Abstract
Background
The antioxidant properties of ethanolic root extract of pepper fruit (Donnetia tripetala), and its effect on lipid peroxidation of some fresh beef tissues during frozen storage were investigated.
Materials and Methods
The antioxidant parameters were assessed using standard methods, while malondialdehyde levels of different fresh beef tissue sections treated with the extract prior to freezing, were estimated in a colorimetric reaction with thiobarbituric acid.
Results
The H2O2-scavenging ability of the extract was similar to that of ascorbic acid, with a maximum scavenging power of 55.61 ±4.98%, and an IC50 value of 86µg/ml. The extract exhibited a concentration-dependent ferric ion-reducing power, although this was significantly lower relative to that of the ascorbic acid (p < 0.05). The total phenolic content was 212.5 ± 0.002 mg/g, while the nitric oxide-scavenging ability was 64.33 ± 0.2% after 150 min. The capacity of the extract to inhibit lipid peroxidation in frozen heart muscle slices was significantly higher than that of vitamin C (p < 0 .05), but comparable to vitamins C and E in frozen testes and kidney slices.
Conclusion
These results suggest that the root extract of D. tripetala is rich in antioxidants which can be applied to meat preservation during refrigerated storage.
Keywords: Donnetia tripetala, Root Extract, Antioxidants, lipid peroxidation, Beef
Introduction
Studies on biochemical basis for therapeutic properties of medicinal plants have become popular in recent times. These investigations aim at unraveling the scientific basis for various claims of traditional healers on the therapeutic effects of roots and herbs. Dennetia tripetala G. Baker (family Annonaceae), is a well-known forest and spicy medicinal plant found in the rainforest zone of Africa (Ejechi et al. 1999; Okwu and Morah, 2004). The fruits of the tree plant, popularly called pepper fruit in Nigeria, are edible and have a fiery but spicy taste. In some Nigerian communities, the fruits are used for the treatment of cough, fever and toothache (Adebayo et al. 2010). It also serves as mild stimulant, source of some vitamins, appetite stimulant, mouth wash and anti-pyretic (Gill, 1992; Enwere, 1998; Keay, 1989). The fruits are rich in antioxidants. A survey of existing literature shows that pepper fruits contain Dennettia essential oils and phenolic acids (Anyaele and Amusan, 2003) as well as alkaloids (Rossanan et al. 2003). Vanillin, dennettine (a new 2, 6-dimethoxy-chrome), argentinine and uvariopsine have been isolated from D. tripetala (Janview et al. 2002). The antioxidant properties of either the ripe and unripe fruits of D. tripetala have been reported (Adebayo et al. 2010; Oboh and Rocha, 2007). In addition, the fruit extracts have been shown to be active antifungal agents (Adedire and Akinkurolere, 2005).
Although the roots of D. tripetala are used ethno-medicinally for the treatment of cough, inflammation, and as vitamin supplement (Iwu, 1993), scientific investigations have so far focused mainly on the edible fruits. Thus not much is known about phytochemical composition of the roots and their antioxidant properties. The present study was therefore carried out to determine the phytochemical composition and the antioxidant potential of roots of D. tripetala.
Material and Methods
Collection of plant material
Fresh sample of D. tripetala roots were obtained from Utteh Village, behind Ugbowo Campus of the University of Benin, Benin City, Nigeria. The identification and authentication of the plant, was done by Dr.O. Osawaru, of the Department of Plant Biology & Biotechnology, University of Benin.
Preparation of plant extract
The roots of D. tripetala (voucher No. 15356) were washed with distilled water and chopped into small pieces using a table knife. The pieces were then sun-dried and ground to powder in a grinder. 2kg of the powdered sample was suspended in 5L of 98% (v/v) ethanol for 24 hrs. Thereafter, the suspension was filtered through a clean piece of cheese cloth. The resultant ethanolic extract was concentrated in a rotary evaporator at 40°C. Using the dry extract, a stock concentration of 1,000µg/ml was prepared in methanol. From the stock solution, different concentrations viz. 10, 20, 40, 60, 80 and 100µg/ml were prepared in methanol and used for the antioxidant studies.
Phytochemical screening
The extract was screened for presence of alkaloids, saponins, tannins and flavonoids using the colorimetric methods outlined in Trease and Evans (1989).
Determination of Hydrogen Peroxide Scavenging Capacity
The hydrogen peroxide (H2O2)-scavenging activity of the extract was determined by the method of {Srinivasan et al. 2007}. A solution of hydrogen peroxide (20 mM), was prepared in phosphate buffered saline (pH 7.4). Different concentrations of plant extract and standard ascorbic acid solution viz: 10, 20, 40, 60, 80 and 100 µg/ml in methanol (1 ml), were separately added to H2O2 solution (2 ml), and mixed. Each tube was allowed to stand for 10 min after which absorbance at 230 nm was read against a blank solution containing phosphate buffer in place of H2O2.The percentage inhibition activity was calculated as follows:
- % inhibition = [(A0−A1)/A0]× 100,
- Where A0 is the absorbance of the control, and
- A1 is the absorbance of extract/standard.
The antioxidant activity of the extract was expressed as IC50. All the tests were performed in triplicate determination.
Determination of ferric reducing power
The method described by Kumaran and Karunakaran (2007) was employed. Different concentrations of plant extract and standard ascorbic acid solution viz. 10, 20, 40, 60, 80 and 100 µg/mL in 1 mL of methanol were mixed with 0.2M phosphate buffer (2.5 mL, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50° C for 20 min. A portion (2.5 mL), of trichloroacetic acid (10%), was added to the mixture, which was then centrifuged at 3,000 g for 10 min at room temperature. The upper layer of solution (2.5 mL), was mixed with distilled water (2.5 mL), and 0.5 mL of 0.1% FeCl3 solution. The absorbance, which reflects increase in reducing power, was measured at 700 nm. All the tests were performed in triplicate.
Determination of Nitric Oxide (NO) scavenging activity
The NO scavenging activity of the extract was determined by the method of Daljit and Priyanka (2010). An aliquot (6 mL), of 5 mM sodium nitroprusside solution was mixed with 6 ml of extract and incubated at 25°C for two and half hours. 0.5 mL of the reaction mixture was removed at 30 min intervals, and mixed with an equal 0.5 mL of Griess reagent (1 % sulphanilamide, 2 % phosphoric acid, and 0.1 % napthylethylene diamine dihydrochloride). The absorbance of the mixture was then read at 546 nm and compared with absorbance of 1 mg/mL standard solution (sodium nitrite), treated in the same way with Griess reagent.
Determination of Total Phenolic Content (TPC)
The total polyphenolic content was determined colorimetrically using the Folin-Ciocalteau (FC), method of Daljit and Priyanka, 2010, with some modifications. Test sample (0.5 mL), was mixed with 0.2 mL of FC reagent and allowed to stand for 10 min. Then 0.6 mL of 20 % sodium carbonate was added with mixing, and the reaction mixture was incubated at 40°C for 30 min. before absorbance was measured at 765 nm. The polyphenolic contents were expressed as gallic acid equivalents. The determination was carried out in triplicates.
Determination of Ascorbic acid
Ascorbic acid was determined using dinitrophenylhydrazine (DNPH), as described previously (Omaye et al. 1979). To each 1.5 mL of 6 % trichloroacetic acid (TCA), was added 0.5 mL of sample and the mixture centrifuged at 3500 g for 20 min. 0.5 mL of the supernatant was then added to 0.5 mL of DNPH reagent (2 % DNPH and 4 % thiourea in 9 M sulphuric acid). The solution was incubated for 3 hrs at room temperature, followed by the addition of 2.5 mL of 85 % H2SO4. Absorbance was read at 530 nm after 30 minutes. The assay was done in triplicates, and ascorbic acid content was calculated using the relation:
[OD of test/OD of standard] × concentration of standard
Values were expressed in ug/g of extract.
Determination of Vitamin E
Vitamin E was assayed by the method of Desai (1984). It was first extracted by the addition of 1.6 mL ethanol and 2.0 mL petroleum ether to 0.5 mL of sample. The mixture was stirred and allowed to stand for 10 min at room temperature. It was then clarified by centrifugation, and the supernatant obtained was evaporated to dryness. To the residue, 0.2 mL of 0.2 % 2, 2-dipyridyl and 0.2 mL of 0.5 % ferric chloride were added and the mixture was kept in the dark for 5 mins. Thereafter 4 mL of butanol was added, and the absorbance of the resultant intense red colored layer was read at 520 nm. The vitamin E content was extrapolated from a standard curve in which serial dilutions of vitamin E were similarly treated as extract. Assays in extract were done in triplicate and results were expressed in mg/g of extract.
Determination of Inhibitory Capacity of Extract on Lipid Peroxidation
This was measured against vitamin C and vitamin E with proven capacities as antioxidants and inhibitors of lipid peroxidation (Padayatty et al. 2003). Different organs -liver, testis, kidney, heart and muscle from a freshly slaughtered cow were obtained from an abattoir in Benin City, Edo State, Nigeria. The samples were transported to our laboratory in ice packs, and used immediately on arrival. 1 g portions of each sample were separately placed in 50 mL of each of the following solutions: 5 % solution of D. tripetala root extract, 5 % ascorbic acid, 5% vitamin E, and distilled water. The samples were kept frozen at 4°C in a deep freezer, and analyzed for malondialdehyde (MDA), levels at 48 hour-intervals for 35 days. MDA was determined colorimetrically using thiobarbituric acid (TBA), as described by Okolie et al. (2009). In essence, 1 g of the chilled tissue sample was homogenized with 100 mL of physiological saline for 15 min. The homogenate was centrifuged at 8000 g for 5 min. 1 mL of the supernatant was then added to 2.0 mL of TCA-TBA -HCl reagent (15 % TCA, 0.375 % TBA, 0.25 M HCl), and the solution was mixed thoroughly and placed in a boiling water bath for 15 min. On cooling, the protein precipitate was removed by centrifugation at 10,000 g for 5 min and the absorbance of the clear, pink supernatant fraction was read at 535 nm against reagent blank. MDA values were calculated from the molar extinction coefficient and expressed as µmole/g fresh weight.
Statistics
All assays were performed in triplicates, and results expressed as Mean ± SD. Where appropriate, analysis of variance, ANOVA was used for determination of statistical differences between samples. P values < 0.05 were taken as significant.
Results and Discussion
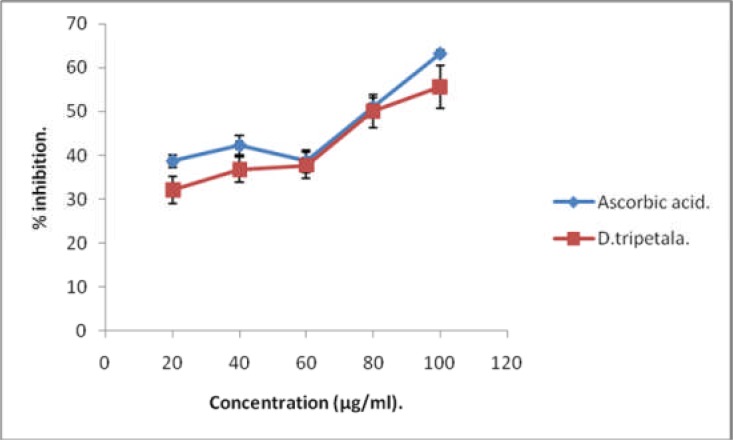
The biochemical basis for medicinal properties of plants has continued to engage the attention of researchers. In these investigations, antioxidant parameters are frequently focused on. This is informed by the fact that the pathogenesis of many human diseases is traceable to imbalance between pro-oxidants and anti-oxidants (Rosen et al. 2001; Poulsen, 2005; Rahman, 2005; Paravicini et al. 2008; Zhang et al. 2009). Several studies have consistently reported that the medicinal properties of plants are largely attributable to their phytochemical antioxidant contents (Choo and Yong, 2011; Kim et al. 2007). Table 1 shows results of qualitative analysis of phytochemical composition of the ethanolic root extract of D. tripetala. The extract contains alkaloids, flavonoids, tannins and saponins. The levels of vitamins\ C and E, as well as the total phenolic content of the extract are shown in Table 3.The extract is rich in polyphenolic and vitamin E, but the level of vitamin C is very low. The total phenolic content value of 212 mg gallic acid equivalent/g obtained for the root extract of D. tripetala in this study is quite higher than values of 1.4 and 1.0 mg/g reported for the ripe and unripe fruits of the plant respectively (Adebayo et al. 2010). It is also higher than the values of total phenols reported for ripe and unripe fruits of Capsicum pubescens (Oboh and Rocha, 2007). This shows that the roots of D. tripetala which are often neglected may be of greater medicinal value than the fruits. Phenolic compounds act as antioxidants through mechanisms which include donation of protons, quenching of singlet oxygen and chelating of metals (Rice-Evans et al., 1996; Heim et al. 2004; Abdel-Hameed, 2009; Abdel-Hameed, 2012). Figure 1 depicts the H2O2 - scavenging capacity of the extract relative to that of standard ascorbate. The extract showed concentration-dependent H2O2 -scavenging capacity which was comparable to that of the ascorbic acid, with an IC50 value of 86.79 µg/ml.
Table 1.
qualitative phytochemical composition of root extract of D. tripetala
| Phytochemical class | Status |
| Benzylisoquinoline alkaloids | +ve |
| Indole alkaloids | +ve |
| Tropane alkaloids | +ve |
| Alkaloids | +ve |
| Tannins | +ve |
| Saponins | +ve |
| Flavonoids | +ve |
+ve = present
Table 3.
Nitric oxide (NO) scavenging activity of D. tripetala extract (% activity)
| Time (min) | % Activity (Mean ± SD) |
| 30 | 49.47 ± 0.70 |
| 60 | 53.19 ± 0.90 |
| 90 | 57.49 ± 0.20 |
| 120 | 61.09 ± 0.20 |
| 150 | 64.33 ± 0.20 |
Values are mean ± SD (n = 3)
Figure 1.
H2O2-scavenging activity of D. tripetala root extract relative to ascorbic acid
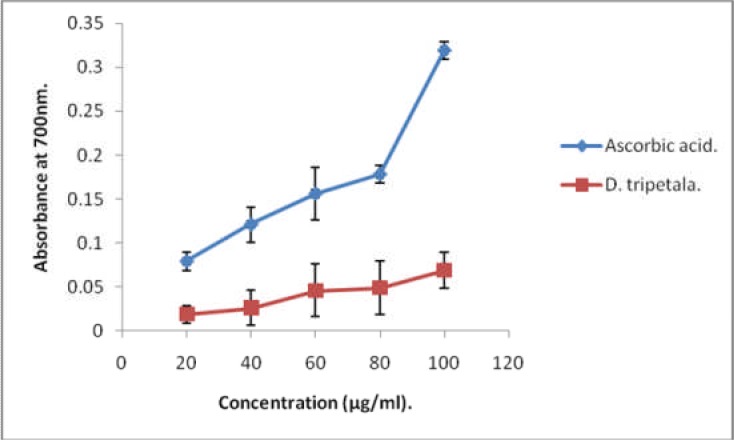
Thus, the high levels of these polyphenols in the root extract of D. tripetala may account for the fact that the H2O2 scavenging activity of the extract was comparable to that of standard ascorbic acid. In addition, the vitamin E content of D. tripetala root extract (2.67 ± 0.02 mg/g,) was very high, considering the notion that roots, with a few exceptions are generally poor sources of the vitamin. However the root content of vitamin C (0.14 µg/g), was much lower than that reported for the fruits (Adebayo et al. 2010). This is not surprising, since fruits are generally better sources of vitamin C than roots. Figure 2 shows the ferric ion reducing potential of the ethanolic root extract of D. tripetala. The ability of the extract to reduce ferric ions was concentration-dependent, but it was significantly lower than that of standard ascorbic acid (P < 0.05). The NO-scavenging activity of the extract is shown on Table 2. This was time-dependent, with a maximum value of 64 % after 150 min. Thus, the various antioxidant properties exhibited by the root extract such as ferric ion reducing, as well as NO -and H2O2 -scavenging activities may be due mainly to vitamin E and polyphenols. Tables' 4–8 show results obtained in the comparison of the capacity of the extract to inhibit lipid peroxidation in some beef tissue slices with those of vitamin C, vitamin E and distilled water. The ability of the extract to inhibit lipid peroxidation (i.e. decrease MDA levels) in frozen heart muscle slices within 10 days was significantly higher than that of vitamin C (p < 0.05; Table 7), but comparable to those of vitamin C and vitamin E in frozen testes and kidney slices (Table 6 and Table 8 respectively). However, the ability of the extract to inhibit MDA formation was significantly lower in beef liver and muscle when compared with either vitamin C or vitamin E (Table 4 and Table 5 respectively). Tissues frozen in water medium had the highest MDA in virtually all cases.
Figure 2.
Ferric reducing power of D. tripetala extract and ascorbic acid
Table 2.
vitamin C, vitamin E and total phenolic content (tpc) of D. tripetala oots
| Compounds | Concentration |
| Vitamin C | 0.14 ± 0.020 µg/g |
| Vitamin E | 2.67 ± 0.002 mg/g |
| Total Phenolic Content (TPC) | 212.50 ± 0.002 mg gallic acid/g |
(Values are mean ±SD of three determinations for each parameter)
Table 4.
MDA levels in liver tissue slices frozen in various media (µmole MDA/g)
| Days of incubation |
Incubation Medium | |||
| D. tripetala | Vitamin E | Vitamin C | Distilled Water | |
| 0 | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND |
| 4 | ND | ND | ND | 0.25 ± 0.10 |
| 6 | 0.26 ± 0.01 | ND | ND | 0.25± 0.10 |
| 8 | 0.26 ± 0.02 | ND | ND | 0.45 ± 0.10 |
| 10 | 0.26 ± 0.01a | 0.32 ± 0.01b | 0.26 ± 0.10a | 0.58 ± 0.20c |
| 35 | 4.49 ± 1.10a | 2.56 ± 1.10b | 4.49 ± 1.10a | 6.41 ± 1.10c |
Values are mean ± SD (n = 3). At each incubation period, values with different superscripts across differ significantly (p < 0.05). ND: not detected.
Table 8.
MDA content of kidney sections frozen in various media (µmole/g)
| Days of incubation |
Incubation Medium | |||
| D. tripetala | Vitamin E | Vitamin C | Distilled Water | |
| 0 | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND |
| 6 | ND | ND | ND | ND |
| 8 | 0.25 ± 0.01a | 0.26 ± 0.03a | 0.31 ± 0.02a | 0.32 ± 0.01a,b |
| 10 | 0.26 ± 0.03a | 0.25 ± 0.01a | 0.25 ± 0.01a | 0.26 ± 0.01a |
| 35 | 4.49 ±1.06a | 1.92 ± 1.10b | 2.50 ± 1.10c | 6.49 ± 1.10d |
Values are mean ± SD (n = 3). At each incubation period, values with different superscripts across differ significantly (p < 0.05). ND: not detected.
Table 7.
MDA content of heart muscle slices frozen in different media (µmole /g)
| Days of incubation |
Incubation Medium | |||
| D. tripetala | Vitamin E | Vitamin C | Distilled Water | |
| 0 | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND |
| 6 | ND | ND | 0.26 ± 0.01 | 0.45 ± 0.10 |
| 8 | 0.25 ± 0.03a | ND | 0.32 ± 0.01b | 0.45 ± 0.10c |
| 10 | 0.25 ± 0.01a | 0.26 ± 0.01a | 0.32 ± 0.02a,b | 0.51 ± 0.01c |
| 35 | 4.49 ±1.00a | 3.21 ± 0.02b | 3.21 ±0.10b | 5.78 ± 1.11c |
Values are mean ± SD (n =3). At each incubation period, values with different superscripts across differ significantly (p < 0.05). ND: not detected.
Table 6.
MDA content of testes slices frozen in various media (µmole /g)
| Days of incubation |
Incubation Medium | |||
| D. tripetala | Vitamin E | Vitamin C | Distilled Water | |
| 0 | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND |
| 6 | 0.25 ± 0.01 | ND | 0.26 ± 0.01 | 0.45 ± 0.02 |
| 8 | 0.26 ± 0.03a | 0.26 ± 0.02a | 0.32 ± 0.01a | 0.45 ± 0.01b |
| 10 | 0.32 ± 0.01a | 0.26 ± 0.01a | 0.32 ± 0.02a | 0.51 ± 0.01b |
| 35 | 3.20 ± 0.11a | 3.20 ± 0.08a | 3.20 ± 0.10a | 5.76 ± 1.20b |
Values are mean ± SD (n = 3). At each incubation period, values with different superscripts across differ significantly (p < 0.05). ND: not detected.
Table 5.
MDA content of muscle slices frozen in various media (µmole/g)
| Days of incubation |
Incubation Medium | |||
| D. tripetala | Vitamin E | Vitamin C | Distilled Water | |
| 0 | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND |
| 6 | 0.26 ± 0.10 | ND | ND | 0.26 ± 0.01 |
| 8 | 0.26 ± 0.10 | ND | 0.26 ± 0.10 | 0.32 ± 0.10 |
| 10 | 0.51 ± 0.10a | 0.26 ± 0.01b | 0.26 ± 0.10b | 0.45 ± 0.10c |
| 35 | 5.15 ±1.10a | 0.45 ± 0.01b | 3.21 ± 1.10c | 6.41 ± 1.21d |
Values are mean ± SD (n = 3). At each incubation period, values with different superscripts across differ significantly (p < 0.05). ND: not detected.
The ability of the extract to inhibit lipid peroxidation in some frozen beef tissue slices is particularly interesting, and further confirms its significant antioxidant potential. Although the extract was not as effective as the antioxidant vitamins in arresting MDA formation in beef muscle and liver, it was nonetheless better than vitamin C in inhibiting lipid peroxidation in heart muscle slices, and exhibited the same level of protection as vitamins C and E with respect to kidney and testes. This shows that the extract may be of beneficial application in refrigerated storage of meat and meat products in homes and cold storage facilities. This is based on the fact that lipid peroxidation was regarded as a major source of quality deterioration, not only in meat and meat products but also in fish and fish products. The disparity with respect to ability of the extract to inhibit lipid peroxidation in liver and muscle is consistent with the finding that the susceptibility to lipid peroxidation was a function of muscle type and anatomical location (Rhee and Ziprin, 2000; Rhee et al. 1996). Aside from the production of undesirable changes in flavor and loss of food quality, lipid peroxidation increases the level MDA in foods. Studies have revealed that MDA is one of the most abundant lipid peroxidation cytotoxins formed in foods, especially in meat (Stocker and Keaney, 2005; Gorelick et al. 2007). Earlier reports on the mutagenic and carcinogenic potentials of MDA (Shamberger et al. 1970; Hadley and Drager, 1989), have since led to increased interest in its implication for human health. Following ingestion of peroxidised foods, humans and lower animals have been shown to excrete increased amounts of MDA in the urine (Hadley and Drager, 1989). Indeed it is now known that MDA can be absorbed from tainted foods when these foods are ingested (Gopaul et al. 2000; Giron-Calle et al. 2002). MDA is genotoxic, reacting with DNA to form highly mutagenic adducts in human cells (Riggins et al. 2001; Del-Rio et al. 2005). Consequently lipid peroxidation in foods is deleterious to human health. Thus the ability of the root extract of D. tripetala to inhibit lipid peroxidation in the frozen beef tissues is highly desirable, especially since it has been demonstrated that lipid peroxidation also occurs even during refrigerated storage of meat and fish (Okolie et al. 2009; Okolie et al. 2013).
Conclusion
This study has for the first time, demonstrated experimental evidence for antioxidant properties of roots of D. tripetala. These properties can be ascribed to the high levels of antioxidant phytochemicals present in the root. These findings are considered crucial, for they may provide a biochemical basis for some of the medicinal uses of the roots of D. tripetala.
Acknowledgement
Special thanks to the University of Benin and STEP B for grants to execute the research work.
References
- 1.Abdel-hameed E S. Total phenolic contents .and free radical scavenging activity of certain Egyptian Ficus species leaf samples. J Biogeography. 2009;31:475–485. [Google Scholar]
- 2.Abdel-hameed ES, Salih A B, Shohayeb MM. Total Phenolics and antioxidant activity of defatted fresh taif rose, Saudi Arabia. Br J Pharm Res. 2012;2:129–140. [Google Scholar]
- 3.Adedire CO, Akinkurolere RO. Bioactivity of four plants extracts on Coleopterous pests of stored cereals and grain legumes in Nigeria. Zool Res. 2005;26:234–239. [Google Scholar]
- 4.Anyaele OO, Amusan AAS. Toxicity of hexanolic extract of Donnetia tripetala (G.Baxer) on larvae of Aedes aegypti (L) Afr J Biomed Res. 2003;6:49–53. [Google Scholar]
- 5.Adedayo B C, Oboh G, Akindahunsi A. Changes in the total phenolic content and antioxidant properties of pepperfruit (Dennettia tripetala) with ripening. Afr J Food Sci. 2010;46:403–409. [Google Scholar]
- 6.Choo WS, Yong WK. Antioxidant properties of two species of Hylocereus fruits. Adv Appl Sci Res. 2011;2:418–425. [Google Scholar]
- 7.Daljit S A, Priyanka C. Assay of antioxidant potential of two Aspergillus isolates by different methods under various physiochemical conditions. Braz J Microbiol. 2010;41:465–467. doi: 10.1590/S1517-83822010000300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del-rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiov Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Desai FD. Vitamin E analysis methods for animal tissues. Methods Enzymol. 1984;105:138–147. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- 10.Enwere N J. Foods of plant origin. Nsukka: Afro-Orbit Publications Ltd., University of Nigeria; 1998. [Google Scholar]
- 11.Ejechi BO, Nwafor O E, Okoko F J. Growth inhibition of tomato rots fungi by phenolic acids and essential oil extracts of pepperfruit (Dennetia tripetala G. Barker) Food Res Int. 1999;32:395–399. [Google Scholar]
- 12.Gill LS. Ethnomedical uses of plant in Nigeria. Benin-city, Nigeria: University of Benin Press, Benin-City; 1992. [Google Scholar]
- 13.Gopaul NK, Halliwell B, Anggard EE. Measurement of plasma F2 isoprostanes as an index of lipid peroxidation does not appear to be compounded by diet. Free Rad Res. 2000;33:115–127. doi: 10.1080/10715760000300671. [DOI] [PubMed] [Google Scholar]
- 14.Gorelick S, Kohen R, Ligumsky M, Kanner J. Saliva plays a dual role in oxidative process in stomach medium. Arch Biochem Biophys. 2007;458:236–243. doi: 10.1016/j.abb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Giron-calle J, Alaiz M, Millan M, Ruiz-gutienez V, Viague E. Bound malondialdehyde in foods: bioavailability of N-2-propenals of lysine. J Agric Food Chem. 2002;50:6149–6198. doi: 10.1021/jf025681r. [DOI] [PubMed] [Google Scholar]
- 16.Hadley M, Draper HH. Identification of N-(2-propenal) ethanolamine as a urinary metabolite of malondialdehyde. Free Rad Biol Med. 1989;6:49–52. doi: 10.1016/0891-5849(89)90159-7. [DOI] [PubMed] [Google Scholar]
- 17.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: Chemistry,metabolism and structure-activity relationships. J Nutr Biochem. 2004;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 18.Iwu MM. Handbook of African Medicinal Plants. 1st edn. Boca Raton, Florida: CRC Press; 1993. [Google Scholar]
- 19.Janview L M, Edet M, Herminio B, Sanz MJ, Maria A. Chromone and phenathrene alkaloids from Dennettia tripetala. Chem Pharm Bull. 2002;20:1613–1617. doi: 10.1248/cpb.50.1613. [DOI] [PubMed] [Google Scholar]
- 20.Keay R W J. Trees in Nigeria. UK: Clarendon Press Oxford; 1989. pp. 19–30. [Google Scholar]
- 21.Kim YY, Lim TT, Tee JJ. Antioxidant properties of several tropical fruits: A comparative study. Food Chemistry. 2007;103:1003–1008. [Google Scholar]
- 22.Kumaran A, Karunakaran JR. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. Food Sci Technol. 2007;40:344–352. [Google Scholar]
- 23.Oboh G, Rocha J B T. Polyphenols in red pepper [Capsicum annuum var. aviculare (Tepin)] and their protective effect on some pro-oxidants induced lipid peroxidation in brain and liver in vitro. Eur Food Res Technol. 2007b;225:247. [Google Scholar]
- 24.Okolie NP, Okugbo TO. A comparative study of malondialdehyde contents of some meat and fish samples processed by different methods. J Pharm Sci Innov. 2013;2:26–29. [Google Scholar]
- 25.Okolie N P, Akioyamen M O 1, Okpoba N, Okonkwo C. Malondialdehyde levels of frozen fish, chicken and turkey on sale in Benin City markets. Afr J Biotech. 2009;8:6638–6640. [Google Scholar]
- 26.Okwu D E, Morah FN I. Mineral and nutritive value of Dennettia tripetala fruits. Fruit Paris. 2004;59:439–442. [Google Scholar]
- 27.Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 28.Padayatty S, Katz A, Wang Y, Eck P, Kwon O, Lee J, Chen S, Corpe C, Dutta A, Dutta S, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 29.Paravicini TM, Touyz R. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;2:170–180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 30.Poulsen HE. Oxidative DNA modifications. Exp Toxicol Pathol. 2005;57:161–169. doi: 10.1016/j.etp.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Rahman I. The role of oxidative stress in the pathogenesis of COPD: implications for therapy. Treat Respir Med. 2005;4:175–200. doi: 10.2165/00151829-200504030-00003. [DOI] [PubMed] [Google Scholar]
- 32.Rhee KS, Ziprin YS. Lipid peroxidation in retail beef, pork and chicken muscles as affected by concentration of heme pigments and non-heme iron and microsomal enzymic lipid peroxidation activity. Food Biochem. 2000;11:1–15. [Google Scholar]
- 33.Rhee KS, Anderson LM, SAMS AR. Lipid peroxidation potential of beef, chicken and pork. J Food Sci. 1996;61:8–12. [Google Scholar]
- 34.Rice-Evans C, Miller NJ, Paganga G. Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radical Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 35.Riggins JN, Marnett LJ. Mutagenicity of the malondialdehyde oligomerisation products 2-(3-oxo-1-propenyl) malondialdehyde and 2, 3-dihydroxymethylene-3-(2,2-dimethoxyethyl) glutaraldeyhde in Salmonella. Mut Res. 2001;497:153–157. doi: 10.1016/s1383-5718(01)00253-4. [DOI] [PubMed] [Google Scholar]
- 36.Rossana E, Javier L, Lara M, José-enrique O, Magdalena M, Miguel C, Edet MA, María D I, Andrew C I, Julio C, Esteban JM, María AB, María-jesús S. Effect of two phenanthrene alkaloids on angiotensin II-induced leukocyte-endothelial cell interactions in vivo. Br J Pharmacol. 2003;140:1057–1067. doi: 10.1038/sj.bjp.0705525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen P, Nawroth King PP, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a congress series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes/Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 38.Shamberger R, Andreone T, Willis C. Antioxidants and cancer. IV. Initiating activity of malondialdehyde as a carcinogen. J Nat Cancer Inst. 1974;53:1771–1773. [PubMed] [Google Scholar]
- 39.Srinivasan R, Chandrasekar MJN, Nanjan M J, Suresh B. Free Radical scavenging activity of Ipomoea obscura (L.) Ker-Gawl. J Nat Remed. 2007;7:184–188. [Google Scholar]
- 40.Stocker R, Keaney JF JR. New insight on oxidative stress in the artery wall. J Thromb Haemost. 2005;3:1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 41.Trease G E, Evans WC. Pharmacognosy. 13th ed. Bailliere Tindall, Britain: English Language Book Society; 1989. pp. 378pp. 386–480. [Google Scholar]
- 42.Zhang L, Wang M, Kang X, Boontheung P, LI N, Nel AE, Loo JA. Oxidative stress and asthma: proteome analysis of chitinase-like proteins and FIZZI in lung tissue and bronchoalveolar lavage fluid. J Proteome Res. 2009;8:1631–1638. doi: 10.1021/pr800685h. [DOI] [PMC free article] [PubMed] [Google Scholar]