Abstract
Background
Ganoderma lucidum (Ling Zhi), a basidiomycete white rot macrofungus has been used extensively for therapeutic use in China, Japan, Korea and other Asian countries for 2,000 years. The present study is an attempt to investigate its DNA protecting property in human lymphocytes.
Materials and methods
Beta glucan (BG) was isolated by standard procedure and the structure and composition were studied by infrared radiation (IR) and nuclear magnetic resonance (NMR) spectroscopy, gel filtration chromatography and paper chromatography. The radioprotective properties of BG isolated from the macro fungi Ganoderma lucidum was assessed by single cell gel electrophoresis (comet assay). Human lymphocytes were exposed to 0, 1, 2 and 4 Gy gamma radiation in the presence and absence of BG.
Results
The comet parameters were reduced by BG. The results indicate that the BG of G. lucidum possessed significant radioprotective activity with DNA repairing ability and antioxidant activity as the suggestive mechanism.
Conclusions
The findings suggest the potential use of this mushroom for the prevention of radiation induced cellular damages.
Keywords: Fungi, polysaccharides, Ganoderma lucidum, DNA damage, radioprotection, comet assay, gel filtration
Introduction
Macrofungi are distinguished as important natural resources of immunomodulating and anticancer agents and with regard to the increase in diseases involving immune dysfunction, cancer, autoimmune conditions in recent years, applying such immunomodulator agents especially with the natural original is vital. Beta glucans (BG) are only recently recognized as the effective ingredients (1,2). Subsequently, detailed investigations into their influence on health have been reported, mainly using animal models (3). Several fungal BG appear to be effective immunomodulators, and they appear to impact positively on cancers and several bacterial infections (3,4). In the last three decades, numerous polysaccharides and polysaccharide-protein complexes have been isolated from mushrooms and used as a source of therapeutic agents. A polysaccharide peptide (PSP), isolated from a strain of Coriolus versicolor in China, has been widely used as an anti-cancer and immunomodulatory agent (5). In the present study polysaccharides from G. lucidum were isolated and the radioprotective ability is studied.
Ionizing radiation is one of the well-established and widely used therapeutic modalities either for curative or palliative treatment of tumors in man. In radiotherapy of cancer, normal tissues need to be protected while cancers are exposed to high doses of radiation. A large number of compounds natural and synthetic have been evaluated for this purpose (6). However, most of them were not successful clinically because of toxicity and side effects. Hence, search for an ideal radioprotector without side effects is a compelling urgency. The present study is an attempt to find out the structure and to investigate the DNA protecting property of BG in human lymphocytes.
Materials and methods
Chemicals
Tris base, high melting agarose, sephadex G-200, low melting point agarose, Na2-ethylene diamine tetraacetic acid (EDTA), TritonX-100, sodium sarcosinate, dimethylsulfoxide (DMSO) and propidium iodide were obtained from Sigma Chemicals (St. Louis, Missouri, USA). All other chemicals used were of analytical grade and procured locally.
Collection of human blood
Human blood samples were collected from three healthy non-smoking volunteers with a mean age of 25 (±2 years).
Irradiation
60Co-gamma rays in a Gamma Cell 220 [Atomic Energy of Canada Limited (AECL), Canada] at a dose rate of 5.3 Gy/min and Junoir Theratron unit (AECL, Ottawa, Canada) with a dose rate of approximately 0.35 Gy/min at 38 cm was used for irradiation purpose.
Isolation of polysaccharides
The fruiting bodies of G. lucidum were collected from the outskirts of Thrissur district, Kerala, South India. The type specimen was deposited in the herbarium of Center for Advanced Studies in Botany, University of Madras, Chennai, India (HERB. MUBL. 3175). BG was isolated by the method of Mizuno (7) with slight modification (8). The confirmation of BG was done by anthrone (9) and phenol sulphuric acid test (10). Structural confirmation of BG was done by infrared radiation (IR) and nuclear magnetic resonance (NMR) spectrum which were recorded at Sophisticated Analytical Instrument Facility, Indian Institute of Technology, Bombay, India. The molecular wt of BG was determined by gel filtration chromatography.
Identification of sugar components (complete hydrolysis and chromatography) was done. A total of 20 mg of BG was hydrolysed with 1 N HCl at 100 °C for 10 hours and also by 1 N Trifluro acetic acid at 120 °C for 3 hours. After cooling, the excess acid was removed by passage over a small column of DEAE cellulose and eluted with deionised water. The eluate was concentrated. The hydrolysates were applied on Whatman No:1 filter paper and the chromatogram was developed using isopropanol and water (4:1). For identification of the spots, aniline: diphenylamine—Phosphoric acid spray reagent was used. Constituent saccharides were identified by comparison with Rf values of spots obtained by hydrolysis of Ganoderma BG and also by co-chromatography with standard monosaccharides-glucose, fructose, galactose, mannose and rhamnose.
Comet assay
Comet assay was performed using the method of Singh (11) with minor modifications (12,13). In order to estimate DNA damage in blood leukocytes, 10 µL heparinised whole blood was mixed with 200 µL of low melting point agarose at 37 °C and layered on frosted slides pre-coated with 200 µL high melting point agarose. After solidification of agarose, the cover slips were removed and the slides were kept in pre-chilled lysing solution containing 2.5 M NaCl, 100 mM Na2-EDTA: pH10.0, 10 mM Tris HCI, 1% sodium sarcosinate with freshly added 1% Triton X-100 and 1% DMSO at 4 °C for 1 hour. The slides were removed from the lysis solution and placed on a horizontal electrophoresis tank filled with the alkaline buffer (300 mM NaOH, 1 mM Na2-EDTA, 0.2% DMSO, pH >13.0). The slides were equilibrated in the same buffer 20 minutes. Electrophoresis was carried out for 20 minutes at 25 V, 300 mA using a compact power supply. After electrophoresis, the slides were stained by layering on the top with 50 µL of propidium iodide (20 µg/mL) and visualized using a Carl Ziess Axioskop microscope with bright field, phase contrast and epifluorescence facility (HBO 50 high pressure mercury lamp), 40× camera adaptor lens. The quantitation of the DNA strand breaks of the stored images was done using the imaging software CASP by which the percentage DNA in tail, tail length, tail moment, and olive tail moment could be obtained directly (14). The tail length of comet indicated the extent of damage because the smaller molecules moves faster on the agarose gel. Thus, the longer tails of the comets indicated that the strand breaks were more frequent and the DNA was fragmented into several small molecules. The tail moment was a commonly accepted unit of DNA damage that normalizes the difference in the size of the nucleus studied (e.g., blood leukocytes) (12,13). It was the product of the percent DNA in the tail of the comet and tail length. For olive tail moment, distance of center of gravity of DNA was considered instead of usual tail length.
Results
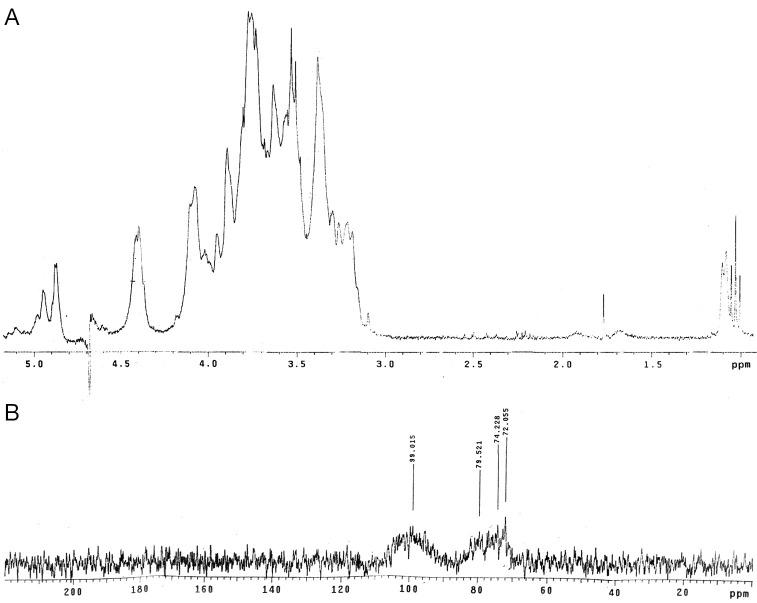
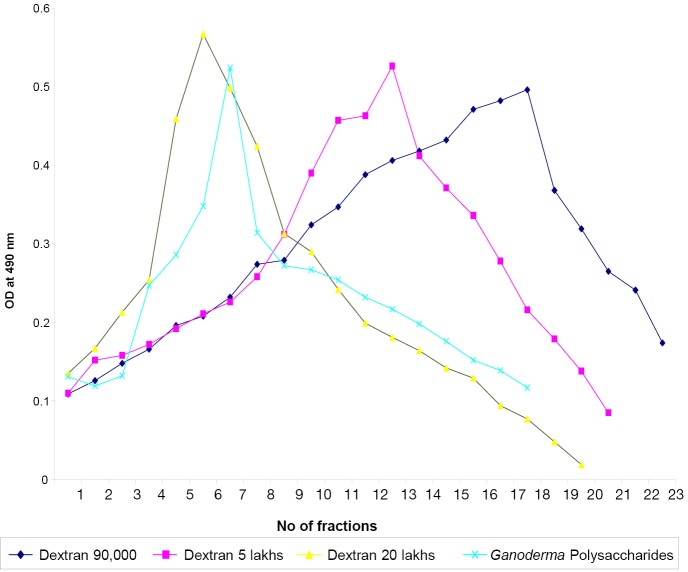
BG answered anthrone and phenol sulphuric tests giving typical color reactions indicating the presence of carbohydrates. From the IR spectrum, pyranoid form was presented due to the presence of three absorption bands at 1,153.4, 1,091.6 and 1,029.9 cm.sup.-1 (Furanose form has only two absorption bands in the region). Typical bands for C-O stretching (1,028 cm–1), C-O-C stretching (1,153 cm–1) and the band between 1,000-1,100 (1,080 cm–1) is characteristic for the presence of BG (Figure 1A,B). In the HNMR spectrum, H.sup.-1 signals were observed at less than 4.8 ppm (4.762, 4.683, 4.667, 4.658, 4.402 ppm), which suggest that component sugars have beta configuration (Figure 2A). In the 13CNMR spectrum, C-4 and C-5 signals were observed at less than 80 ppm. This result suggests that component sugars have pyranoid form (Figure 2B). From gel filtration chromatography, the molecular weight of BG was found to be 1.5×106 Daltons (Figure 3). From the acid hydrolysis treatment for the detection of monosaccharides, the Rf values of hydrolysate was compared with standard monosaccharides. The Rf values of monosaccharides D-glucose, D-fructose, D-galactose, D-mannose and D-rhamnose were 0.47, 0.54, 0.44, 0.53 and 0.65 respectively. Comparing the Rf values, the sugar present in the Ganoderma BG was found to be glucose, mannose and rhamnose (Figures 4,5).
Figure 1.
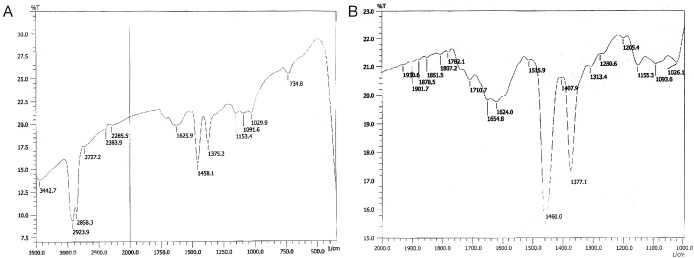
Infrared spectrum of polysaccharide from G. lucidum.
Figure 2.
(A) HNMR spectrum of polysaccharide from G. lucidum; (B) 13CNMR spectrum of polysaccharide from G. lucidum. HNMR, H nuclear magnetic resonance; 13CNMR, 13C nuclear magnetic resonance.
Figure 3.
Absorbance of different fractions of three dextrans and Ganoderma beta glucan at 490 nm.
Figure 4.
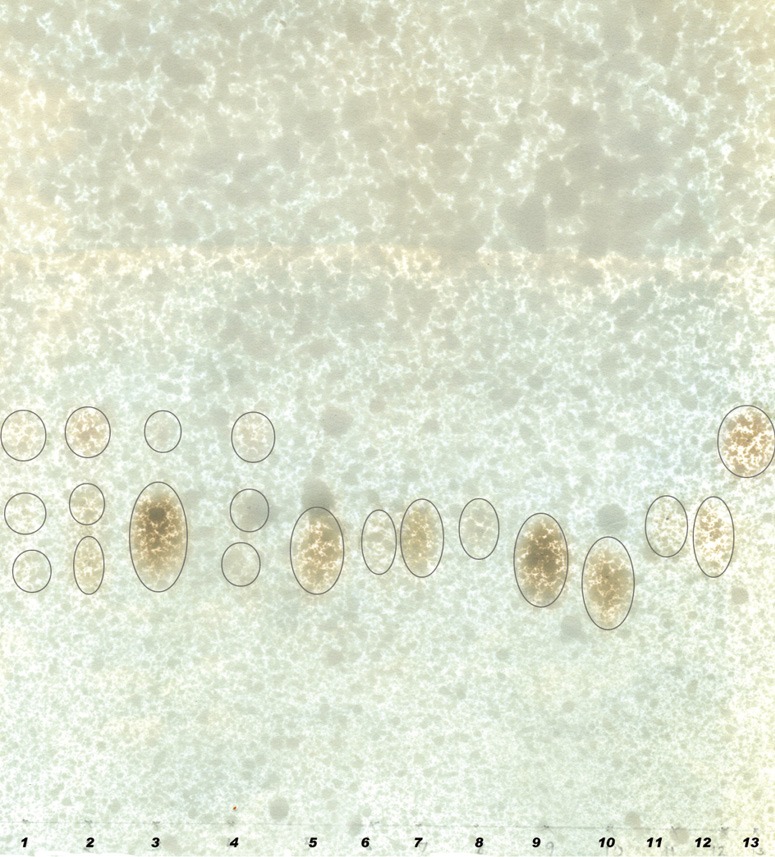
Paper chromatogram showing monosaccharide profile of G. lucidum polysaccharides with trifluoacetic acid. The first 8 spots are samples and spots from 9 to 13 are standards. 9, glucose; 10, fructose; 11, galactose; 12, mannose; 13, rhamnose.
Figure 5.
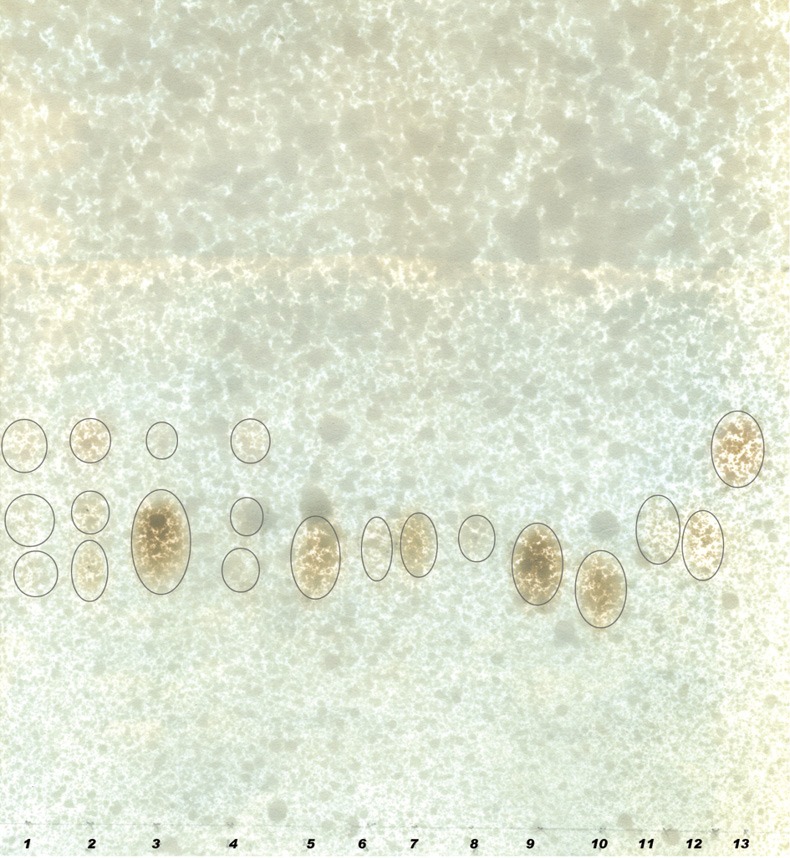
Paper chromatogram showing monosaccharide profile of G. lucidum polysaccharides with HCL. The first 8 spots are samples and spots from 9 to 13 are standards. 9, glucose; 10, fructose; 11, galactose; 12, mannose; 13, rhamnose.
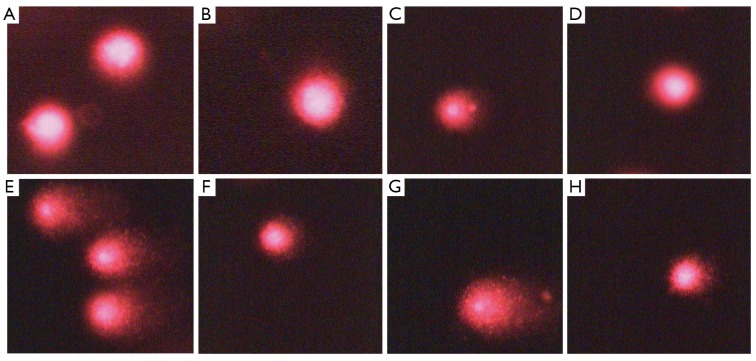
An exposure of human peripheral blood leukocytes to 0, 1, 2 and 4 Gy γ-radiation ex vivo, resulted in increase of comet parameters such as % DNA in tail, tail length, tail moment and olive tail moment. The presence of BG at 50 and 100 µg/mL during irradiation reduced these parameters (Figure 6, Table 1). The % DNAs at 0, 1, 2 and 4 Gy were reduced by BG from 1.9718±0.21096, 2.7315±0.4736, 3.426±0.3952 and 6.6679±0.3743 to 1.0258±0.2008, 2.1259±0.1806, 2.7436±0.3610, 5.2055±0.3728 at 50 µg/mL and to 0.8718±0.1707, 0.91974±0.2842, 2.1560±0.4541 and 4.3003±0.5664 at 100 µg/mL respectively. Similarly the tail lengths at 0, 1, 2 and 4 Gy were reduced by BG from 9.7137±0.3456, 13.1368±1.182, 11.8648±0.61625 and 21.7296±0.6288 to 8.2476±0.6154, 11.1730±0.7463, 9.3694±0.7929 and 19.8316±0.7294 at 50 µg/mL and to 6.7807±0.3258, 9.5267±0.4194, 7.0196±1.0938 and 16.5423±1.1118 respectively at 100 µg/mL. The tail moments were reduced by BG from 0.3051±0.0463, 0.9531±0.5304, 0.5925±0.0908, 2.0148±0.1665 at 0, 1, 2 and 4 Gy to 0.2324±0.0501, 0.6531±0.5327, 0.4260±0.978 and 2.011±0.2160 at 50 µg/mL and to 0.2161±0.0311, 0.4110±0.1105, 0.2575±0.1055 and to 0.6887±0.1243 at 100 µg/mL. The olive tail moments at 0, 1, 2 and 4 Gy were reduced by BG from 0.7326±0.0637, 1.5144±0.4783, 1.0849±0.1197 and 2.7278±0.14507 to 0.3617±0.0691, 1.288±0.2876, 0.9041±0.1345 and 2.2012±0.2299 at 50 µg/mL and to 0.1327±0.0691, 0.7790±0.1202, 0.4017±0.1025, 1.1450±0.1434 at 100 µg/mL.
Figure 6.
Comet assay of BG. (A) 0 Gy; (B) 0 Gy + BG; (C) 1 Gy; (D) 1 Gy + BG; (E) 2 Gy; (F) 2 Gy + BG; (G) 4 Gy; (H) 4 Gy + BG. BG, beta glucan (100 µg/mL).
Table 1. Comet parameters in the presence and absence of polysaccharides at different doses of gamma irradiation.
| Treatment | 0 Gy | 1 Gy | 2 Gy | 4 Gy |
|---|---|---|---|---|
| % DNA | ||||
| RT alone | 1.9718±0.21096 | 2.7315±0.4736 | 3.426±0.3952 | 6.6679±0.3743 |
| RT + PS 50 µg/mL | 1.0258±0.2008 | 2.1259±0.1806 | 2.7436±0.3610 | 5.2055±0.3728 |
| RT +100 µg/mL | 0.8718±0.1707 | 0.91974±0.2842 | 2.1560±0.4541 | 4.3003±0.5664 |
| Tail length | ||||
| RT alone | 9.7137±0.3456 | 13.1368±1.182 | 11.8648±0.61625 | 21.7296±0.6288 |
| RT + PS 50 µg/mL | 8.2476±0.6154 | 11.1730±0.7463 | 9.3694±0.7929 | 19.8316±0.7294 |
| RT +100 µg/mL | 6.7807±0.3258 | 9.5267±0.4194 | 7.0196±1.0938 | 16.5423±1.1118 |
| Tail moment | ||||
| RT alone | 0.3051±0.0463 | 0.9531±0.5304 | 0.5925±0.0908 | 2.0148±0.1665 |
| RT + PS 50 µg/mL | 0.2324±0.0501 | 0.6531±0.5327 | 0.4260±0.978 | 2.011±0.2160 |
| RT +100 µg/mL | 0.2161±0.0311 | 0.4110±0.1105 | 0.2575±0.1055 | 0.6887±0.1243 |
| Olive tail moment | ||||
| RT alone | 0.7326±0.0637 | 1.5144±0.4783 | 1.0849±0.1197 | 2.7278±0.14507 |
| RT + PS 50 µg/mL | 0.3617±0.0691 | 1.288±0.2876 | 0.9041±0.1345 | 2.2012±0.2299 |
| RT +100 µg/mL | 0.1327±0.0691 | 0.7790±0.1202 | 0.4017±0.1025 | 1.1450±0.1434 |
Abbreviations: RT, radiation alone; PS, polysaccharides.
Discussion
BG has shown good antioxidant activity in our earlier studies by scavenging of hydroxyl radical generated by Fenton reaction, inhibition of lipid peroxidation and reduction of Fe3+-TPTZ complex to Fe2+-TPTZ in Ferric reducing antioxidant property (FRAP) assay (15), inhibited thiobarbituric acid reactive substance (TBARS) formation in mice liver microsomal membrane (16). BG is highly effective in reducing both simple and complex chromosomal aberrations, indicating significant protection against single-strand breaks and double-strand breaks in the DNA (15), and also shown may enhance DNA repair in human lymphocytes (16). The antioxidant activity of BG, together with its DNA repairing ability may be the contributing factor for BG’s DNA protecting property. The solubility of BG in water completely helps in easy administration.
Polysaccharides widely exist in the plants, microorganism (fungi and bacteria), algae, and animals. Together with proteins and polynucleotides, they are essential biomacromoleules in the life activities and play important roles in cell-cell communication, cell adhesion, and molecular recognition in the immune system (17). In recent years, some bioactive polysaccharides isolated from natural sources have attracted much attention in the field of biochemistry and pharmacology. Chemical modification is often carried out in polysaccharides to improve the antitumor activity of polysaccharides and their clinical qualities (mostly water solubility). Their activity is especially beneficial in clinics when used in conjunction with chemotherapy. Mushroom polysaccharides prevent oncogenesis, show direct antitumor activity against various allogeneic and syngeneic tumors, and prevent tumor metastasis.
The damages by ionizing radiation to DNA can cause the loss of viability of the cells exposed to radiation. One of the deleterious consequences of DNA damage from exposure to ionizing radiation is the induction of cancer. Protecting cellular DNA from radiation damage might result in the prevention of the cancers induced by the radiation. Protection against ionizing radiation is of paramount importance during accidental and unavoidable exposures to radiation Upadhyay (18), and development of novel and effective approaches to combat radiation damage using non-toxic radioprotectors are of considerable interest for defense, nuclear industries, radiation accidents, space travels, etc., besides protection of normal tissues during radiotherapy of tumors and other medical exposures Upadhyay (18).
The bioactive glucanes and proteoglucans isolated from medicinal mushrooms are the most promising class of immunoceutics. Many of its active mycotic components are chemically related with the structure β-D-glucan or β-D-glucose linked to proteins. The ability of bioactive polysaccharides and polysaccharide-bound proteins to modulate so many important immune cells may be due to the structural diversity and variability of these macromolecules. Unlike proteins and nucleic acids, polysaccharides contain repetitive structural features which are polymers of monosaccharide residues joined to each other by glycosidic linkages (5). Among these macromolecules, polysaccharides offer the highest capacity for carrying biological information because they have the greatest potential for structural variability. For example, the number of possible permutations for four different sugar monomers can be up to 35,560 unique tetrasaccharides, whereas four amino acids can form only 24 different permutations (5). Therefore, this enormous potential variability in polysaccharide structure gives the necessary flexibility for the precise regulatory mechanisms of various cell-cell interactions in higher organisms. Evidence suggests that the activity of polysaccharides is also dependent on their conformation, composition and size, with high molecular weight fraction being more active, while fractions with low molecular weight from same source show no activity (19). Immunomodulating and antitumor activity of these metabolites related to their effects to act of immune effecter cells such as hematopoietic stem cells, lymphocytes, macrophages, T cells, dendritic cells (DCs), and natural killer (NK) cells involved in the innate and adaptive immunity, results in the production of biologic response modifiers.
The result of present investigation reveals the potentials of BG from G. lucidum in radiation protection. Fungal BG appear to be beneficial to humans with impaired immune systems, and those suffering from infectious diseases and cancer, as well as in helping patient recovery from chemotherapy and radiotherapy (20). As a traditional medicine this mushroom is used for its therapeutic significance and is freely available. It is worthwhile to do further studies to reveal the mechanism of action of BG which may show light in the full potential of this mushroom polysaccharides both in medical and non-medical exposure.
Acknowledgements
The author is grateful to Staff of SAIF, IIT Bombay for helping in getting the structural details of the compound. The authors are also grateful to Subin Kumaran for helping in Photography.
Disclosure: The authors declare no conflict of interest.
References
- 1.Lucas EH, Byerrum RU, Clarke DA, et al. Production of oncostatic principles in vivo and in vitro by species of the genus Calvatia. Antibiot Annu 1958-1959;6:493-6. [PubMed] [Google Scholar]
- 2.Williams DL, Di Luzio NR. Glucan-induced modification of murine viral hepatitis. Science 1980;208:67-9. [DOI] [PubMed] [Google Scholar]
- 3.Vetvicka V, Yvin JC. Effects of marine beta-1,3 glucan on immune reactions. Int Immunopharmacol 2004;4:721-30. [DOI] [PubMed] [Google Scholar]
- 4.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature 2001;413:36-7. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Zhou S.Cancer prevention and treatment by Ganoderma, a mushroom with medicinal properties. Food Rev Int 2003;19:275-325 [Google Scholar]
- 6.Ooi VE, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem 2000;7:715-29. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno T.Development of an antitumor biological response modifier from Phellinus linteus (Berk. et Curt.) Teng (Aphyllophoromycetideae) (review). Int J Med Mushrooms 2000;2:21-33 [Google Scholar]
- 8.Pillai TG, Nair CKK, Janardhanan KK. Enhancement of repair of radiation induced DNA strand breaks in human cells by Ganoderma mushroom polysaccharides. Food Chem 2010;119:1040-3 [Google Scholar]
- 9.Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J 1954;57:508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DuBois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem 1956;28:350-6 [Google Scholar]
- 11.Singh NP. Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat Res 2000;455:111-27. [DOI] [PubMed] [Google Scholar]
- 12.Maurya DK, Salvi VP, Krishnan Nair CK. Radioprotection of normal tissues in tumor-bearing mice by troxerutin. J Radiat Res 2004;45:221-8. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi NM, Maurya DK, Salvi V, et al. Radioprotection of DNA by glycyrrhizic acid through scavenging free radicals. J Radiat Res 2004;45:461-8. [DOI] [PubMed] [Google Scholar]
- 14.Końca K, Lankoff A, Banasik A, et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res 2003;534:15-20. [DOI] [PubMed] [Google Scholar]
- 15.Pillai TG, Uma Devi P. Mushroom beta glucan: potential candidate for post irradiation protection. Mutat Res 2013;751:109-15. [DOI] [PubMed] [Google Scholar]
- 16.Pillai TG, Nair CK, Janardhanan KK. Polysaccharides isolated from Ganoderma lucidum occurring in Southern parts of India, protects radiation induced damages both in vitro and in vivo. Environ Toxicol Pharmacol 2008;26:80-5. [DOI] [PubMed] [Google Scholar]
- 17.Dwek RA. Glycobiology: toward understanding the function of sugars. Chem Rev 1996;96:683-720. [DOI] [PubMed] [Google Scholar]
- 18.Upadhyay SN, Dwarakanath BS, Ravindranath T, et al. Chemical radioprotectors. Defence Sci J 2005;55:403-25 [Google Scholar]
- 19.Bohn JA, BeMiller JN. (1-3)-β-D-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr Pol 1995;28:3-14 [Google Scholar]
- 20.Chen J, Seviour R.Medicinal importance of fungal beta-(1→3), (1→6)-glucans. Mycol Res 2007;111:635-52. [DOI] [PubMed] [Google Scholar]