed normal hepatic lobular architecture with hepatocytes arranged in thin plates. The portal tracts were within normal limits and contained arteries, veins and bile ducts. The hepatocytes contained rounded, regular nuclei with lymphocytes scattered between hepatocytes and the sinusoids. Moreover, no hydropic, steatotic, feathery or ballooning changes, degeneration or apoptosis were observed (Fig 3 a&b). Liver response to infection was characterized with necrosed hepatocytes infiltrated and prominent Kuppfer cells with microabscesses, composed mainly of neutrophils (Fig 3c). Large rmicroabscesses were found at day 3 (Fig 3d). Compared to the infected untreated group, treatment with AMB (150 mg/kg) or IE (200 mg/kg) at 24 h or incombination reduced the sizes of the microabscesses (Fig 3e&h). Moreover, we observed normal hepatic parenchyma in groups treated with IE (200 mg/kg) at 24 and 72 hs post-inoculation in comparison with groups treated with AMB at 24 h or in combination with IE (Figure 3g&h).
Figure 3.
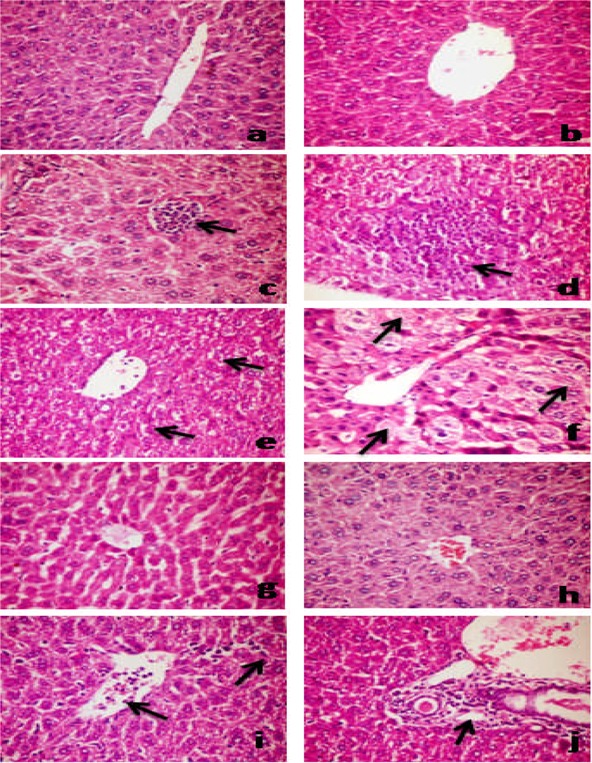
Haematoxylin and eosin stained liver sections from (a & b) uninfected untreated mouse showing normal hepatic architecture and normal hepatocytes; (c) infected untreated mice (24 h post-inoculation) showing small focal mononuclear cells infiltration (arrow); (d): infected untreated mice (72 hs post-inoculation) showing large focal area of necrosed hepatocytes infiltrated and replaced by mononuclear cells infiltration (arrows); (e): infected treated with AMB (150 mg/kg) showing severely degenerated hepatic cells at 24 h post-inoculation (arrows); (f): infected treated with AMB (150 mg/kg) showing massive necrosed hepatic cells at 72 hs post-inoculation (arrows); (g &h): infected treated with IE (200 mg/kg) (24 h and 72 hs post-inoculation, respectively) showing apparently normal histology of hepatic parenchyma; (I): infected treated with AMB (150 mg/kg) + IE (200 mg/kg) (24 h post-inoculation) showing leucocytic cells permeate the central vein (arrow) and blood sinusoids (Arrow head); (j): infected treated with AMB (150 mg/kg) + IE (200 mg/kg) (72 hs post-inoculation) showing leucocytic cells permeate the portal tract (arrow) (400X).
Estimation of fungal load in liver tissue
Figure 4 shows the microbial burden in the autopsied livers. Liver cultures had minimal fungal load in AMB-group. Mean burden of fungal organisms in the livers (cfu/g liver tissue) after 72 hs of inoculation was 205× 106 (IPA group), 66 × 106 (IE group), 34 × 106(AMB group), and 56× 106 (AMB+IE). Compared with IPA group; IE-treated, AMB-treated, and AMB+IE-treated animals had a 67.80%, 83.41%, and 72.68% reduction in fungal burden, respectively.
Figure 4.
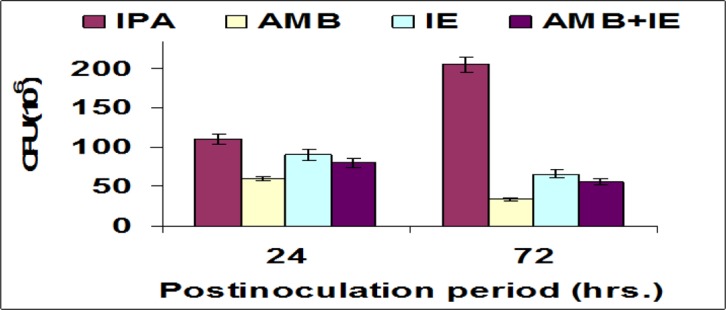
Screening of fungal load in liver homogenate of mice infected with Aspergillus fumigatus. IPA: invasive pulmonary aspergillosis (neutropenic, infected with A. fumigatus conidia); AMP: amphotericine B (neutropenic, infected with A. fumigates conidia, and injected with amphoteicine B); IE: ink extract (neutropenic, infected with A. fumigates conidia, and injected with ink extract); AMP+IE: amphotericine B and ink extract (neutropenic, infected with A. fumigates and injected with amphoteicine B and ink extract).
Detection of gliotoxin in liver tissue
Gliotoxin was found in liver tissue samples from all mice inoculated with A. fumigatus (Table 2). It is obvious from the results that concentration of gliotoxin in liver tissues varies significantly in all samples according to treatment type and days after inoculation. The highest concentration of gliotoxin was recorded in sample No. 2 in which mice were infected with A. fumigatus conidia and didn't subjected to any treatment while the lowest concentration was observed in sample No. 6 where mice were treated with a amphotericin B for 72 hs. It is also clear from the results that in sample No. 8 where combination treatment was used the concentration of gliotoxin significantly decreased as compared with infected group but the reduction is less than that when amphotericin B used separately.
Table 2.
Liver gliotoxin concentration in mice with invasive pulmonary aspergillosis (IPA) treated with Sepia officinalis ink extract (IE) and Amphotericin B (AMB).
| Sample no. | Neutropenia | Infection | Treatment | Post inoculation period (hrs.) |
Gliotoxinconcn. (µg/ g liver) |
| 1 | Yes | Yes | Saline | 24 | 5374b±3.1 |
| 2 | Yes | Yes | Saline | 72 | 6172a±2.8 |
| 3 | Yes | Yes | IE | 24 | 3221c±1.7 |
| 4 | Yes | Yes | IE | 72 | 2010f±2.0 |
| 5 | Yes | Yes | AMB | 24 | 2100e±1.9 |
| 6 | Yes | Yes | AMB | 72 | 1030h±0.9 |
| 7 | Yes | Yes | IE and AMB | 24 | 2623d±1.4 |
| 8 | Yes | Yes | IE and AMB | 72 | 1324g±0.8 |
Data are presented as mean ± SEM (n=3 in each group). The means followed by different letters in the same column are significantly different according to ANOVA and Duncan's multiple range tests.
Toxicity study (OECD 420)
Oral administration of IE at the higher dose (5 g/kg body weight) caused mortality in the first 24h. At the limiting dose (2 g/kg body weight) of IE, there was no mortality and the mice did not show any sign of toxicity in the first 24h or during the experiment. The single lethal dose of IE that kills half of the animals (LD50) was therefore taken as above 2000 mg/kg, P.O. The median effective dose (ED50) was selected based on the proposed LD50 obtained from the acute toxicity study. This dose was considered one tenth of the proposed LD50 (200mg/kg body weight, P.O).
Effect of the IE or/and AMB on serum aminotransferase enzymes (AST& ALT)
As shown in Table 3, the serum AST activity of mice was significantly (P< 0.05) decreased after 24 h and 72 hs of treatment, the neutropenic infected mice with the ink extract (IE) (200 mg/kg body weight i.p), amphotericin B (AMB) (150 mg/ kg body weight i.p), and their combinations (IE + AMB), as compared with infected untreated mice. Meanwhile, IE treatment only for 72 hs induced significant (P< 0.05) decrease in the serum AST activity, as compared with the corresponding AMB treated mice (Table 3). Serum ALT activity of IE group was significantly (P< 0.05) lower than the infected untreated mice after both time periods of treatment. Meanwhile, AMB treatment at the selected periods of time was found to cause significant (P< 0.05) decrease in the serum ALT activity, as compared to the infected untreated mice (Table 3). However, combined treatment with IE and AMB insignificantly changed the activity of serum ALT, as compared to the infected untreated or AMB groups.
Table 3.
Effect of Sepia officinalis ink extract (IE) and Amphotericin B (AMB) on AST and ALT activities in neutropenic mice with invasive pulmonary aspergillosis (IPA).
| Groups | AST (U/ml) | ALT (U/ml) | ||
| 24 h | 72 hs | 24 h | 72 hs | |
| Infected untreated group | 156.8 ± 8.37 | 147.6 ± 2.15 | 112 ± 1.64 | 112.4 ± 4.13 |
| AMB group | 73.4 ± 5.48a | 85.8 ± 4.56a | 87.4 ± 2.83a | 85.2 ± 9.47a |
| IE group | 87.4 ± 7.97a | 92.2 ± 3.96ab | 65.2 ± 3.42a | 68.4 ± 12.86a |
| IE + AMB group | 70.6 ± 7.31a | 64.8 ± 10.37a | 91 ± 8.34 | 93 ± 5.87 |
| ANOVA Two way | ||||
| Treatment | p<0.0001 | p<0.0001 | ||
| Time | NS | NS | ||
| Interaction | p<0.01 | NS | ||
| (treatment X time intervals) | ||||
Data are presented as mean ± SEM (n=5 in each group).
Significantly different from infected untreated group at p<0.05.
Significantly different from AMB treated group at p<0.05.
Effect of the IE or/and AMB on liver oxidative stress markers liver malondialdehyde (MDA)
Liver MDA levels of IE, AMB, and IE + AMB groups were significantly (P< 0.05) decreased after 24 h and 72 hs of treatment, as compared with the infected untreated group (Table 4). Meanwhile, significant decrease (P< 0.05) was noticed in the level of liver MDA only after 72 hs of combined IE and AMB administration, as compared to the corresponding AMB group.
Table 4.
Effect of Sepia officinalis ink extract (IE) and Amphotericin B (AMB) on some hepatic oxidative stress markers in neutropenic mice withinvasive pulmonary aspergillosis (IPA).
| Groups | MDA | SOD | GSH | |||
| 24 h | 72 hs | 24 h | 72 hs | 24 h | 72 hs | |
| Infected untreated | 26.45 ± 0.72 | 21.43 ± 1.16 | 24.28 ± 1.25 | 21.92 ± 1.18 | 11.74 ± 0.46 | 8.92 ± 0.94 |
| AMB group | 7.28 ± 0.71a | 5.56 ± 0.36a | 38.1 ± 2.37a | 45.96 ± 1.80a | 19.52±1.18a | 24.74±2.45a |
| IE group | 5.36 ± 0.31a | 5.38 ± 0.61a | 41.52 ± 2.64a | 37.68 ± 2.36ab | 24.22±2.40a | 29.66±0.94ab |
| IE + AMB group | 5.86 ± 0.41a | 8.68 ±0.45ab | 48.02 ± 2.85ab | 47.24 ± 1.71a | 24.26±0.72a | 21.59 ±1.13a |
| ANOVA Two way | ||||||
| Treatment | p<0.0001 | p<0.0001 | p<0.0001 | |||
| Time | p<0.05 | NS | NS | |||
Data are presented as mean ± SEM (n=5 in each group).
Significantly different from infected untreated group at p<0.05.
Significantly different from AMB treated group at p<0.05.
Liver glutathione reduced (GSH)
Table 4 shows that, treatment with IE, AMB, and IE+ AMB for 24 h and 72 hs significantly (P< 0.05) increased the liver GSH levels, as compared with the infected untreated group (Table 4). Additionally, the liver GSH level of IE group was significantly (P< 0.05) higher than that of AMB group only after 72 hs of treatment.
Liver superoxide dismutase (SOD)
Liver SOD activities of all experimental groups at the two selected time periods were significantly (P< 0.05) higher than those of the infected untreated group (Table 4). However, in comparing with AMB group, the liver SOD activity of mice treated with IE for 72 hs and IE+ AMB for 24 h were only statistically of higher significant (P< 0.05).
Discussion
Invasive pulmonary aspergillosis (IPA) is one of the most frequent life-threatening fungal infections in immunocompromised patients, with a reported mortality rate of approximately 60% (Mucha et al., 2013). In the present study, invasive pulmonary aspergillosis (IPA) pathogenesis was induced by the intranasal instillation which, best mimics the natural route of infection in humans, in whom the lung is the primary target organ following conidia inhalation, especially during immunosuppression (Pasqualotto, 2008). At the site of infection, alveolar macrophage and polymorphonuclear cells, cellular components of innate defense of the lung cooperate to control and eliminate the fungus in the airways. Macrophages eliminate conidia, and protection against the hyphal form is mediated by polymorphonuclear (Dagenais and Keller, 2009).
Recently, Aspergillus fumigatus was demonstrated as it disseminated to liver following intravenous injection causing an intense inflammation (Mirkov et al., 2013). Moreover, A. flavus, another aspergillus species, has been reported as a potent hepatotoxic and hepatocarcinogenic agent in humans and various animal species (Ricordy et al., 2002). Liver failure occurs in severely immunocompromised patients and is accompanied by various infections, including IPA, is frequently fatal (Polson and Lee, 2005).
For the therapeutic strategies of IPA, murine models of aspergillosis have been used extensively in the study of antifungal drug efficacy (Xu et al., 2009). During the course of evolution, many invertebrates have established as a selective advantage by endogenous production of protective chemicals which may have a deleterious effect on other animals (Fahmy and Hamdi, 2011). So, the present study speculated that the ink extract (IE) of Sepia officinalis has antifungal efficacy against IPA, because it has radical scavenging ability based on its antioxidant activity (Fahmy and Soliman, 2013). The present study demonstrated good antifungal potential of IE of Sepia officinalis as compared to amphotericin B against A. fumigatus in vitro, as observed by a reduction in percentage of spore germination and a reduction in hepatic fungal burden. Marine mollusks are protecting themselves from predators through their unique way; one of them is releasing ink when disturbed (Vennila et al., 2011). The antimicrobial activity of Sepia officinalis ink extract may be attributed to the presence of active antimicrobial compounds in the extract. Our results went parallel with those obtained by Peruru et al. (2012), who concluded that the eumelanin extracted from Sepia officinalis( cuttle fish) ink showed strong antimicrobial activity. Our results are more or less similar to those obtained by Shrivastava et al. (2006), who documented broad antifungal efficacy of Sepia against A. niger. Moreover, the ink extract of Sepia pharaonis showed inhibitory effect against Pseudomonas aeruginosa, Staphylococcus epidermidis, Klebsiella pneumoniae (Nithya et al., 2011).
Aspergillus fumigatus produces a number of toxins. The most abundant mycotoxin produced by A. fumigatus is gliotoxin (GT) (Waring and Beaver, 1996). In murine models of IPA, GT was shown to inhibit macrophage and polymorphonuclear cell function, including phagocytosis and bactericidal activity (Watanabe et al., 2003). The results from the present work revealed that, ink extract and AMB significantly reduced the concentration of gliotoxin secreted by A. fumigatus in the liver tissues. Our results are in harmony with those obtained by Lewis et al. (2005), who documented that gliotoxin levels in mice with IPA reduced with antifungal therapy.
In the assessment of liver damage, the determination of enzyme levels such as serum AST and ALT is largely used. Their quantification in plasma is useful biomarkers of the extent and type of hepatocellular damage (Pari and Murugan, 2004). Therefore, they can be measured in the serum. High levels of serum AST indicate liver damage, such as that due to viral hepatitis as well as cardiac infraction and muscle injury. Serum ALT is more specific to the liver, and is thus a better parameter for detecting liver injury (Williamson et al., 1996). In conjunction with the reports of Davidson and Pappagianis (1995) and Shafiq and Al-Joofy (2010), data from the present study showed that IPA for 24 h and 72 hs, caused hepatic damage with a significant increase in serum levels of AST and ALT. Treatment with ink extract (IE) of Sepia officinalis alone or in combination with AMB at the tested dose significantly decreased the levels of serum AST and ALT activities, indicating maintenance of functional integrity of hepatic cell membrane. The hepatoprotective role of IE may be due to their antioxidant effects (Fahmy & Soliman, 2013). The present study showed that, at both 24 and 72 hours post-inoculation, microscopic examination of mice with invasive aspergilllosis revealed prominent microabcsses as well as diffuse inflammatory cell infiltrates with foci of cellular necrosis throughout the hepatic lobule, these cellular infiltrates together with the hepatocellular necrosis were maximal in the IPA-untreated control, while there are minimal in the AMB and IE-AMB-treated groups or absence in the IE-treated group of these features. It has been reported that, liver abscesses result from fungus are common, the organisms reach the liver through one of the following pathways: 1-Ascending infection in biliary tract. 2-Vascular seeding, either portal or arterial, 3-Direct invasion of the liver from a nearby source, or 4-a penetrating injury (Kumar et al., 2008). The magnitude of visible hepatocellular damage correlated well with the serum levels of the liver-associated enzymes ALT and AST, which were markedly increased in the infected untreated mice. It was also reported that at later stages of infection, the microabcesses enlarge into necrotic lesions that are poorly populated with neutrophils or monocytic cells (Zhang and Bliska, 2005).
Accumulating evidence suggests that, powerful reactive oxygen intermediates (ROS) are involved in many of the complex interactions between the invading microorganisms and its host (Miller and Britigan, 1997). Asperigillus fumigatus conidias are phagocytosed and killed by alveolar macrophages, using ROS (Xu et al., 2009). In apparent support of the present study, Xu et al. (2009) and Mahmoud et al. (2011) reported that exposure to A. fumigatus for 24 h and 72 h induced significant increase in the hepatic MDA level. It has been reported that increased MDA level suggests enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms to prevent formation of excessive free radicals (Park et al., 2010). Treatment with IE and/or AMB significantly decreased MDA levels, suggesting that the hepatic curative effect of them against IPA oxidative stress-induced injury might be involved in decreasing lipid peroxide generation and stimulating antioxidant enzyme. The present study extended the previously reported finding that, antioxidant and anti-inflammatory agents play a critical role in body protection by scavenging active oxygen and free radicals and neutralizing lipid peroxides (Fahmy & Soliman, 2013).
Glutathione (GSH) is the most abundant non-protein thiol compound present in mammalian cells and serves many important physiological roles, particularly as cellular antioxidant in peripheral tissue (Liu and Pravia, 2010). It acts as an electron donor in the glutathione peroxidase-catalysed reduction of organic and hydrogen peroxides. Insufficiency in non-enzymatic antioxidant GSH following A. fumigatus infection could be the consequence of increased utilization for trapping free radicals. The present study confirmed the finding of Thomas et al. (2009), who suggested that enhancement of lipid peroxidation is a consequence of depletion of GSH to certain critical levels. In the present study, the reduced glutathione level was significantly decreased in liver of IPA-untreated mice; whereas treatment of IE to IPA infected mice increased the GSH level. Furthermore, the toxic and immunosuppressive characteristics of GT towards the host's immune effector cells (Orciuolo et al., 2007) implied a substantial role of this compound in fungal pathogenicity. Bernardo et al. (2003) reported that, cellular uptake of GT is GSH-dependent as GSH is suggested to be involved in the possible detoxification of GT. It has been reported that, hepatocytes with high GSH levels rapidly and extensively metabolize GT, and proposed metabolites are the reduced dithiol form of GT and GT-GSH conjugates (Hagens et al., 2006).
The normal sequence of events in the release of reactive oxygen intermediates by phagocytic cells is the production of superoxide anion, which can either spontaneously or with the help of superoxide dismutase (SOD) form hydrogen peroxide (Klebanoff, 1980). A. fumigatus does not trigger an increased release of detectable amounts of superoxide anion (Robertson et al., 1987). Our results showed a significant decrease in SOD activity in liver of IPA-untreated group; whereas treatment of IE to IPA infected mice significantly increased the SOD activity. The present study extended the previously reported finding that SOD activity significantly decreased following A. fumigatus infection (Xu et al., 2009). It was found that great loss of SOD activity occurs with enhanced production of oxygen radicals by inflammatory cells (Comhair et al., 2000). SOD, a member of the first line of defense in the antioxidant system of living cells, works at preventing the production of free radicals (Halliwell and Gutteridge, 1999). Superfluous free radicals may damage protein and nucleic acids, and induces lipid peroxidation to produce large amounts of MDA that injures cells and results in disequilibrium of the internal environment and diseases (Liu et al., 2011). Consequently, SOD activity and MDA levels can reflect the antioxidant ability of body. In recent years, the antioxidant ability of squid ink was discovered (Zhong et al., 2009; Liu et al., 2011). Chen et al. (2007), showed that melanin of squid ink, like superoxide dismutase, can catalyze O2-to H2O2, and thus avoid the free radical chain reaction triggered by O2-. Moreover, (Zhang et al., 2003) reported that squid ink elevated SOD activity in the liver and kidney of mice in a dose-dependent manner. In consonance with our study Liu et al. (2009) ; Wang et al. (2009); Wang et al. (2010) and Zhong et al. (2009) have reported that, squid ink up regulated SOD activities and down regulated MDA contents in kidney, spleen, heart, lung and brain of mice. Therefore, IE-treated group showed significant antioxidant activity when compared with the IPA-untreated groups.
In conclusion, the present study demonstrated good in vitro and in vivo activity of ink extract of Sepia officinalis against A. fumigatus. IE significantly reduces hepatic fungal burden and returns liver histology and function to normal levels. The concomitant use of AMB with IE showed decreased therapeutic efficacy compared with that of each drug alone. This was evidenced by the nearly complete eradication of fungi, healing of hepatic granulomatous lesions and normalization of liver serum enzyme levels. Therefore, the IE treatment therapy may represent a cost-effective treatment regimen in developing countries. In combination with antifungal therapy (AMB), IE treatment can reduces hepatic fungal burden, alleviate hepatic granulomatous lesions and oxidative stress associated with IPA in neutropenic mice. Antioxidants in conjunction with antifungal treatment may thus be a more effective means of treating IPA in neutropenic hosts.
References
- 1.Aykaç G, Uysal M, Yalçin A, Koçak-Toker N, Sivas A, Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicol. 1985;36(1):71–76. doi: 10.1016/0300-483x(85)90008-3. [DOI] [PubMed] [Google Scholar]
- 2.Bai Q X, Huan Y, Wang J H, Yang L J, Dong H J. Successful treatment of liver aspergilloma by caspofungin acetate first-line therapy in a non-immunocompromised patient. Int J Mol Sci. 2012;13(9):11063–11070. doi: 10.3390/ijms130911063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker M J, de Marie S, Fens M H, Verbrugh H A, Bakker-Woudenberg I A. Effect of amphotericin B treatment on kinetics of cytokines and parameters of fungal load in neutropenic rats with invasive pulmonary aspergillosis. J Antimicrob Chemother. 2003;52(3):428–434. doi: 10.1093/jac/dkg367. [DOI] [PubMed] [Google Scholar]
- 4.Bernardo P H, Brasch N, Chai C L, Waring P. A novel redox mechanism for the glutathione-dependent reversible uptake of a fungal toxin in cells. J Biol Chem. 2003;278(47):46549–46555. doi: 10.1074/jbc.M304825200. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell RL. An observation of inking behavior protecting adult Octopus bocki from predation by Green Turtle (Cheloniamydas) hatchlings. Pac Sci. 2005;59:69–72. [Google Scholar]
- 6.Chauhan N, Latge J, Calderone R. Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat Rev Microbio. 2006;l4(6):435–444. doi: 10.1038/nrmicro1426. [DOI] [PubMed] [Google Scholar]
- 7.Chen S G, Xue C H, Xue Y, Li Z J, Gao X, Ma Q. Studies on the free radical scavenging activities of melanin from squid ink. Chin J Mar Drugs. 2007;26(1):24–27. [Google Scholar]
- 8.Comhair S A, Bhathena P R, Dweik R A, Kavuru M, Erzurum S C. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355(9204):624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- 9.Conn H J. Biological Stains: A handbook on the nature and uses of the dyes employed in the biological laboratory. 5ed ed. Biotech publication; 1946. p. 98. [Google Scholar]
- 10.Dagenais T R, Keller N P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev. 2009;22(3):447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson A P, Pappagianis D. Treatment of nasal aspergillosis with topical clotrimazole. In: Bonagura J D, editor. Kirk's current veterinary therapy xll: small animal practice. Philadelphia: W. B. Saunders; 1995. pp. 899–901. [Google Scholar]
- 12.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 13.Fahmy SR, Hamdi SAH. Curative effect of the Egyptian marine Erugosquilla massavensis extract on carbon tetrachloride-induced oxidative stress in rat liver and erythrocytes. Eur Rev Med Pharmacol Sci. 2011;15(3):303–312. [PubMed] [Google Scholar]
- 14.Fahmy S R, Soliman A M. In vitro antioxidant, analgesic and cytotoxic activities of Sepia officinalis ink and Coelatura aegyptiaca extracts. Afr J Pharm Pharmacol. 2013;7(22):1512–1522. [Google Scholar]
- 15.Funatsu Y, Fukami K, Kondo H, Watabe S. Improvement of “KurozukuriIka-Shiokara” (Fermented Squid Meat with Ink) odor with Staphylococcus Nepalensis isolated from the fish sauce mush of frigate mackerel Auxis Rochei. Bull Jpn Soc Fish. 2005;71:611–617. [Google Scholar]
- 16.Gerson SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, Cassileth PA. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100(3):345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- 17.Guang W, Jiang-Qiu P, Jie-Ping Z, Hua-Zhong L, Yan H, Jin-Long H, Kun L. Protective effect of Sepia ink extract against cyclophosphamideinduced oxidative damage in mice spleen [J] Food Sci. 2009;30(11):219–222. [Google Scholar]
- 18.Hagens W I, Olinga P, Meijer D K, Groothuis G M, Beljaars L, Poelstra K. Gliotoxin non-selectively induces apoptosis in fibrotic and normal livers. Liver Int. 2006;26(2):232–239. doi: 10.1111/j.1478-3231.2005.01212.x. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. third ed. Oxford: Oxford University Press; 1999. [Google Scholar]
- 20.Howard S J, Cerar D, Anderson M J, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009;15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim-Granet O, Philippe B, Boleti H, Boisvieux-Ulrich E, Grenet D, Stern M, Latgé JP. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect Immun. 2003;71:891–903. doi: 10.1128/IAI.71.2.891-903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff S J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Abbas A K, Fausto N, Mitchell R N. Robbins Basic Pathology. Philadelphia: Saunders Elsevier; 2008. Liver, Gallbladder, and Biliary Tract. [Google Scholar]
- 24.Kupfahl C, Heinekamp T, Geginat G, Ruppert T, Härtl A, Hof H, Brakhage A A. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol Microbiol. 2006;62(1):292–302. doi: 10.1111/j.1365-2958.2006.05373.x. [DOI] [PubMed] [Google Scholar]
- 25.Latgé J P. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–389. doi: 10.1016/s0966-842x(01)02104-7. [DOI] [PubMed] [Google Scholar]
- 26.Latgé J P. Aspergillus fumigatus and Aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leenders A C, de Marie S, ten Kate M T, Bakker-Woudenberg I A, Verbrugh H A. Liposomal amphotericin B (AmBisome) reduces dissemination of infection as compared with amphotericin B deoxycholate (Fungizone) in a rate model of pulmonary aspergillosis. J Antimicrob Chemother. 1996;38(2):215–225. doi: 10.1093/jac/38.2.215. [DOI] [PubMed] [Google Scholar]
- 28.Lei M, Wang J F, Pang L, Wang Y M, Chen S G, Xue C H. Effects of sepia on the metabolization of blood lipid and antioxidant ability in hyperlipidemia rats. Chin J Mar Drugs. 2007;3:30–33. [Google Scholar]
- 29.Lewis R E, Wiederhold N P, Chi J, Han X Y, Komanduri K V, Kontoyiannis D P, Prince R A. Detection of gliotoxin in experimental and human aspergillosis. Infect Immun. 2005;73(1):635–637. doi: 10.1128/IAI.73.1.635-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindequist U, Schweder T. Marine Biotechnology. In: Rehm HJ, Reed G, editors. Biotechnology. Vol. 10. Wiley-VCH, weinheimpp; 2001. pp. 441–484. [Google Scholar]
- 31.Liu H Z, Wang G, Guo Y Z, Pan J Q, Huang Y, Zhong J P, Li K. Protective effect of squid ink on cyclophosphamide-induced oxidative injury of kidney in mice. Chin J Nephrol. 2009;25(10):804–805. [Google Scholar]
- 32.Liu H, Luo P, Chen S, Shang J. Effects of squid ink on growth performance, antioxidant functions and immunity in growing broiler chickens. Asian-Aust J Anim Sci. 2011;24(12):1752–1756. [Google Scholar]
- 33.Liu RM, Pravia KAG. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med. 2010;48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmoud Y A, Al-Ghamdi A Y, Abd El-Zaher E H. A protective mechanism in lungs of rats experimentally infected with Aspergillus fumigatus. Mycobiology. 2011;39(1):40–44. doi: 10.4489/MYCO.2011.39.1.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manavathu E, Cutright J, Chandrasekar PH. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J Clin Microbiol. 1999;37:858–861. doi: 10.1128/jcm.37.3.858-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marr K A, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant. Clin Infect Dis. 2002;34(7):909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 37.Mayer AMS, Hamann MT. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol, (NY) 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller R A, Britigan B E. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev. 1997;10(1):1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirkov I, Glamoclija J, Stosic-Grujicic S, Zolotarevski L, Kataranovski D, Kataranovski M. Differential strain-related tissue immune response to sublethal systemic Aspergillus fumigatus infection in mice. APMIS. 2013;121(3):211–220. doi: 10.1111/j.1600-0463.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- 40.Morcillo E J, Estrela J, Cortijo J. Oxidative stress and pulmonary inflammation: pharmacological intervention with antioxidants. Pharmacol Res. 1999;40(5):393–404. doi: 10.1006/phrs.1999.0549. [DOI] [PubMed] [Google Scholar]
- 41.Mucha K, Foroncewicz B, Orłowski T, Religioni J, Bobek-Billewicz B, Jarząb B, Raczyńska J, Krawczyk M, Pączek L. A typical presentation of invasive pulmonary aspergillosis in a liver transplant recipient. Ann Transplant. 2013;18:238–242. doi: 10.12659/AOT.883921. [DOI] [PubMed] [Google Scholar]
- 42.Nair M G, Safir G R, Siqueira J O. Isolation and identification of vesicular arbuscular mycorrhiza stimulatory compounds from clover (Tnifoliumrepens) roots. Appl Environ Microbiol. 1991;57:434–439. doi: 10.1128/aem.57.2.434-439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neifar A, Ben Rebeh F, Gargouri A, Abdelmouleh A. Physicochemical characterisation of Sepia officinalis ink and the effects of storage conditions on the coagulation process. J Mar Biol Assoc UK. 2009;89(4):803–807. [Google Scholar]
- 44.Neifar A, Borgi I, Mnif R, Rebah F B, Abdelmouleh A, Gargouri A. Inhibitory effects of extracts from Tunisian marine species on serine-protease from a local Beauveria bassiana strain. Afr J Biotechnol. 2012;11(10):2504–2512. [Google Scholar]
- 45.Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 46.Nithya M, Ambikapathy V, Panneerselvam A. Effect of pharaoh's cuttlefish ink against bacterial pathogens. Asian J Plant Sci Res. 2011;1(4):49–55. [Google Scholar]
- 47.Obar J, Lehmann M, Caffrey A, Watschke C, Shepardson K, Barker B, Cramer R. IL-1α and IL-1β play non-redundant roles in limiting in vivo Aspergillus fumigatus pulmonary growth (P3092) J Immunol. 2013;190:125.20. [Google Scholar]
- 48.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 49.Orciuolo E, Stanzani M, Canestraro M, Galimberti S, Carulli G, Lewis R, Petrini M, Komanduri K V. Effects of Aspergillus fumigatus gliotoxin and methylprednisol24 h on human neutrophils: implications for the pathogenesis of invasive aspergillosis. J Leukoc Biol. 2007;82:839–848. doi: 10.1189/jlb.0207090. [DOI] [PubMed] [Google Scholar]
- 50.Pahl H L, Krauss B, Schulze-Osthoff K, Decker T, Traenckner E B, Vogt M, Myers C, Parks T, Warring P, Mühlbacher A, Czernilofsky A P, Baeuerle P A. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappa B. J Exp Med. 1996;183(4):1829–1840. doi: 10.1084/jem.183.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park H K, Kim S J, Kwon do Y, Park J H, Kim Y C. Protective effect of quercetin against paraquat-induced lung injury in rats. Life sci. 2010;87(5–6):181–186. doi: 10.1016/j.lfs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Pari L, Murugan P. Protective role of tetrahydrocurcumin against erythromycin estolate-induced hepatotoxicity. Pharmacol Res. 2004;49(5):481–486. doi: 10.1016/j.phrs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Pasqualotto A. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med Mycol. 2008:S1–S10. doi: 10.1080/13693780802247702. [DOI] [PubMed] [Google Scholar]
- 54.Peruru D, Ramesh S, Noor Ahmed V H, Sandeep, Priya S, Raju S, Nazan S, Begum S. Isolation of eumelanin from Sepia officinalis and investigation of its antimicrobial activity by ointment formulation. Int J Pharm. 2012;2(2):67–72. [Google Scholar]
- 55.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 56.Rajaganapathy J, Thyagarajam SP, Edward JK. Study on cephalopod's ink for anti-retroviral activity. Indian J Exp Biol. 2000;38:519–520. [PubMed] [Google Scholar]
- 57.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 58.Richarad J L, DeBey M C. Production of gliotoxin during the pathogenic state in turkey poults by Aspergillus fumigatus Fresenius. Mycopathologia. 1995;129:1110–115. doi: 10.1007/BF01103470. [DOI] [PubMed] [Google Scholar]
- 59.Ricordy R, Gensabella G, Cacci E, Augusti-Tocco G. Impairment of cell cycle progression by aflatoxin B1 in human cell lines. Mutagenesis. 2002;17(3):241–249. doi: 10.1093/mutage/17.3.241. [DOI] [PubMed] [Google Scholar]
- 60.Robertson M D, Seaton A, Milne L J, Raeburn J A. Suppression of host defences by Aspergillus fumigatus. Thorax. 1987;42:19–25. doi: 10.1136/thx.42.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russo A, Falc24 h M, Vena A, Venditti C, Mancini C, Morelli A, Venditti M. Invasive pulmonary aspergillosis in non-neutropenic patients: analysis of a 14-month prospective clinical experience. J Chemother. 2011;23(5):290–294. doi: 10.1179/joc.2011.23.5.290. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki J, Ishita K, Takaya Y, Uchiswa H, Matsue H. Antitumor activity of squid ink. J Nutr Sci Vitaminol. 1997;43:455–461. doi: 10.3177/jnsv.43.455. [DOI] [PubMed] [Google Scholar]
- 63.Sbaraglia G, D'Errico P, Serafini S, Vecchiarelli L, Perito S. Pathogenicity of various species of Candida in mice immunodepressed with cyclophosphamide. Boll SocItal Biol Sper. 1984;60:1421–1426. [PubMed] [Google Scholar]
- 64.Scott E M, Tariq V N, McCrory R M. Demonstration of synergy with fluconazole and either ibuprofen, sodium salicylate, or propylparaben against Candida albicans in vitro. Antimicrob Agents Chemother. 1995;39:2610–2614. doi: 10.1128/aac.39.12.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shafiq S A, Al-Joofy A K. Histopathological and enzymatic study on the effect of Aspergillus fumigatus in mice. J Fac Med Baghdad. 2010;52(4):480–483. [Google Scholar]
- 66.Shrivastava M K, Lipsky E M, Stanier C O, Robinson A L. Modeling semi volatile organic aerosol mass emissions from combustion systems. Environ Sci Technol. 2006;40(8):2671–2677. doi: 10.1021/es0522231. [DOI] [PubMed] [Google Scholar]
- 67.Stanzani M, Orciuolo E, Lewis R, Kontoyiannis D P, Martins S L, St John L S, Komanduri K V. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood. 2005;105:2258–2265. doi: 10.1182/blood-2004-09-3421. [DOI] [PubMed] [Google Scholar]
- 68.Thomas M, Sujatha K S, George S. Protective effect of Piper longum Linn. on monosodium glutamate induced oxidative stress in rats. Indian J Exp Biol. 2009;47:186–192. [PubMed] [Google Scholar]
- 69.Vadlapudi V. Antifungal resistance of few Aspergillus species. Pharmacophore. 2011;2(3):163–167. [Google Scholar]
- 70.Vanden Heuvel MJ, Clark DG, Fielder RJ, Koundakjian PP, Oliver GJA, Pelling D, Tomlinson NJ, Walker A. The international validation of a fixed-dose procedure as an alternative to the classical LD50 test. Food Chem Toxicol. 1990;28:469–482. doi: 10.1016/0278-6915(90)90117-6. [DOI] [PubMed] [Google Scholar]
- 71.Vennila R, Rajeshkumar R K, Kanchana S, Arumugam M, Balasubramanian T. Investigation of antimicrobial and plasma coagulation property of some molluscan ink extracts: Gastropods and cephalopods. Afr J Biochem Res. 2011;5(1):14–21. [Google Scholar]
- 72.Walsh T J, Anaissie E J, Denning D W, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 73.Wang G, Guo Y Z, Guan S B, Zhong J P, Pan J Q, Huang Y, Liu H Z. Protective effect of sepia ink extract on cyclophosphamide-induced pulmonary fibrosis in mice. Chin J Mar Drugs. 2009;28(1):36–40. [Google Scholar]
- 74.Wang G, Liu H Z, Wu J L, Zeng Q W, Chen Y P, Yang C L, Zhong J P. Study of sepia ink extract on protection from oxidative damage of cardiac muscle and brain tissue in mice. Chin JMAP. 2010;27(2):95–98. [Google Scholar]
- 75.Waring P, Beaver J. Gliotoxin and related epipolythiodioxopiperazines. Gen Pharmacol. 1996;27:1311–1316. doi: 10.1016/s0306-3623(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe A, Kamei K, Sekine T, Waku K, Nishimura M, Miyaji, Kuriyama T. Immunosuppressive substances in Aspergillus fumigatus culture filtrate. J Infect Chemother. 2003;9:114–121. doi: 10.1007/s10156-002-0227-1. [DOI] [PubMed] [Google Scholar]
- 77.Whitehead A, Curnow RN. Statistical evaluation of the fixed dose procedure. Food Chem Toxicol. 1992;30:313–324. doi: 10.1016/0278-6915(92)90009-a. [DOI] [PubMed] [Google Scholar]
- 78.Williamson E M, Okpako D T, Evans F J. Selection, preparation and pharmacological evaluation of plant material. England: John Wiley; 1996. p. 13. [Google Scholar]
- 79.Wu Z, Ling Z, Shao F, Sheng J, Li L. Invasive pulmonary aspergillosis in patients with acute-on-chronic liver failure. J Int Med Res. 2012;40(5):1958–1965. doi: 10.1177/030006051204000537. [DOI] [PubMed] [Google Scholar]
- 80.Xie G L, He S. Study of sepia improving natural killer cell activity in mice. J Chin Med Univ. 2002;1:23–24. [Google Scholar]
- 81.Xu P, Qu J M, Xu J F, Zhang J, Jiang H N, Zhang H J. NAC is associated with additional alleviation of lung injury induced by invasive pulmonary aspergillosis in a neutropenic model. Acta Pharmacol Sin. 2009;30(7):980–986. doi: 10.1038/aps.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Bliska J B. Role of Macrophage Apoptosis in the Pathogenesis of Yersinia. In: Griffin D E, editor. Role of apoptosis in infection. 2005 ed. Berlin Heidelberg: Springer; 2005. pp. 151–173. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y J, Xu X Y, Wang B Q. The influence of compound sepia capsules on SOD activity in mice. Chin New Med. 2003;2(1):23–27. [Google Scholar]
- 84.Zhao Y, Park S, Warn P, Shrief R, Harrison E, Perlin D S. Detection of Aspergillus fumigatus in a rat model of invasive pulmonary aspergillosis by real-time nucleic acid sequence-based amplification. J Clin Microbiol. 2010;48(4):1378–1383. doi: 10.1128/JCM.02214-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong J-P, Wang G, Shang J-H, Pan J-Q, Li K, Huang Y, Liu H-Z. Protective effects of squid ink extract towards hemopoietic injuries induced by cyclophosphamine. Mar Drugs. 2009;7(1):9–18. doi: 10.3390/md7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]