Abstract
Background:
Free radicals are the known mechanisms responsible for inducing colitis with two origins: Inflammatory cells and tissues. Only the inflammatory cells can be controlled by corticosteroids. Our aim was to assess the importance of neutrophils as one of the inflammatory cells in inducing colitis and to evaluate the efficacy of corticosteroids in the treatment of inflammatory bowel disease (IBD).
Materials and Methods:
Thirty-six mice were divided into six groups of six mice each. Colitis was induced in three groups by exposing them to acetic acid through enema (group 1), ex vivo (group 3), and enema after immune suppression (group 5). Each group had one control group that was exposed to water injection instead of acetic acid. Tissue samples were evaluated and compared based on macroscopic damages and biochemical and pathological results.
Results:
Considering neutrophilic infiltration, there were significant differences between groups 1, 3, 5, and the control of group 1. Groups 3, 5, and their controls, and group 1 and the control of group 3 had significant differences in terms of goblet depletion. Based on tissue originated H2O2, we found significant differences between group 1 and its control and group 3, and also between groups 5 and the control of group 3. All the three groups were significantly different from their controls based on Ferric Reducing Ability of Plasma (FRAP) and such differences were also seen between group 1 with two other groups.
Conclusion:
Neutrophils may not be the only cause of oxidation process in colitis, and also makes the effectiveness of corticosteroids in the treatment of this disease doubtful.
Keywords: Acetic acid-induced colitis, corticosteroid, inflammatory bowel disease, neutrophil infiltration
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic disease commonly seen in all ages from infancy to adulthood.[1,2] This disease is clinically presented with abdominal pain, diarrhea, and sometimes accompanied by mucus, blood, or pus secretion.[3] The disease occurs worldwide,[4] and there is no difference in incidence between males and females.[1] Results of different investigations show that incidence of the disease is more among South Europeans, Asians, and in many other developing countries, especially among children.[5,6]
The etiology of this disease is still unknown.[4,7,8,9,10,11] Current treatments such as corticosteroids[12] are not completely promising[13,14,15] and their side effects remain a major clinical problem.[16,17] Many researches are under way to control the disease, and one of the suggested approaches in this regard is focusing on free radicals [Reactive Oxygen Species (ROS)].[13,18,19,20,21] Reactive oxygen metabolites which are generated via inflammatory and also oxidation–reduction reactions (in normal physiologic processes or enzymatic metabolism of endogenous materials) play a crucial role in the development and persistence of this disease.[18,21,22,23] In addition to the free radicals that are generated in the injured tissue, inflammatory cells which are recalled through chemotaxis to the target tissue play a pivotal role in the pathogenesis of the disease.[24] Neutrophils may play a key role in this regard by producing reactive peroxides that are involved significantly in the tissue necrosis and mucosal dysfunction.[17]
Although we can control inflammatory cells by using anti-inflammatory drugs such as corticosteroids, tissue oxidant formation is not affected by these agents.[25,26] Defining the role of each of the two parts (inflammatory cells and oxidant formation) can be of benefit in the management of IBD. If the main role in the pathogeneses is assigned to inflammatory cells, the treatment strategy should be focused on preventing chemotaxis and inflammatory mediator secretion, and if not, it should be focused on antioxidants to reach treatment goals. Various animal models of experimental colitis have been designed and used to define the mechanisms of IBD and to evaluate effective drugs for its treatment. Acetic acid-induced colitis, which is our model in this investigation, is an animal model that imitates some of the acute inflammatory responses of ulcerative colitis and is used wildly in this regard.[17]
The aim of this study was to investigate the role of neutrophils as one of the inflammatory cells in the pathogenesis of IBD and also to assess the efficacy of corticosteroids which are one of the widely used drugs for IBD in the treatment of this disease, in the acetic acid-induced colitis model.
MATERIALS AND METHODS
Animals
Thirty-six NMRI male mice of weight 25-30 g and age 5 weeks were divided into six groups of six members each. Mice were normal without any prominent gastrointestinal problems such as diarrhea or mucus secretion. Presenting any disease, especially gastrointestinal problems, was the exclusion criterion of the study. Mice were kept in antirust cages at a temperature of 24 ± 1°C and in 12-h light-dark cycle, moisture of 65-70%, and with standard food and water.[26]
Induction of colitis
Following 24 h fasting (receiving nothing except water) and under light ether anesthesia, colonic inflammation was induced by acetic acid. After mechanical anal lavage with normal saline (in order to clean their colons), group 1, named “exposed group,” was treated with 0.1 ml acetic acid 4% enema, through a polypropylene trocar cannula which was inserted into the colon via the anus. Group 2 (named “exposed control group”) was treated with 0.1 ml distilled water instead of acetic acid with the same method to serve as the control of the exposed group. In a sterile environment, we resected the distal end of the colon of six mice in group 3 and added 0.1 ml of acetic acid 4% on them. This group was designed to evaluate the direct chemical effect of acetic acid on tissue and is called as “ex vivo group.” Tissues were kept for 15 sec with acetic acid[27] and then washed with warm normal saline.
In order to assess the effect of resection, after resection of the distal colon of group 4 mice (called “ex vivo control group”), they were treated with 0.1 ml distilled water instead of acetic acid for at least 15 sec.
In order to suppress neutrophil infiltration, group 5 mice were immune suppressed with daily intra peritoneal injection of 20 mg/kg hydrocortisone.[28,29,30] After 7 days, the mice of this group, called “prevention group,” were exposed to acetic acid, like those in exposed group. Mice of group 6 (prevention control group) were immune suppressed like group 5 mice and were treated with distilled water similar to those in exposed control group. The groupings and the aim of designing them are presented in Table 1.
Table 1.
The names and the aim of the six study groups
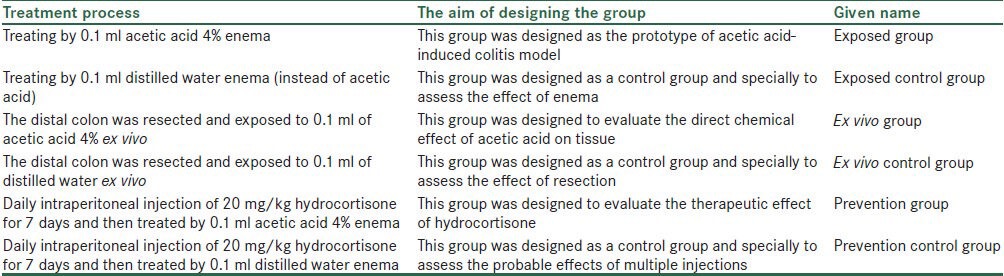
In order to prevent liquid excretion, all mice were positioned supine in trendelenburg position,[14] and at least 15 sec later, their colons were processed with warm normal saline.[7] After 4 days, the mice were sacrificed, and through laparotomy, 10 cm of each terminal colon was removed,[7] the tissue borders were detached, and all the tissues were treated with phosphate buffered saline.[14] After that, all the tissue samples were compared based on their tissue macroscopic damages and pathological and biochemical results.
Macroscopic analysis
Macroscopic mucosal damages were evaluated, scored, and quantified by Morris et al. scoring system.[31] One centimeter of each damaged tissue was extracted and the extent of damage was evaluated by the light microscope.
Pathological analysis
Samples were stained with H and E, and neutrophil infiltration, goblet depletion, and crypt abscess of sampled tissues were assessed.[32] Neutrophil infiltration assessment was based on a qualitative scoring of 0-3 (absence of neutrophil = 0 and presence of it in all histological layers = 3). Regarding goblet depletion, 0 was for samples with normal goblet and 1 for samples with pathologically depleted goblet. And finally, crypt abscess was assessed based on the presence and absence of it, wherein cases with crypt abscess were scored 1 and the ones without them were scored 0. All evaluations and scorings were done by a blinded pathologist.
Biochemical analysis
In a blinded manner, the rest of each colon was dissected longitudinally into small pieces and a homogenized tissue section was obtained from each colon part.[14]
The total antioxidant capacity of each sample was assessed by Ferric Reducing Ability of Plasma (FRAP) method[33,34,35] and by evaluating tissue H2O2 level with ferrous ion oxidation xylenol orange (FOX) method.[36,37]
To equalize homogenized tissue samples, tissue protein level was measured in all samples. Also, the amounts were counted and compared per sample protein unit by FRAP and H2O2. All measurements were done by two laboratory technicians in a blinded manner.
Data analysis
To assess each two groups based on their FRAP and H2O2 averages, we used t-test, and to assess and compare them based on goblet depletion and grade of neutrophil infiltration, we used χ2 and Fisher's exact tests. All data were analyzed through SPSS 14 and P < 0.05 was considered as statistical significance.
RESULTS
We had 36 mice without diarrhea and bloody feces, until the end of the study.
Macroscopic results
Among all cases, only three samples showed stage 1 damage and there were no significant differences between groups with respect to macroscopic results (P = 0.69).
Pathological results
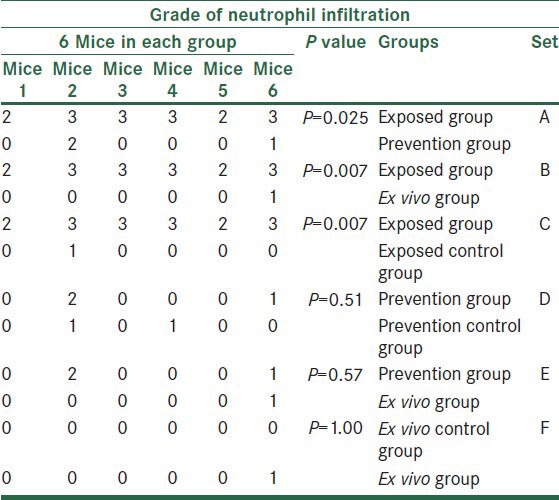
Regarding neutrophil infiltration, exposed group was significantly different from its control group (P = 0.007), prevention group (P = 0.025), and also ex vivo group (P = 0.007). We did not find any statistically significant differences in neutrophil infiltration among other groups [Table 2].
Table 2.
The comparison of all groups based on grade of neutrophil infiltration
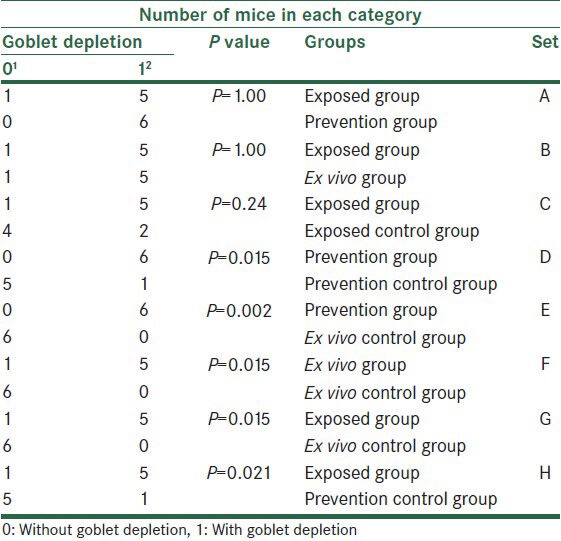
Considering goblet depletion, we found significant differences between ex vivo group and its control (P = 0.015), between prevention group and its control (P = 0.015), and also between prevention group and ex vivo control group (P = 0.002). There were also significant differences on comparing exposed group with ex vivo control group (P = 0.015) and prevention control group (P = 0.021). We did not find any other significant differences [Table 3].
Table 3.
The comparison of all groups based on goblet depletion
Also, we found crypt abscess in only two samples without any significant difference among the groups (P = 0.56).
Biochemical results
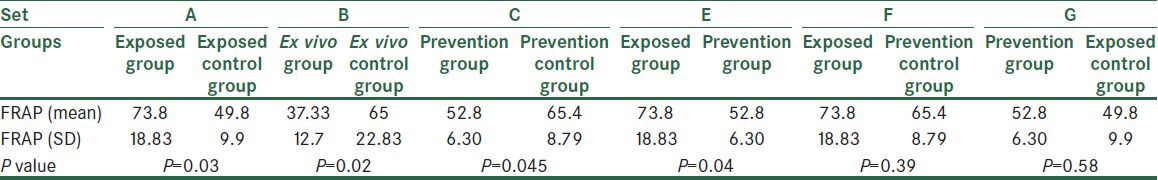
With respect to FRAP concentration averages, all the three acetic acid treated groups (exposed group, ex vivo group, and prevention group) were significantly different from their controls [(P = 0.03), (P = 0.02), and (P = 0.04), respectively]. Also, exposed control group was significantly different from prevention (P = 0.04) and ex vivo groups (P = 0.01). Besides, there were significant differences between ex vivo group and two immunosuppressed groups [prevention group (P = 0.03) and its control (P = 0.02)]. There was no other significant difference among the other groups [Table 4].
Table 4.
The comparison of all groups based on their FRAP averages
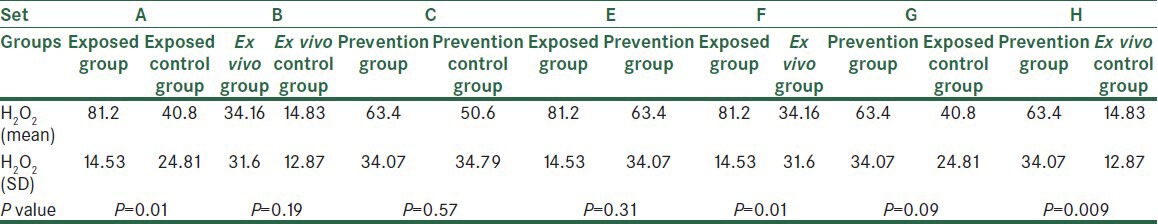
Regarding tissue H2O2 concentration, we found significant difference between exposed group and its control (P = 0.01). Also, we found significant difference between two groups treated only with acetic acid (exposed group and ex vivo group) (P = 0.01) and also between prevention group and ex vivo control group (P = 0.009). Finally, among the three control groups, prevention control group showed significant differences from exposed control group (P = 0.048) and ex vivo control group (P = 0.02) [Table 5].
Table 5.
The comparison of all groups based on their H2O2 averages
DISCUSSION
Results of this study show that activated neutrophils may not be the main cause of oxidation process in tissue and makes the effectiveness of the corticosteroids in the treatment of this disease doubtful. Acetic acid-induced colitis is one of the commonly used experimental models of colitis that allows many studies to be done on this disease.[17,38] The significant differences in neutrophil infiltration between exposed group and its control, prevention group and ex vivo group, and also the goblet depletion difference between prevention control group and ex vivo control group showed that the model used was successful in inducing mild acute colitis in this study. Also, as there was a significant difference in goblet depletion of the prevention group with prevention control group and ex vivo control group, and also a significant difference between the ex vivo group and its control, we concluded that acetic acid had an acceptable impact on samples, and this would be confirmed when considering the non-significant differences among the exposed, prevention, and ex vivo groups. Besides, regarding macroscopic view, hyperemia, which was only seen in exposed group and prevention group, may help to reaffirm the presence of induced colitis in samples.
On the other hand, significant difference in neutrophil infiltration between exposed group and prevention group, and nonsignificant differences between prevention group and its control group, exposed control group, ex vivo group, and ex vivo control group may confirm the effect of corticosteroid on the prevention of neutrophil infiltration in this animal model.
Nonsignificant difference in the variable of goblet depletion between exposed group and its control may be justified as due to the colon mechanical lavage before enema, which may result in goblet depletion.
On comparing H2O2 concentration between ex vivo group and its control, it showed no significant difference, and thus may reveal that the impact of acetic acid on tissue H2O2 concentration is not simply due to its local chemical effect on colon. Significant difference between exposed and ex vivo groups also can confirm this conclusion. Considering significant difference between exposed group and its control, it can be concluded that colitis may increase tissue H2O2 concentration. Lack of significant difference between exposed group and prevention group shows that although corticosteroid decreases neutrophil count, it does not decrease tissue H2O2 concentration in colitis model.
We derived two conclusions from the comparison between the prevention group and its control group, which did not show a significant difference based on H2O2 concentrations:
Corticosteroid may prevent the effect of acetic acid and cause decrease in H2O2, or
Corticosteroid may spontaneously increase H2O2
Regarding the fact that prevention group showed significant differences from ex vivo and exposed control groups, it can reinforce the second conclusion.
On the other hand, as H2O2 production in prevention group is significantly different from its production in exposed group but there is no difference between the two aforementioned groups with regard to neutrophil infiltration, we may criticize the role of neutrophil in the oxidation process which induces colitis.
By considering the significant difference in FRAP concentration between exposed group and its control, we can conclude that colitis is accompanied by increased FRAP in tissue. Increasing FRAP results in reducing H2O2 to H2O and consequently decreasing tissue H2O2 concentration (may be in order to balance the increased H2O2 level). Comparing ex vivo group with its control based on FRAP concentration, it showed significant difference, but by considering the fact that exposing ex vivo tissue to acetic acid may generate a permanent acidic environment and because the isolated tissue is not able to neutralize it and subsequently may lead to acid–base equation imbalance, we cannot have any discussion on this pair. In this regard, comparing exposed group with prevention group showed statistical difference as a result of FRAP average decreasing in the prevention group. As there was also a significant difference between prevention group and its control based on a decline in the FRAP concentration average in the former group, we can hypothesize that in colitis model, corticosteroid may decrease FRAP or prevent it from increasing. Nonsignificant difference between ex vivo group and prevention group may help to confirm this hypothesis.
As colitis may increase H2O2 in tissue, if something decreases FRAP or prevents it from increasing, it can result in increasing H2O2 and exacerbates the inflammation process and its consequences and complications.
So, based on all aforementioned discussions, we should take this possibility into account that corticosteroids may increase free radical formation and oxidant factors and also decrease antioxidants in this model.
IBD has still saved its importance as a subject of many investigations to discover its exact mechanisms and treatments. The results of the study published by Bilsela and colleagues showed that inflammatory cells are not the sole factors in inducing and exacerbating colitis.[14] In 2011, a study considering the main roles of increased free-radical production, decreased antioxidant capacity, and excessive inflammation in the pathogenesis of IBD proposed that melatonin as a powerful antioxidant may be a hopeful therapeutic agent for ulcerative colitis.[23]
The acetic acid-induced colitis model which is used in this study is a commonly used model, and in recent years, some studies have accomplished to investigate the effects of new proposed drugs and treatments like royal jelly,[39] budesonide-succinate-dextran,[38] and angiotensin converting enzyme inhibitors[40] on this disease through this (acetic acid-induced colitis) model.
This study had some limitations such as small sample size and confined available techniques, which hampered researchers from using miscellaneous confirmatory techniques and methods. Also, all of these may end in some kinds of biases and misconceptions in deriving the conclusions.
CONCLUSION
In summary, the results of this study make suspicious the pivotal role of neutrophils as the main cause of oxidative reactions and free radical formation in colitis. Also, due to increased H2O2 concentration in the presence of corticosteroid and decrease or no change in samples’ FRAP concentration averages, it may be possible to doubt the confirmed role of corticosteroids in the management of colitis. Further studies with greater sample size and the possibility of taking advantages of more advanced techniques are warranted to support this idea. Also, it is suggested to repeat this study with other animal models for colitis and to run some more studies for evaluating the effectiveness of antioxidant drugs on the treatment of this disease.
ACKNOWLEDGMENTS
We are extremely thankful to Dr. Fatemeh Ghassami and Dr. Arash Hadadgar for their kind help.
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Edward V, Loftus JR. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Jan Praskoa J, Jelenovaa D, Mihal V. Psychological aspects and psychotherapy of inflammatory bowel diseases and irritable bowel syndrome in children. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154:307–14. doi: 10.5507/bp.2010.046. [DOI] [PubMed] [Google Scholar]
- 3.Rufo PA, Bousvaros A. Current therapy of inflammatory bowel disease in children. Paediatr Drugs. 2006;8:279–302. doi: 10.2165/00148581-200608050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–81. doi: 10.1016/s0889-8553(05)70056-x. [DOI] [PubMed] [Google Scholar]
- 5.Karlinger K, Gyorke T, Mako E, Mester A, Tarjan Z. The epidemiology and the pathogenesis of inflammatory bowel disease. Eur J Radiol. 2000;35:154–67. doi: 10.1016/s0720-048x(00)00238-2. [DOI] [PubMed] [Google Scholar]
- 6.Sood A, Midha V. Epidemiology of inflammatory bowel disease in Asia. Indian J Gastroenterol. 2007;26:285–9. [PubMed] [Google Scholar]
- 7.Harputluoglu MM, Demirel U, Yucel N, Karadag N, Temel I, Firat C, et al. The effects of Gingko biloba extract on acetic acid induced colitis in rats. Turk J Gastroenterol. 2006;17:177–82. [PubMed] [Google Scholar]
- 8.Jahanshahi G, Motavase V, Hashtroudi A, Daryani N, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2005;49:1752–7. doi: 10.1007/s10620-004-9564-5. [DOI] [PubMed] [Google Scholar]
- 9.Roediger WE. Review article: Nitric oxide from dysbiotic bacterial respiration of nitrate in the pathogenesis and as a target for therapy of ulcerative colitis. Aliment Pharmacol Ther. 2008;27:531–41. doi: 10.1111/j.1365-2036.2008.03612.x. [DOI] [PubMed] [Google Scholar]
- 10.Pavlick KP, Laroux S, Fuseler J, Wolf RE, Gray L, Hoffman J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–22. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 11.Rezaie A, Parker R, Abdollahi M. Review article: Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015–21. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 12.Reuter KC, Grunwitz CR, Kaminski BM, Steinhilber D, Heinfried H, Radeke HH, et al. Selective glucocorticoid receptor agonists for treatment of inflammatory bowel disease-studies in mice with acute TNBS colitis. J Pharmacol Exp Ther. 2012;341:68–80. doi: 10.1124/jpet.111.183947. [DOI] [PubMed] [Google Scholar]
- 13.Wallace JL, Mcknight W, Asfahs S, Liu YD. Reduction of acute and reactivated colitis in rats by an inhibitor of neutrophil activation. Am J Physiol Gastrointest Liver Physiol. 1998;274:802–8. doi: 10.1152/ajpgi.1998.274.5.G802. [DOI] [PubMed] [Google Scholar]
- 14.Bilsel Y, Bugra D, Yamaner S, Buluta T, Cevikbas U, Turkogluc U. Could honey have a place in colitis therapy? Effects of honey, prednisolone, and disulfiram on inflammation, nitric oxide, and free radical formation. Dig Surg. 2002;19:306–12. doi: 10.1159/000064580. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer SB. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:53–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 16.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, et al. Animal model; CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paiva LA, Gurgela LA, Silva RM, Tomé AR, Gramosa NV, Silveira ER, et al. Anti-inflammatory effect of kaurenoic acid, a diterpene from Copaifera langsdorffii on acetic acid-induced colitis in rats. Vascul Pharmacol. 2002;39:303–7. doi: 10.1016/s1537-1891(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 18.Araki Y, Sugihara H, Hattori T. The free radical scavengers edaravone and tempol suppress experimental dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:331–4. [PubMed] [Google Scholar]
- 19.Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, et al. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol. 2005;207:164–76. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- 20.Kohen R, Nyska A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 21.Rezaie A, Ghorbani F, Eshghtork A, Zamani M, Dehghan G, Taghavi, et al. Alterations in salivary antioxidants, nitric oxide, and transforming growth factor-β1 in relation to disease activity in crohn's disease patients. Ann N Y Acad Sci. 2006;1091:110–22. doi: 10.1196/annals.1378.060. [DOI] [PubMed] [Google Scholar]
- 22.Tahan G, Gramignoli R, Marongiu F, Aktolga S, Cetinkaya A, Tahan V, et al. Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig Dis Sci. 2011;56:715–20. doi: 10.1007/s10620-010-1364-5. [DOI] [PubMed] [Google Scholar]
- 23.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: Impact on histone acetylation and deacetylation, NF-B and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–67. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Keshavarzian A, Fusunyan RD, Jacyno M, Winship D, MacDermott RP, Sanderson IR. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: Evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 2004;94:704–12. doi: 10.1111/j.1572-0241.1999.00940.x. [DOI] [PubMed] [Google Scholar]
- 25.Ho G, Chiam P, Drummond H, Loane J, Arnott R, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: Analysis of a 5- year UK inception cohort. Aliment Pharmacol Ther. 2006;24:319–30. doi: 10.1111/j.1365-2036.2006.02974.x. [DOI] [PubMed] [Google Scholar]
- 26.Kang JW, Kim TW, La JH, Sung TS, Kim HJ, Kwon YB, et al. Electroacupuncture ameliorates experimental colitis induced by acetic acid in rat. J Vet Sci. 2004;5:189–95. [PubMed] [Google Scholar]
- 27.Graybill RJ, Bocanegra R, Najvar LK, Loebenberg D, Luther MF. Granulocyte colony-stimulating factor and azole antifungal therapy in murine aspergillosis: Role of immune suppression. Antimicrob Agents Chemother. 1998;42:2467–73. doi: 10.1128/aac.42.10.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolic B, Zhao G, Swenson K, Sykes M. A novel application of cyclosporine A in nonmyeloablative pretransplant host conditioning for allogeneic BMT. Blood. 2000;96:1166–72. [PubMed] [Google Scholar]
- 29.Mosier HD, Jr, Smith FG, Jr, Schultz MA. Failure of catch-up growth after Cushing's syndrome in childhood. Am J Dis Child. 1972;124:251–3. doi: 10.1001/archpedi.1972.02110140101015. [DOI] [PubMed] [Google Scholar]
- 30.Holbrook WP, Sofaer JA, Southam JC. Experimental oral infection of mice with a pathogenic and a non-pathogenic strain of the yeast Candida albicans. Arch Oral Biol. 1983;28:1089–91. doi: 10.1016/0003-9969(83)90163-2. [DOI] [PubMed] [Google Scholar]
- 31.McCafferty DM, Miampamba M, Sihota E, Sharkey KA, Kubes P. Role of inducible nitric oxide synthase trinitrobenzene sulphonic acid induced colitis in mice. Gut. 1999;45:864–73. doi: 10.1136/gut.45.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 33.Benzil I, Strain J. Ferric reducing/antioxidant power assey: Direct measure of total antioxidant activity of biological fluids and modified version for stimultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 34.Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44:1309–15. [PubMed] [Google Scholar]
- 35.Bleau G, Giasson C, Brunette I. Measurement of hydrogen peroxide in biological samples containing high levels of ascorbic acid. Anal Biochem. 1998;263:13–7. doi: 10.1006/abio.1998.2801. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee D, Jacob J, Kunjamma G, Ghos S. Measurement of urinary hydrogen peroxide by FOX-1 method in conjunction with catalase in diabetes mellitus-sensitive and specific approach. Clin Chim Acta. 2004;350:233–6. doi: 10.1016/j.cccn.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Noa M, Más R, Carbajal D, Valdés S. Effect of D-002 on acetic acid-induced colitis in rats at single and repeated doses. Pharmacol Res. 2000;41:391–5. doi: 10.1006/phrs.1999.0596. [DOI] [PubMed] [Google Scholar]
- 38.Varshosaz J, Emami J, Fassihi A, Tavakoli N, Minaiyan M, Ahmadi F, et al. Effectiveness of budesonide-succinate-dextran conjugate as a novel prodrug of budesonide against acetic acid-induced colitis in rats. Int J Colorectal Dis. 2010;25:1159–65. doi: 10.1007/s00384-010-1026-2. [DOI] [PubMed] [Google Scholar]
- 39.Karaca T, Şimşek N, Uslu S, Kalkan Y, Can I, Kara A, et al. The effect of royal jelly on CD3(+), CD5(+), CD45(+) T-cell and CD68(+) cell distribution in the colon of rats with acetic acid-induced colitis. Allergol Immunopathol (Madr) 2012;40:357–61. doi: 10.1016/j.aller.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 40.El-Medany AH, Guemei AA, Hagar HH, El-Medany JH, Baraka AM. Comparative study between effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on acetic acid-induced ulcerative colitis in rats. Int Res J Pharm Pharmacol. 2011;1:100–8. [Google Scholar]


