Abstract
Background:
There are considerable attempts worldwide on herbal and traditional compounds to validate their use as anti-cancer drugs. Plants from Moringaceae family including Moringa oleifera possess several activities such as antitumor effect on tumor cell lines. In this study we sought to determine if callus and leaf extracts of M. oleifera possess any cytotoxicity.
Materials and Methods:
Ethanol-water (70-30) extracts of callus and leaf of M. oleifera were prepared by maceration method. The amount of phenolic compounds of the extracts was determined by Folin Ciocalteu method. The cytotoxicity of the extracts against Hela tumor cells was carried out using MTT assay. Briefly, cells were seeded in microplates and different concentrations of the extract were added. Cells were incubated for 48 h and their viability was evaluated by addition of tetrazolium salt solution. After 3 h medium was aspirated, dimethyl sulfoxide was added and absorbance was determined at 540 nm with an ELISA plate reader. Cytotoxicity was considered when more than 50% reduction on cell survival was observed.
Results:
Callus and leaf extracts of M. oleifera significantly decreased the viability of Hela cells in a concentration-dependent manner. However, leaf extract of M. oleifera were more potent than that of callus extract.
Conclusion:
As the content of phenolic compounds of leaf extract was higher than that of callus extract, it can be concluded that phenolic compounds are involved in the cytotoxicity of M. oleifera.
Keywords: Cytotoxicity, Hela, Moringa oleifera
INTRODUCTION
Cancer has become a prominent cause of death throughout the world which has caused about 7.6 million deaths in 2008 and will probably rise to over 11 million in 2030.[1] Apart from death, it is indicated that cancer pain significantly decreases the global quality of life scores of patients.[2] In spite of the ongoing progress in the field of treatment, we still face the problem of drug resistance and side effects with the present chemotherapeutic compounds; therefore, the trend towards scientific exploring in natural compounds has significantly increased recently.[3]
Moringa oleifera Lam, also known as the ‘drumstick tree’ or the ‘horse radish tree’, is the most widely distributed and cultivated member of Moringaceae family. This highly valued plant is native to western and sub-Himalayan tracts, India, Pakistan, Asia Minor, Bangladesh, Africa and Arabia and has become naturalized in many tropical and subtropical areas. M. oleifera is considered as ‘natural nutrition of the tropics’, since almost all parts of this tree possess high nutritional value and even have been used to resist malnutrition in breast-feeding mothers.[4,5]
Furthermore, this tree is traditionally believed and scientifically proven to have numerous medicinal traits which have drawn more attention towards it. Some examples of such traits include antibacterial, antitrypanosomal, hypotensive, antispasmodic, antiulcer, anti-inflammatory, hypo-cholesterolemic, and hypoglycemic activities.[4] Besides, M. oleifera leaves are found to have antitumor activities which is probably due to the presence of O-Ethyl-4-(α-L-rhamnosyloxy) benzyl carbamate, (α-L-rhamnosyloxy)-benzyl isothiocyanate, niazimicin, and 3-O-(6’-O-oleoyl-β-D-glucopyranosyl)-β-sitosterol together with some natural antioxidants such as phenolic compounds.[5,6]
With regard to the problems of whole plant culture including environmental demands and variation in the production of secondary metabolites, plant cell culture is an agreeable alternate. The advantages of this procedure include cost-effective downstream process and being independent of geographical, environmental, and seasonal factors which can affect both type and amount of the metabolites.[7]
In this study we investigated the cytotoxic activity of the calli extract gained through plant cell culture of M. oleifera leaves in comparison to the leaf extract of this plant against Hela cell line.
MATERIALS AND METHODS
Callus establishment
M. oleifera leaves were collected from Bushehr Jahad Keshavarzi farm in September 2010. Young leaves of M. oleifera were sterilized by ethanol 70% and sodium hypochlorite, for one and five minutes, respectively; then, washed with sterile distilled water and transferred into culture jars containing sterile solid medium. The whole procedure was done under aseptic condition using class II laminar air flow hood.
Solid culture medium containing 30 g/l of sugar, 4.4 g/l of MS basal medium, 8 g/l of agar and also growth regulators including 0.25 mg/l of kinitine, 0.25 mg/l of 2,4-dichlorophenoxy acetic acid and 0.5 mg/l of α-naphthyl acetic acid was used.
The pH of the medium was adjusted at 5.7 ± 0.1. It was sterilized by autoclave at the condition of 121°C with 1/8 barr pressure for 15 minutes.[8] Culture jars were incubated at 25 ± 2°C in a dark place to establish the callus. Approximately every 1-2 months, when the jars were fully occupied, subculture was done. Each time, the calli were collected and freeze dried.
Extraction of cultured cells
Five gram of leaves or 5 g of freeze dried callus was ground and extracted through maceration in 160 ml and 240 ml of methanol:water 80:20, respectively. After three days the extracts were shaken, filtered and methanol was evaporated under reduced pressure using a rotary evaporator at 40°C.[9] Afterwards, the remained water content was removed by two days of freeze drying.
Measurement of phenolic compounds
Folin Ciocalteu method was applied to measure and compare the amount of the phenolic compounds in the callus and leaf extracts. Using pre-defined concentrations of gallic acid, as the standard solutions, a calibration curve was plotted and based on it, the concentrations of the phenolic compounds in the leaf and callus samples were measured spectrophotometrically at 765 nm.[10] The test was performed three times.
Maintenance of human cell lines
Human cervix carcinoma cell line (Hela) obtained from the Pasture Institute, Tehran, Iran. The medium used for maintenance of this cell line was roswell park memorial institute (RPMI) 1640 supplemented with 10% fetal calf serum and combination of penicillin/streptomycin (100 IU/ml and 100 μg/ml). Incubation was performed at 37°C in a humidified atmosphere with 5% CO2 condition.[11] The growth curve was plotted over five days.
Preparation of different concentrations of extracts
Stoke solutions of the leaf and callus extract of M. oleifera were obtained by dissolving the powder in dimethyl sulfoxide (DMSO) and then adding culture media. The maximum amount of DMSO was 10%, so that the final concentration of DMSO in the culture medium did not exceeded 1% in the wells. Based on the results of a pilot study using 1, 5, 10, 20, 50, 100, 200 and 500 μg/ml of leaf and callus extracts, it was decided to apply 1000, 1500, 2000, 2500 and 3000 μg/ml of callus extract and 100, 200, 220, 240, 260, 280, 300, 320, 340, 360, 380, 400 and 500 μg/ml of leaf extracts for the main test. The test procedure was performed in tetraplicate and repeated three times different days.
MTT cell viability assay
The cytotoxic effect of the extracts of M. oleifera leaf and callus against Hela human tumor cell line was determined by a rapid colorimetric assay, using Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT).[12] This assay is based on the reaction between mitochondrial dehydrogenase enzymes in viable cells and the tetrazolium rings of a soluble reagent which results in the production of violet formazan crystals. Crystals are solubilized in DMSO. The number of surviving cells is proportional to the amount of formazan and is represented by the color of the solution. The color is quantified using multi-well scanning spectrophotometer (ELISA reader). Briefly, 180 μl of cell suspension (2 × 104 cell/ml) was seeded in 96-well microplates except for the first row which contained only 180 μl of Roswell Park Memorial Institute medium (RPMI) 1640 which was considered as the blank. After 24 h of incubation in the previously mentioned conditions, 20 μl of specific concentrations of the leaf and callus extracts, were added. For the positive and negative controls doxorubicin 20 μg/ml and DMSO 10% were added instead of extracts, respectively. All plates were incubated for 48 h and using the ELISA reader the absorbance was measured. To evaluate cell survival, 20 μl of MTT solution (5 mg/ml in phosphate buffer solution) was added to each well and incubated for 3 h. Then gently 150 μl of old medium containing MTT was replaced by DMSO and pipetted to dissolve any formed formazan crystals. Absorbance was then determined at 540 nm by an ELISA plate reader. Each extract concentration was assayed in 4 wells and repeated 3-times. Standard curve (absorbance against the number of cells) was plotted. Intraday and interday variations were determined. Based on the standard curve percent cell survival was calculated. Percent of cell survival in the negative control was assumed 100.
Statistical analysis
SIGMASTAT™ (Jandel Software, San Raphael, CA) was used to perform statistical tests. Analyze-of-variance followed by the Student-Newman-Keuls test was used to see the differences among the groups. Significance was assumed at the 5% level.
RESULTS
Extract yeilds
The yield of the extracts was 19.6% and 18.2% for leaf and callus, respectively.
Callus formation
Fresh healthy creamy calli was established. The callus was compact in appearances.
Quantification of phenolic compounds
The standard curve for gallic acid, as indicator for phenolic compounds is shown in Figure 1. There was a linear relationship between the absorbance and gallic acid concentration.
Figure 1.
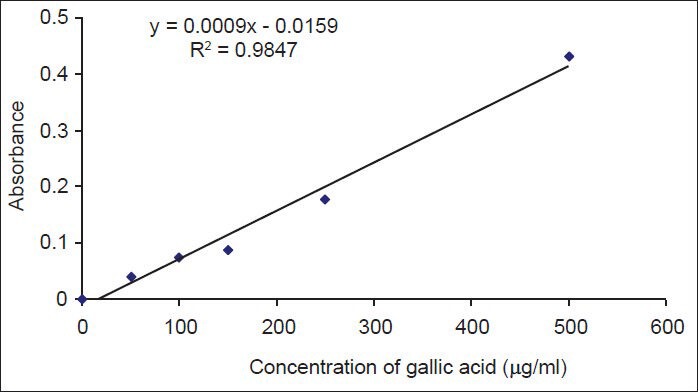
The standard curve of gallic acid. Different concentrations of gallic acid were prepared and their absorbances were determined at 765 nm. Data are shown as mean ± SD of three experiments
Based on the standard curve, the percent of phenolic compounds in dry powder of callus and leaf extracts was estimated as 0.237 ± 0.04% and 0.746 ± 0.09%, respectively.
Cytotoxic effect of extracts of M. oleifera
A good relationship between absorbance and the number of cells was observed for Hela cells, (r2 = 0.974) [Figure 2]. Intraday and interday variations for all standard curves were acceptable (%CV<15).
Figure 2.
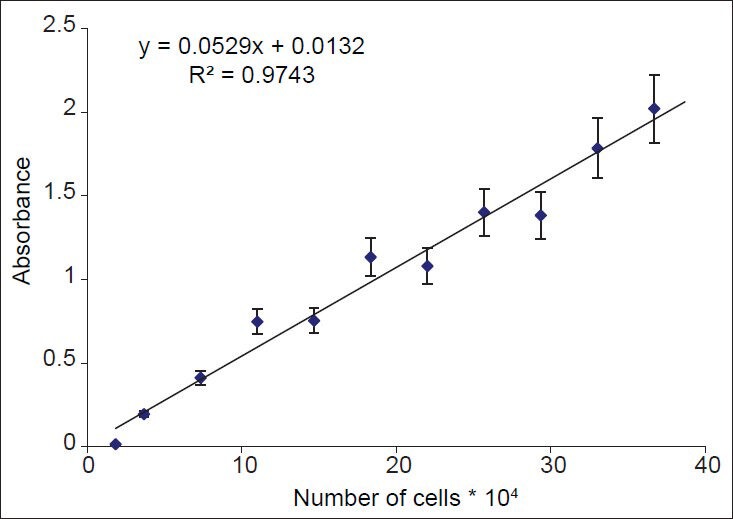
Relationship between absorbance and numbers of Hela cells. Number of viable cells was assessed using MTT assay. Absorbance was determined at 540 nm. Data are presented as mean ± SD, n = 9
To evaluate appropriate time of incubation of Hela cells with tested compounds, the growth curve of Hela cells over 120 h was plotted [Figure 3]. Based on the data, 48 h of incubation was used for further cytotoxicity tests.
Figure 3.
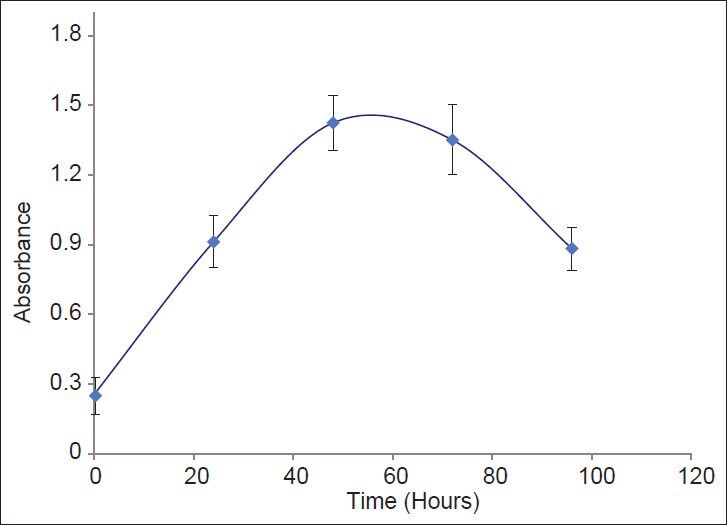
Growth curve for Hela cells. Cell culture medium containing 5* 104 cells/ml were added to plate and their absorbance was detemined up to 94 h. Data are presented as Mean ± SD of three experiments
Doxorubicin, a known cytotoxic antibiotic at concentration of 20 μg/ml, as a positive control significantly reduced the cell viability to less than 25%. Extracts were considered cytotoxic when they reduced cell viability to less than 50%. Callus extract of M. oleifera significantly and concentration-dependently decreased the viability of Hela cells at concentrations above 2000 μg/ml [Figure 4]. Also, leaf extract of M. oleifera significantly and concentration-dependently decreased the viability of Hela cells at concentrations above 260 μg/ml [Figure 5].
Figure 4.
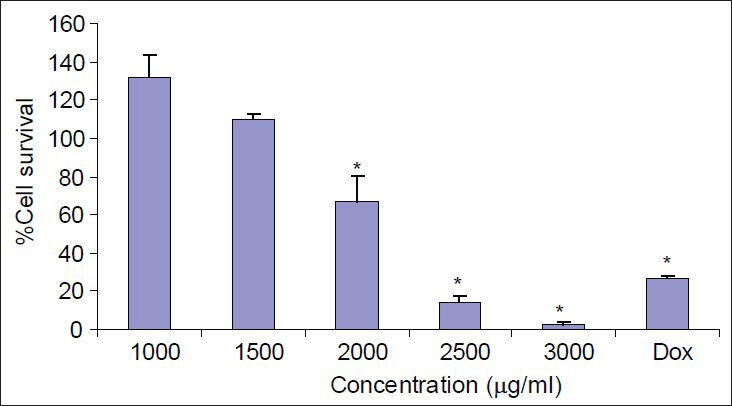
The effects of different concentration of callus extracts of M. oleifera on Hela cells. Cell viability was assessed using MTT assay. Percent survival in the control group was assumed 100. Dox = Doxorubicin, * = P < 0.05, n = 9
Figure 5.
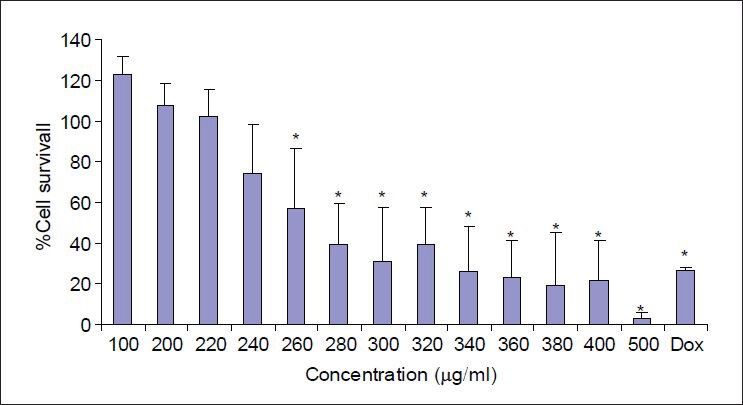
The effects of different concentration of Leaf extracts of M. oleifera on Hela cells. Cell viability was assessed using MTT assay. Percent survival in the control group was assumed 100. Dox = Doxorubicin, * = P < 0.05, n = 9
DISCUSSION
Cancer is one of the most important diseases of the 21st century. The incidence of cancer is increasing during the last decades in most countries. In spite of numerous efforts to combat this disease, world medicine is facing with the lack of perfect cure for it. In the last decades there has been a great emphasis towards the researches on complementary and alternative medicine for cancer management.[6] Several studies have been conducted on herbs to find new anti-tumor compounds. It has been shown that about 3000 plants, which possess anticancer properties, subsequently have been used as anticancer drugs.[13,14]
It has been shown that plants from Moringaceae family including M. oleifera possess antitumor activities.[5,6] Using MTT assay, we sought to determine if the extracts of callus and leaves of M. oleifera have cytotoxic activity against Hela cells. Doxorubicin, a known cytotoxic drug, significantly decreased the number of Hela cells indicating accuracy of the method used in this study. Our findings showed that leaf and callus extracts of M. oleifera significantly reduced viability of Hela cells in a concentration-dependent manner. However, the IC50 of leaf extract of M. oleifera was lower than that of the callus extract. Several studies have been shown that phenolic compounds are involved in cytotoxic activity of plants.[15,16,17,18,19,20]
It has been shown by Dehshahri et al. that leaf extract of M. peregrina possess antioxidant activity. They concluded that phenolic compounds in this plant were involved in its antioxidant activity.[21] As the content of phenolic compounds in the leaf extract of M. oleifera was higher than that of the callus extract it may be concluded that at least in part they are involved in the cytotoxicity of the extracts. However, the differences between the ratio of cytotoxicity and phenolic constituents of callus and leaf extracts of M. oleifera indicates that the cytotoxic effect of this plant is not merely due to the phenolic compounds. Studying on the volatile glucosinolate content of seed and leaf of M. peregrina showed that isobutyl isothiocyanate was the main glucosinolate degradation product of aerial parts of this plant, while no isothiocyanates were found in cell cultures of M. peregrina.[8] The difference in the constituents of plant and callus of M. peregrina may explain the difference in the cytotoxicity of their extracts. More investigation is needed to find out which components are responsible for cytotoxicity of M. oleifera. M. oleifera has several therapeutic and nutritional applications. Although its callus did not have desirable cytotoxic activity, it can be applied for other medical applications. Regarding to the numerous advantages of plant cell culture, it worth's to investigate the callus for further useful compounds and traits.
CONCLUSIONS
It can be concluded from this study that phenolic compounds are involved in the cytotoxicity of M. oleifera and callus extract of M. oleifera is less cytotoxic than leaf extract.
ACKNOWLEDGMENT
This study was supported by a grant from the Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: The Research Council of Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.2013. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/index. html .
- 2.Tavoli A, Montazeri A, Roshan R, Tavoli Z, Melyani M. Depression and quality of life in cancer patients with and without pain: The role of pain beliefs. BMC Cancer. 2008;8:177. doi: 10.1186/1471-2407-8-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efferth T, Kahl S, Paulus K, Adams M, Rauh R, Boechzelt H, et al. Phytochemistry and pharmacogenomics of natural products derived from traditional chinese medicine and chinese materia medica with activity against tumor cells. Mol Cancer Ther. 2008;7:152–61. doi: 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- 4.Fahey JW. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005;1:5–9. [Google Scholar]
- 5.Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: A food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 6.Guevara AP, Vargas C, Sakurai H, Fujiwara Y, Hashimoto K, Maoka T, et al. An antitumor promoter from Moringa oleifera Lam. Mutat Res. 1999;440:181–8. doi: 10.1016/s1383-5718(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 7.Raoa SR, Ravishankar GA. Plant cell culture: Chemical factories of secondary metabolites. Biotechnol Adv. 2002;20:101–53. doi: 10.1016/s0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 8.Dehshahri S, Afsharypuor S, Asghari G, Mohagheghzadeh A. Determination of volatile glucosinolate degradation products in seed coat, stem and in vitro cultures of Moringa peregrina Forssk (Fiori) Res Pharmac Sci. 2012;7:51–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Hajhashemi V, Ghannadi A, Sedighifar S. Analgesic and anti-inflammatory properties of the hydroalcoholic, polyphenolic and boiled extracts of stachys lavandulifolia. Res Pharma Sci. 2007;2:92–8. [Google Scholar]
- 10.Rajanandh MG, Kavitha J. Quantitative estimation of β-Sitosterol, total phenolic and flavonoid compounds in the leaves of Moringa oleifera. Int J Pharm Tech Res. 2010;2:1409–14. [Google Scholar]
- 11.Jafarian-Dehkordi A, Emami SA, Saeidi M, Sadeghi H. Cytotoxicologic studies of the extracts of Iranian Juniperus sabina and Platycladus orientalis on cancer cells. J Res Med Sci. 2004;9:7–11. [Google Scholar]
- 12.Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56:279–85. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartwell JL. Plants used against cancer. A survey. Lloydia. 1971;34:103–50. [PubMed] [Google Scholar]
- 14.Pandey G. New Delhi: Sri Satguru Publications; 2002. Anticancer herbal drugs of India with special reference to Ayurveda; pp. 18–121. [Google Scholar]
- 15.Sabzevari O, Galatic G, Moridania MY, Sirakia A, O’Brien PJ. Molecular cytotoxic mechanisms of anticancer hydroxychalcones. Chem Biol Interact. 2004;148:57–67. doi: 10.1016/j.cbi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Nomura1 T, Fukai T, Akiyama T. Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl Chem. 2002;74:1199–206. [Google Scholar]
- 17.Smejkal K, Svacinová J, Slapetová T, Schneiderová K, Dall’acqua S, Innocenti G, et al. Cytotoxic activities of several geranyl-substituted flavanones. J Nat Prod. 2010;73:568–72. doi: 10.1021/np900681y. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigoa G, Almanzab GR, Chengc Y, Penge J, Hamanne M, Duanc RD, et al. Antiproliferative effects of curcuphenol, a sesquiterpene phenol. Fitoterapia. 2010;81:762–6. doi: 10.1016/j.fitote.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakagami H, Jiang Y, Kusama K, Atsumi T, Ueha T, Toguchi M, et al. Induction of apoptosis by flavones, flavonols (3-hydroxyflavones) and isoprenoid-substituted flavonoids in human oral tumor cell lines. Anticancer Res. 2000;20:271–6. [PubMed] [Google Scholar]
- 20.Fukai T, Sakagami H, Toguchi M, Takayama F, Iwakura I, Atsumi T, et al. Cytotoxic activity of low molecular weight polyphenols against human oral tumor cell lines. Anticancer Res. 2000;20:2525–36. [PubMed] [Google Scholar]
- 21.Dehshahri S, Wink M, Afsharypuor S, Asghari G, Mohagheghzadeh A. Antioxidant activity of methanolic leaf extract of Moringa peregrina Forssk (Fiori) Res Pharmac Sci. 2012;7:111–8. [PMC free article] [PubMed] [Google Scholar]


