Abstract
Background:
Cutaneous Leishmaniasis is a self-limiting disease caused by protozoan parasites of the genus Leishmania, which affects the skin with full-thickness wounds, which are prone to scar formation even after treatment. Taurine (Tu) is one of the most abundant amino acids that has antioxidant and anti-inflammatory effects, which play an important role in the process of wound healing. Herein, we have investigated the effects of Tu on cutaneous Leishmaniasis wounds and L. major promastigotes.
Materials and Methods:
Eighteen mice were induced with Leishmaniasis wounds (with L. Major) on the base of their tails and divided into three groups, T1: Treated with Tu injection, T2: Treated with Tu gel, and C: No treatment. Treatments were carried out every 24 hours for 21 days. The volume densities of the collagen bundles and vessels, vessel's length density and diameter, and fibroblast populations were estimated by stereological methods. Flow cytometry was used in order to investigate the direct Tu effect on parasites. The Mann-Whitney U test was used and P ≤ 0.05 was considered to be statistically significant.
Results:
The numerical density of the fibroblasts, volume density of the collagen bundles, and length densities of the vessels in groups T1 and T2 were significantly higher than in group C (P < 0.05). The fibroblast numerical density of group T1 was higher than that of group T2 (P = 0.02). Incidentally, Tu had no direct effect on L. major parasites according to the flow cytometry analysis.
Conclusion:
Tu showed the ability to improve the wound healing process and tissue regeneration although it had no direct anti-leishmaniasis effect.
Keywords: Cutaneous leishmaniasis, mice, stereology, taurine, wound healing
INTRODUCTION
Leishmaniasis is an endemic and self-limiting disease caused by protozoan parasites of the genus Leishmania.[1,2,3] The parasite exists in two developmental phases: The sand fly transmits flagellated promastigote with a bite to the mammalian host and then transforms into the amastigote phase.[2] In this disease an atrophic scar remains after treatment.[3] Acute inflammation caused by the infection leads to collagen destruction that forms scar subsequent to the leishmaniasis wounds.[4] Seventy to seventy-five percent of all cutaneous leishmaniasis (CL) cases in the world are found in ten countries; Iran, as a tropical country, is one of them.[5] Fars, Esfahan, and Kerman provinces are the three regions of Iran with a high prevalence of CL.[6] Amastigote eradication and promoting the healing process with minimal scarring are the goals of CL treatment. Pentavalent antimony compounds (pentostam and Glucantime) have been used for the treatment of leishmaniasis.[7,8] However, they have exhibited various toxic effects on pancreas, heart and liver tissues.[7,9] Moreover, many studies have been conducted with the aim of finding new treatments, with greater healing impacts and fewer side effects, particularly for CL, in order to improve the wound healing process and decrease scar formation;[10,11] thus, investigations for finding more efficient and less toxic agents are still in progress.
Taurine (2-aminoethansulphonic acid; Tu) is one of the most abundant amino acids and plays physiological and pathological roles in the human body, in the form of antioxidant, anti-inflammatory, and anti-apoptotic effects.[12,13] Rapid re-epithelization may be induced by Tu due to its anti-inflammatory and antioxidative properties, the two main reactions that play important roles in the process of wound healing.[14,15]
Finding more beneficial agents to enhance and improve the wound healing process and reduce scar formation in leishmaniasis-induced skin wounds has always been a concern for researchers.
In this study we aim to introduce Tu as a potent healing agent, which is exhibited to be effective on skin wounds caused by CL in mice, based on stereological analysis; we have also presented a flow cytometry (FCM)-based assay for studying the in vitro effects of Tu on L. major promastigotes.
MATERIALS AND METHODS
Animals and wound creation
In an experimental study, Leishmania major amastigotes (MHOM/76/ER) were provided from a group of BALB/c mice that were already infected. We used a final concentration of 4-5000 amastigotes per ml (estimated by means of hemocytometer) to infect the mice in our study. Eighteen mice, aged about four weeks and weighing about 18 g, were obtained from the Pasteur Institute, Tehran, Iran. The animals were randomly divided into three groups (n = 6) with leishmaniasis-induced wounds made on the base of their tails. One group was treated with Tu injection (T1) and the other group was treated with Tu gel (T2), and the control group which received no treatment and had only daily debridement of the wound with distilled water (C). Treatments were carried out every 24 hours for 21 days starting from the day open wounds were observed.
At the end, the animals were sacrificed with a high dose of ether. A full-thickness circular skin sample with a 1 cm margin around the wound area was removed from the wound's site and fixed in buffered formaldehyde for stereological evaluation.
All animal experiments in this study protocol were approved by the Animal Ethics Committee of the Shiraz University of Medical Sciences and the animal care was in accordance with their moral guidelines.
Preparation of agents
Taurine was supplied by Sigma-Aldrich™ (St Quentin Fallavier, France). To facilitate the application of the agent, we provided 5% Tu gel, a concentration that was assigned according to a pilot study, by dissolving 5 g Tu in 2 cc distilled water, and then transferred the solution into 2% carboxymethylcellulose (CMC) (2 g CMC dissolved in 98 cc distilled water) for the topical Tu-treated group. Five percent Tu solution (5 g Tu in 100 cc distilled water) was prepared for the injected Tu treated group. The CMC gel itself was administered on the CL wounds in a pilot study and made no significant difference according to the stereological parameters, when compared with the non-treated group.
Stereological study
Every four days the length and width of each wound were measured by a standard ruler in order to determine the wound closure rate. The area of the wound was measured by the following formula: (Length + width)/2× π; π = 3.14
In a systematic random sampling manner, eight pieces of each skin sample were cut and embedded in a cylindrical paraffin block; 5 μm and 15 μm sections were obtained and stained with both Heidenhain’sazan and Hematoxylin and Eosin (H and E) stains.
Microscopic analysis of the skin samples was done by using a video-microscopy system made up of a microscope (E-200, Nikon™, Japan) linked to a video camera and a flat monitor. The volume densities of the collagen bundles, vessels, and hair follicles were estimated at a magnification of 500 × by using the 5 μm thickness slides and the point counting stereological method.[16,17]
The vascular length density (Lv) and mean diameter of the vessels were estimated at 500 × magnification by employing the 5 μm slides and the stereological method used by Ashkani-Esfahani et al.[16]
The fibroblast numerical density (Nv) (number of the cells per unit volume of the dermis) was estimated by the 15 μm slides at a magnification of 1200 × on the monitor, using the ‘optical dissector’ stereological method.[17] The upper and the lower 5 μm thicknesses of the slides here were considered as an ‘area of safety’.
Flow cytometry
We dissolved Tu in Dimethyl sulfoxide (DMSO) and then Phosphate buffered saline (PBS) to obtain the various concentrations of the compound (0.125-8 mM). The final concentration of DMSO should have exceeded by 0.2%. Promastigotes were incubated in DMSO (as control) at various concentrations of the compound for 2 h at 4°C, and then the promastigotes were collected in Eppendorf tubes and incubated for 30 min at 4°C with 50 g/ml propidium iodide (PI, sigma company, USA) in dark conditions. After incubation, the parasites were kept on ice until analysis. The positive controls for Propidium iodide (PI) staining were acquired by incubating parasites in the presence of 70% alcohol. The cell suspension was transferred into polystyrene flow cytometry tubes (BD Falcon Company, USA). We performed data acquisition and analysis, with a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, USA) and the cell Quest Pro software. A total of 10000 events were acquired in the region that had been previously established as corresponding to the parasites.
Statistical analysis of the data
The data were collected, analyzed, and reported as mean and standard deviation (mean ± SD). Besides, the statistical comparisons between the groups were carried out by the SPSS statistical software (v. 17.0). The Mann-Whitney U test was used to analyze and compare each parameter between the groups. P ≤ 0.05 was considered to be statistically significant.
RESULTS
Wound closure
As shown in Figure 1, the T1 and T2 groups showed a faster wound closure rate in comparison to the C group (P = 0.04). The T1 group even showed a faster wound closure in comparison to the T2 group (P = 0.04).
Figure 1.
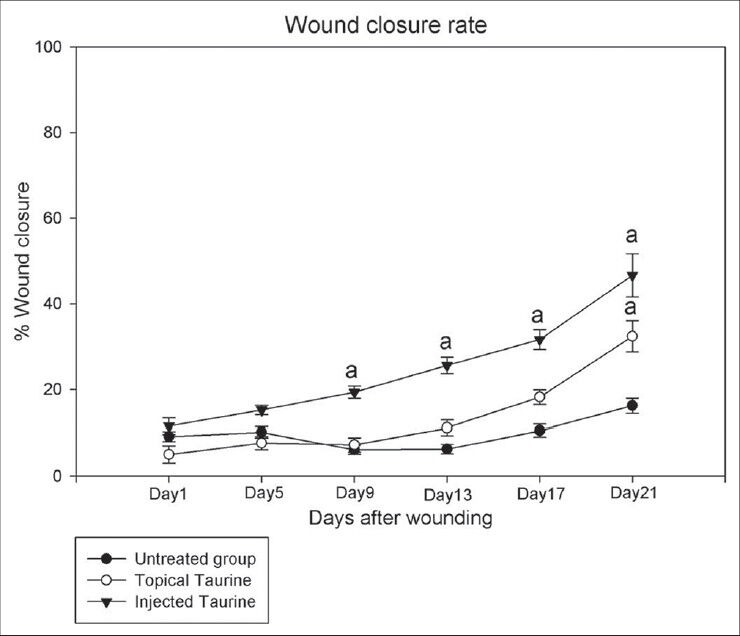
The effect of Taurine (Tu) on the wound closure rate in control, topical Tu-treated, and injected Tu-treated mice, with cutaneous leishmaniasis. Each point represents the mean ± SD of the six wounds. The wound closure rate had significantly increased in both the Tu-treated groups compared to the control group (P < 0.05). The ‘a’ letters show a significant difference compared to the control group on each day
The numerical density of the fibroblasts (Nv) in the dermis of the T1 group was higher than that of the C group and T2 group. As shown in Table 1, the numerical density of the fibroblasts in the T1 group was reported to be 82% higher than the C group (P = 0.003) and 43% higher than the T2 group (P = 0.02). There was no significant difference between the C and T2 groups with regard to the numerical density of fibroblasts.
Table 1.
Mean (SD) of the numerical density of the fibroblasts (×103 per mm3), volume densities of the collagen bundles (Vv collagen/dermis; %), vessels (Vv vessel/dermis; %), and hair follicles (Vv hair follicles/dermis; %), length density (mm/mm3), and mean diameter (μm) of vessels in the dermis of the leishmania induced skin wounds of rats treated with topical taurine gel, taurine injection, and the untreated group
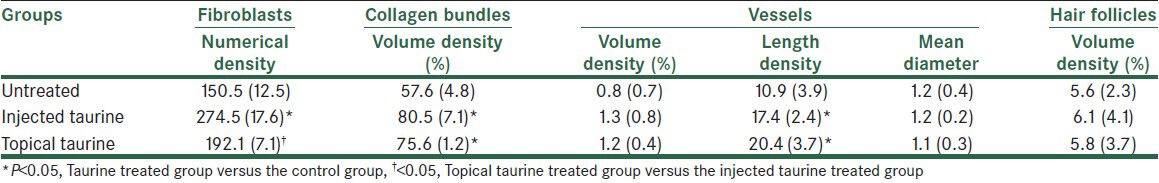
The volume density of the collagen bundles was 80.5% in the T1 group and 57.6% in the C group (P = 0.03). The collagen bundles’ volume density was 1.2% in the T2 group and 0.8% in the C group (P = 0.03). There was no significant difference between the T1, T2, and C groups with regard to the hair follicles’ volume density.
The length density of the vessels was 17.4 in the T1 group and 10.9 in the C group (P = 0.009). The vessel's length density was 20.4 in the T2 group and 10.9 in the C group (P = 0.006). The volume density and mean diameter of the vessels had no significant difference between the T1, T2, and C groups.
According to the result of flow cytometry Tu had no direct effect on this parasite, as it was shown in Figure 2.
Figure 2.
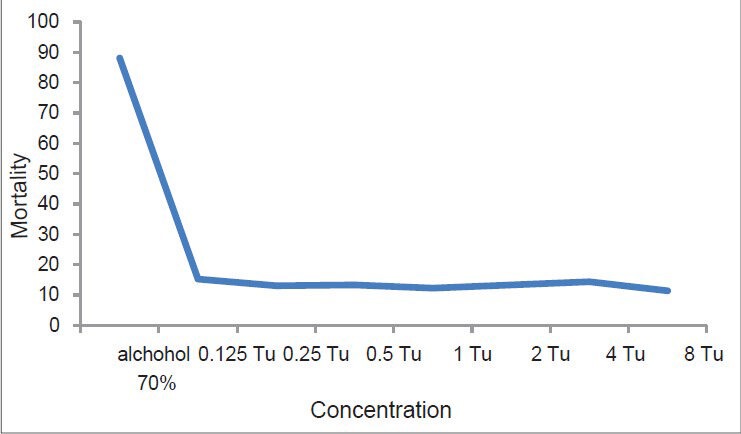
Effect of different concentrations of Tu and 70% alcohol (as a control) on parasite mortality. (Horizontal apex: Concentration, vertical apex: Mortality)
DISCUSSION
Leishmania L. Major induces innate immunity[18] and inflammation by mast cell stimulation and by secreting pro-inflammatory mediators by these cells.[19] Reactive Oxygen Species (ROS) that are produced during an inflammation response lead to oxidative damage to non-infected cells.[20] During oxidative damage some free radicals are released that have an important role in collagen damage.[21]
Similar studies have been done to find reliable drug therapy for cutaneous leishmaniasis. According to a study by Zakai et al., terbinafine and itraconazole had an effect on the treatment of leishmaniasis in infected mice et al.[22] They investigated the efficacy of pentamidine for treatment of leishmaniasis.[23] Soto et al. examined the treatment of American cutaneous leishmaniasis with miltefosine in 72 male soldiers.[24] In the other study, Aguiar et al. investigated the effect of paromomycin and miltefosine on mice experimentally infected with Leishmania.[25]
Previous studies have been conducted to find the Tu's re-epithelization enhancing, anti-inflammatory, and anti-oxidative activities in the process of wound healing.[14] Tu is an antioxidant that can be used in order to reduce oxygen-free radical effects in wound healing and collagen damage.[20] A study by Marcinkiewicz et al. revealed the efficacy of Tu on inflammatory skin diseases like acne vulgaris.[21] In another study, Gültekin et al. investigated the effect of topical Tu on the basement membrane proteins of the regenerating oral gingival epithelium.[13] Tu was shown to have the ability to facilitate the infected wound healing process, in a study by Tian et al.[15] Overall, according to the previous reports, the positive impact of Tu on the inflammatory skin lesions was revealed, and also the results of the present study showed that Tu had enhanced fibroblast proliferation, collagen bundle synthesis, and re-vascularization in the skin wounds of CL. According to the stereological analysis, Tu is supposed to have the ability to be introduced as an alternative effective agent for these kinds of skin lesions. However, further studies are required to determine the adverse effects and also possible alterations made by this agent on intercellular and extracellular signaling pathways, leading to improvement in wound healing, followed by clinical studies, which are also needed to be conducted in order to evaluate the influence of this substance on the human model of CL.
As a limitation of this study, lack of having a group of subjects treated with a common treatment for CL can be mentioned, which could help us to know whether Tu can be introduced as a supportive or alternative treatment for CL wounds.
CONCLUSION
According to this study Tu showed the ability to improve the wound healing process and tissue regeneration, although it had no direct anti-leishmaniasis effect.
ACKNOWLEDGMENT
The authors would like to thank the scientific writing committee of SadraTech® Company supervised by the Student Research Committee of Shiraz University of Medical Sciences for editing and improving the language and structure of the article.
Footnotes
Source of Support: Shiraz University of Medical Sciences, Shiraz, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Leishmaniasis: Worldwide epidemiological and drug access update. Abstract As part of a World Health Organization. [Last accessed on March 2010]. Available from http://www.who.int/leishmaniasis/resources/Leishmaniasis_worldwide_epidemiological_and_drug_access_update.pdf .
- 2.Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Iran J Parasitol. 2007;37:1097–106. doi: 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pourmohammadi B, Motazedian M, Hatam G, Kalantari M, Habibi P, Sarkari B. Comparison of three methods for diagnosis of cutaneous leishmaniasis. Iran J Parasitol. 2010;5:1–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Farahmand M, Nahrevanian H, Shirazi HA, Naeimi S, Farzanehnejad Z. An overview of a diagnostic and epidemiologic reappraisal of cutaneous leishmaniasis in Iran. Braz J Infect Dis. 2011;15:17–21. [PubMed] [Google Scholar]
- 5.Nilforoushzadeh MA, Jaffary F, Ansari N, Moradi S, Siadat AH. The comparison between trichloroacetic acid 50% and co(2) laser in the treatment of cutaneous leishmaniasis scar. Indian J Dermatol. 2011;56:171–3. doi: 10.4103/0019-5154.80411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhavan AA, Yaghoobi-Ershadi MR, Hasibi F, Jafari R, Abdoli H, Arandian MH, et al. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of southern Iran. J Arthrop Borne Dis. 2007;1:1–8. [Google Scholar]
- 7.Markle WH, Makhoul K. Cutaneous leishmaniasis: Recognition and treatment. Am Fam Physician. 2004;69:1455–60. [PubMed] [Google Scholar]
- 8.von Stebut E. Cutaneous leishmania infection: Progress in pathogenesis research and experimental therapy. Exp Dermatol. 2007;16:340–6. doi: 10.1111/j.1600-0625.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 9.Aguiar MG, Pereira AM, Fernandes AP, Ferreira LA. Reductions in skin and systemic parasite burdens as a combined effect of topical paromomycin and oral miltefosine treatment of mice experimentally infected with leishmania (leishmania) amazonensis. Antimicrob Agents Chemother. 2010;54:4699–704. doi: 10.1128/AAC.00809-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010;8:419–33. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 11.Nilforoushzadeh MA, Esfahani MH, Fesharaki MA, Siadat AH, Ansari N, Baradaran EH. Treatment of atrophic cutaneous leishmaniasis scar using autologous fibroblasts and keratinocytes (a case report and literature review) J Res Med Sci. 2010;15:125–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids. 2012 doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcinkiewicz J, Wojas-Pelc A, Walczewska M, Lipko-Godlewska S, Jachowicz R, Maciejewska A, et al. Topical taurine bromamine, a new candidate in the treatment of moderate inflammatory acne vulgaris: A pilot study. Eur J Dermatol. 2008;18:433–9. doi: 10.1684/ejd.2008.0460. [DOI] [PubMed] [Google Scholar]
- 14.Değim Z, Celebi N, Sayan H, Babül A, Erdoğan D, Take G. An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids. 2002;22:187–98. doi: 10.1007/s007260200007. [DOI] [PubMed] [Google Scholar]
- 15.Tian X, Zhang Z, Wang S, Diao Y, Zhao Z, Lv D. Copper-taurine (CT): A potential organic compound to facilitate infected wound healing. Med Hypotheses. 2009;73:1048–50. doi: 10.1016/j.mehy.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Ashkani-Esfahani S, Emami Y, Esmaeilzadeh E, Bagheri F, Namazi MR, Keshtkar M, et al. Silymarin enhanced fibroblast proliferation and tissue regeneration in full thickness skin wounds in rat models; a stereological study. J Saudi Soc Dermatol Dermatol Surg. 2013;17:7–12. [Google Scholar]
- 17.Ashkani-Esfahani S, Imanieh MH, Khoshneviszadeh M, Meshksar A, Noorafshan A, Geramizadeh B, et al. The healing effect of arnebia euchroma in second degree burn wounds in rat as an animal model. Iran Red Crescent Med J. 2012;14:70–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Sakthianandeswaren A, Elso CM, Simpson K, Curtis JM, Kumar B, Speed TP, et al. The wound repair response controls outcome to cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 2005;102:15551–6. doi: 10.1073/pnas.0505630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocyigit A, Keles H, Selek S, Guzel S, Celik H, Erel O. Increased DNA damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat Res. 2005;585:71–8. doi: 10.1016/j.mrgentox.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Dinçer S, Babül A, Erdoğan D, Ozoğul C, Dinçer SL. Effect of taurine on wound healing. Amino Acids. 1996;10:59–71. doi: 10.1007/BF00806093. [DOI] [PubMed] [Google Scholar]
- 21.Gültekin SE, Sengüven B, Sofuoğlu A, Taner L, Koch M. Effect of the topical use of the antioxidant taurine on the two basement membrane proteins of regenerating oral gingival epithelium. J Periodontol. 2012;83:127–34. doi: 10.1902/jop.2011.100568. [DOI] [PubMed] [Google Scholar]
- 22.Zakai HA, Zimmo SK. Effects of itraconazole and terbinafine on leishmaniasis major leisons in BALB/c mice. Ann Trop Med Parasitol. 2000;94:787–91. doi: 10.1080/00034980020027979. [DOI] [PubMed] [Google Scholar]
- 23.Lai A, Fat EJ, Vrede MA, Soetosenojo RM, Lai A, Fat RF. Pentamidine, the drug of choice for the treatment of cutaneous leishmaniasis in Surinam. Int J Dermatol. 2002;41:796–800. doi: 10.1046/j.1365-4362.2002.01633.x. [DOI] [PubMed] [Google Scholar]
- 24.Soto J, Toledo J, Gutierrez P, Nicholls RS, Padilla J, Engel J, et al. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin Infect Dis. 2001;33:E57–61. doi: 10.1086/322689. [DOI] [PubMed] [Google Scholar]
- 25.Aguiar MG, Pereira AM, Fernandes AP, Ferreira LA. Reductions in skin and systemic parasite burdens as a combined effect of topical paromomycin and oral miltefosine treatment of mice experimentally infected with leishmania (leishmania) amazonensis. Antimicrob Agents Chemother. 2010;54:4699–704. doi: 10.1128/AAC.00809-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


