Abstract
Background
Calpurnia aurea is an African medicinal plant used in many countries in Africa to treat a range of medical conditions or disorders. Extracts of the plant were shown to be active in antibacterial and antioxidant assays as well as against lice, ticks and maggots. The aim of the study was to isolate the phytochemical constituents from the plant and to test them in appropriate bioassays dependent on the compounds isolated in order to provide a rationale for the use of the plant in ethno-medicine or to provide some information on its constituents.
Materials and methods
The stem and bark of the plant was extracted with organic solvents of varying polarity and the extracts separated and purified using column chromatography. The isolated compounds were identified by NMR spectroscopy and the compounds were tested for their in vitro anticancer activity against breast (MCF7), renal (TK10) and melanoma (UACC62) human cell lines using an in house method developed at the CSIR, South Africa.
Results
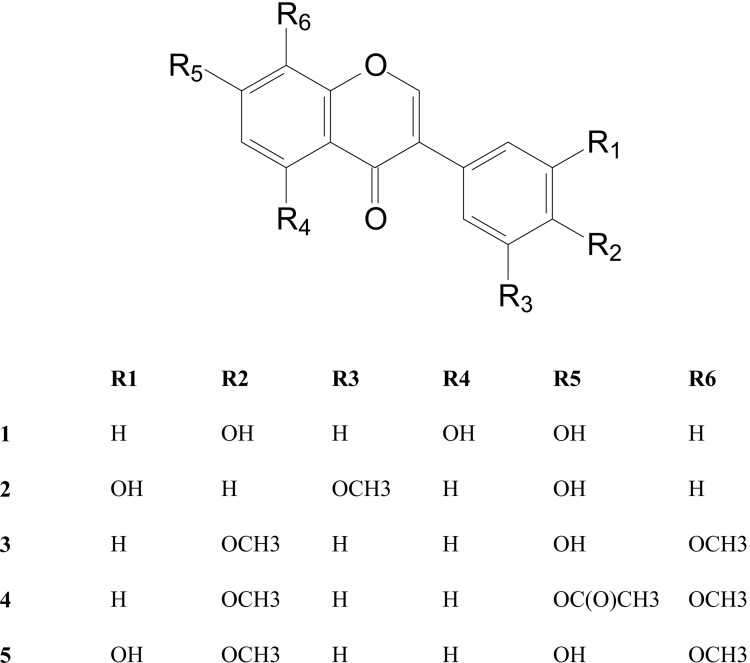
The isoflavones, 4′,5,7-trihydroxyisoflavone (1), 7,3′-dihydroxy-5′-methoxyisoflavone (2), 7-hydroxy-4′,8-dimethoxyisoflavone (3), 7-acetoxy-4′,8-dimethoxyisoflavone (4) and 3′,7-dihydroxy-4′,8-dimethoxyisoflavone (5), a pterocarpan (3-acetoxy-9-methoxypterocarpan) and a quinolizidine alkaloid (calpurnine) were isolated from the stem and bark of Calpurnia aurea. The tetrasubstituted isoflavone 5 was found to be the most active in the three cell lines amongst all the compounds tested. This was followed by trisubstituted isoflavone 2.
Conclusion
The isoflavones showed moderate activity against the renal, melanoma and breast cancer cell lines tested against, with the isoflavones 2 and 5 showing the best activity of the compounds tested. These isoflavones may have a synergistic effect with other anticancer drugs.
Keywords: Calpurnia aurea; Fabaceae; 5,6′-dihydroxy-2′,6-dimethoxyisoflavone; anti cancer
Introduction
Calpurnia aurea (Ait.) Benth, a shrub to slender tree of up to 15 m tall, widespread along the east coast of Africa. The genus Calpurnia E.Mey. was initially considered to belong to the Sophora group of the primitive tribe Sophoreae sensu Polhill (1981) and was later transferred to the tribe Poldalyrieae of the Papilionaceae subfamily (Polhill et al., 1994; Van Wyk and Schutte, 1995; Van Wyk, 2005). There are seven Calpurnia species (eight taxa) (Beaumont et al., 1999) of which only one, C. aurea (syn. Calpurnia subdecandra (L'Hérit.) Schweick.) has been investigated for its phytochemical constituents. There have been several previous investigations of the plant under subspecies aurea and sylvatica. However, C. aurea subsp. sylvatica (Burch.) Brummitt is no longer considered distinct from C. aurea subsp. aurea and has accordingly been synonymised (Beaumont et al., 1999). The Indian endemic C. aurea subsp. indica Brummitt, is though, still recognised.
It is used by the Shinasha people of Northern Ethiopia to treat amoebiasis and giardiasis while the Amhara people from the same region use the leaves to treat malaria and the seeds to treat hypertension while a combination of the leaves and seeds are used to treat diarrhoea, rabies and diabetes (Giday et al., 2007). The plant has also been used as an insecticide to kill lice (Palmer and Pitman, 1972; Waka et al., 2004), to induce uterine contractions (Desta et al., 1994), and to treat coughs, amoebic dysentery, syphilis, leishmaniasis, tapeworm, trachoma, ringworm, scabies, elephantiasis, abscesses and wounds as well as stomach ache, vomiting, headache and eye diseases (Jansen, 1981; Abebe, 1986; Asres et al., 2001; Tadeg et al., 2005; Teklehaymanot and Giday, 2007). Both in East and southern Africa, plant extracts are employed in treating wounds infested with maggots (Palmer and Pitman 1972; Kokwaro, 1976), to the extent that its Zulu name is umKhiphampethu, meaning “maggot-extracter”. Its widespread application for diverse ethno-medicinal uses has made it a subject for pharmacological (Desta et al., 1994) and phytochemical studies.
Pharmocological studies have shown that the methanol extracts of the leaves and stems of C. aurea have good antibacterial and antioxidant properties (Tadeg et al., 2005; Adedapo et al., 2008), validating its traditional use for a range of microbial infections. Insecticidal activity was also shown by the methanol and water extracts against the rice weevil (Sitophilus oryzae) (Louis et al., 2007), in keeping with its ethnobotanical use against lice and maggots. The oil extract of the dried leaves was observed to attract and be toxic to two species of ticks, Rhipicephalus pulchellus and Rhipicephalus appendiculatus, revealing a potential application as an acaricidal trap bait (Nana et al., 2010; Zorloni et al., 2010).
Two early phyto-chemical studies of C. aurea subsp. aurea reported the isolation of agglutinins from the seeds to antigens A and B of human erythrocytes (Bird, 1957; Potapov, 1968), whilst a third (as syn. C. subdecandra) yielded the novel quinolizidine alkaloid, calpurnine (Goosen, 1963). Subsequent investigations reported several more quinolizidine alkaloids, characteristic chemotaxonomic markers for the Fabaceae, bringing the total number of alkaloids isolated to 15 (van Eijk and Radema, 1977; Radema et al., 1979; Asres et al., 1986a; 1986b; Kubo et al., 1984).
Apart from the quinolizidine alkaloids, the flavonoids vicenin-2 (6,8-di-β-D-glucopyranosyl-5,7,4′-trihydroxyflavone), butin (7,3′,4′-trihydroxyflavanone) and 3′-hydroxydaidzein (7,3′,4′-trihydroxyisoflavone) were isolated from the seeds of C. aurea, in keeping with flavonoids being the other major class of compounds consistently found in the Fabaceae (de Nysschen et al., 1998).
Since there have no previous reports on the wood and stem bark of C. aurea, we have carried out a phytochemical analysis of these components to enable a more complete phytochemical analysis of this species. We report herein the isolation of five isoflavonoids, a pterocarpan and a quinolizidine alkaloid from the stem and bark of C. aurea as well as the anticancer activity of the isolated isoflavonoids. Isoflavones and in particular genistein (5,7,4′-trihydroxyisoflavone) are known to possess antitumor effects (Barnes, 1997) by preventing the formation of hormone induced breast cancer (Bruneton, 1995). Since the isoflavones here isolated from C. aurea were all substituted at the 7 and 4′ positions, similar to genistein, they were ideal candidates for the evaluation of their anticancer activity.
Materials and Methods
General Experiment Procedures
The melting points were recorded on an Ernst Leitz Wetzler micro-hot stage melting point apparatus. UV spectra were recorded on a Varian Cary UV-VIS Spectrophotometer and IR spectra were recorded on a Perkin-Elmer Universal ATR Spectrometer. The 1H, 13C and 2D NMR spectra were recorded using a Bruker AvanceIII 400 MHz spectrometer at room temperature using either deuterated methanol (CD3OD) or deuterated chloroform (CDCl3) as solvents. Specific rotations were measured at room temperature in methanol on a PerkinElmerTM, Model 341 Polarimeter with a 10 mm flow tube. For GC-MS analyses, the samples were analysed on an Agilent GC–MSD apparatus equipped with DB-5SIL MS (30 m x 0.25 mm i.d., 0.25 µm film thickness) fused-silica capillary column. Helium (at 2 ml/min) was used as a carrier gas. The MS was operated in the EI mode at 70 eV.
Plant collection and extraction
The stem and bark of Calpurnia aurea (Ait.) Benth. were obtained from a cultivated specimen in Kloof, Durban. A voucher specimen (N. Crouch 1279, NH) was deposited at the KwaZulu-Natal Herbarium, Durban, South Africa for verification purposes. The stem and bark was milled and then extracted separately using a Soxhlet apparatus with hexane, dichloromethane, ethyl acetate and methanol successively for 24 hours each. The dry milled stem and bark (651.8 g mass) yielded 3.2 g, 3.4 g, 10.7 g, and 72.1 g extracts for each of the four solvents mentioned above.
Separation and purification
The separation, isolation and purification of compounds were carried out by gravity column chromatography using Merck silica gel 60 (0.040–0.063 mm) and monitored by thin layer chromatography (TLC; Merck 20 × 20 cm silica gel 60 F254 aluminum sheets). The hexane extract of the stem and bark (3.2 g) was separated successively with 100% hexane and then a hexane : dichloromethane step gradient (10% increments up until 100% dichloromethane), with 20 fractions of 100 ml being collected in each stage off a 4cm diameter column. Further purifications were carried out in 1 cm diameter columns collecting 5 ml fractions. Fraction 10 was purified further with 15% dichloromethane in hexane to produce 7-acetoxy-4′,8-dimethoxyisoflavone (40.1 mg) (4) in fraction 21–22. Fraction 42 was purified further using the same solvent system to afford 3-acetoxy-9-methoxypterocarpan (42.7 mg) in fractions 7–9.
The dichloromethane extract of the stem and bark (3.40 g) was separated on a 3cm diameter column sequentially using 1L each of a dichloromethane: methanol step gradient with 100% dichloromethane, and then 2%, 4%, 6% and 8% methanol in dichloromethane. A total of 50 × 100 mL fractions were collected with ten fractions being collected for each stage. Subsequent purifications were carried out on 1 cm diameter columns collecting 5 ml fractions. Fraction 8 was purified with 1% methanol in dichloromethane, where fraction 3 was further purified with the same solvent system to afford 7-hydroxy-4′,8-dimethoxyisoflavone (3) (49.6 mg) in fractions 14–17. Fraction 32 of the crude column was also purified with 1% methanol in dichloromethane to produce 3′,7-dihydroxy-4′,8-dimethoxyisoflavone (5) (46.7 mg) in fraction 40–48. Fractions 41–50 of the crude column were combined and purified further with 1% methanol in dichloromethane to produce 3′,7-dihydroxy-5′-methoxyisoflavone (2) (37.4 mg) in fraction 3–7.
TLC analysis of ethyl acetate and methanol extracts had similar components and these extracts were combined and separated with a dichloromethane: ethyl acetate step gradient of 100:0, 90:10, 80:20, 60:40, 40:60, 0:100 in a 3 cm column with a total of 120 fractions being collected, (20 x 50 ml fractions for each gradient). Purifications were carried out on 1 cm diameter columns collecting 5 ml fractions. Fractions 12–15 were combined and purified further with 2% methanol in dichloromethane to afford genistein (4′,5,7-trihydroxyisoflavone) (1) (48.9 mg) in fractions 12–15. Fractions 8–10 were combined and purified with 2% methanol in dichloromethane to afford calpurnine (7) (39.8 mg) in fractions 69–74.
Compounds 1–7 were identified from their 1H and 13C NMR, IR, UV and MS data as well as their physical characteristics and mp and verified by comparing the data to those found in the literature.
Anticancer activity
Anti-cancer screening was carried out using a method developed by the CSIR (South Africa) in 1999 and is known as the three cell prescreening method (Fouche et al., 2006; 2008). Breast (MCF-7), renal (TK-7) and melanoma (UACC-62) cell lines were chosen due to their high sensitivity to detect anticancer activity (Fouche et al., 2008). The three cell lines were grown in Roswell Park Memorial Institute 1640 (RPMI 1640) medium containing 5% fetal bovine serum and 2µM L-glutamine. The cells were then inoculated into 96-well micro-titer plates with densities ranging between 5,000 and 40,000 cells per well. A volume of 100 µL of the medium was introduced into the micro-titer plates and subsequently incubated at 37°C in a 5:95 (carbon dioxide: air) atmosphere with 100% relative humidity for 24 hours.
The test compounds were dissolved in dimethyl suphoxide (DMSO) and added to the cells at concentrations ranging between 0.001 µg/ml and 100 µg/ml. The cells were then incubated for 48 hours at 37 °C in a humidified atmosphere, followed by the fixing of the cells in situ with trichloroacetic acid (TCA) and staining with 100 µL sulforhodamine B (SRB) solution. Unbound dye was removed by washing with 1% acetic acid and air drying the plates. Bound stain was solubilized with 10 µM trizma base and the optical density was read on an automated plate reader at a wavelength of 540 nm. The results were analysed according to methodology in the literature (Monks et al., 1991).
Results and Discussion
The stem and bark hexane extract yielded the widely studied genistein (4′,5,7-trihydroxyisoflavone) (1) (Wang et al., 1999; Dixon and Ferreira, 2002), 5′,7-dihydroxy-3′-methoxyisoflavone (2) (An et al., 2008; Li et al., 2009), 7-hydroxy-4′,8-dimethoxyisoflavone (8-O-methylretusin; isoafrormosin) (3) (Jurd et al., 1972; Hayashi and Thomson, 1974; Harper et al., 1976; Chen et al., 1983), 7-acetoxy-4′,8-dimethoxyisoflavone (4) and 3′,7-dihydroxy-4′,8-dimethoxyisoflavone (5) (Harper et al., 1976; de Oliveira et al., 1978; Albuquerque et al., 1981), along with a pterocarpan, 3-acetoxy-9-methoxypterocarpan (6) (Al-Ani et al., 1984) and a quinolizidine alkaloid calpurnine (7) (Asres et al., 1986a).
A search on Scifinder® indicated that 4 had not been isolated from a plant source prior to this work, however it has been prepared by the acetylation of 8-O-methylretusin (3) (Jurd et al., 1972; Hayashi and Thomson, 1974; Harper et al., 1976; Chen et al., 1983). The NMR data reported in Hayashi and Thomson (1974) for both compounds 3 and 4 are erroneous in that the assignments of the two methoxy resonances must be interchanged (8-°CH3 should be at δH 4.06 and 4'-°CH3 at δH 3.90), since our NOESY data shows that the 4′-methoxy resonance shows a NOESY correlation to the H-3′/5′ resonance at δH 6.90.
The five isolated isoflavones were either tri-or tetra-substituted at positions 5, 7 and 8 on the A ring and 3′, 4′ or 5′ on the phenyl ring (ring C). Biosynthetically, substitution at the 5 and 7 positions occur readily because of the polyketide pathway, however species within the Sophoreae have also been popularly substituted at the 7 and 8 positions as well as at the 3′ and 4′ positions on the phenyl ring (Harper et al., 1976; Albuquerque et al., 1981; Bezuidenhout et al., 1988), consistent with the isoflavones isolated from C. aurea in this work. It is highly likely that the isoflavones 3–5 follow the same biosynthetic pathway and most probable that 1 and 2 is also linked to this pathway prior to dehydroxylations and demethoxylations taking place en route to 3–5. The other isoflavonoid isolated from the seeds, 3′-hydroxydaidzen (de Nysschen et al., 1998), is also hydroxylated at the 7, 3′ and 4′ positions. Furthermore, the isolation of 1, 3 and 5 from Monopteryx inpae W.A.Rodrigues (Albuquerque et al., 1981) and 3 and 5 from Xanthocercis zambesiaca (Baker) Dumaz-le-Grand (Harper et al., 1976) of the Sophoreae; demonstrate the relatively close relationship of the tribes Podalyrieae and Sophoreae within the subfamliy Papilionoideae.
The anticancer activity of the isoflavonoids 2–5 are shown in table 1 in the form of the response parameters GI50, TGI and LC50, which are interpolated values from the dose response curves where the net percentage growth is plotted against the concentration of each compound and represent the concentrations of the compounds in µ/ml at which the net percentage growth is +50, 0 and −50, respectively. Genestein (1) was not subject to the anticancer screening as we wanted to explore the effects of the 7,8-dioxygenated isoflavones on the cancer cell lines. The 7-hydroxylated isoflavone (2) was included because of the dioxygenation on the phenyl ring.
Table 1.
Growth Inhibition values for compounds 2–5 against TK-10, UACC-62 and MCF-7 cell lines.
| Compound | Dose-response parameters |
Line 1 (TK-10) Renal |
Line 2 (UACC-62) Melanoma |
Line 1 (MCF-7) Breast |
| 2 | GI50 | 50.57 | 31.01 | 45.51 |
| TGI | N/A | 60.28 | 78.40 | |
| LC50 | N/A | 89.55 | N/A | |
| 3 | GI50 | 55.44 | 40.14 | 69.05 |
| TGI | N/A | 90.41 | N/A | |
| LC50 | N/A | N/A | N/A | |
| 4 | GI50 | 69.89 | 53.34 | 66.19 |
| TGI | N/A | N/A | N/A | |
| LC50 | N/A | N/A | N/A | |
| 5 | GI50 | 45.81 | 27.35 | 31.92 |
| TGI | 91.06 | 52.49 | 57.22 | |
| LC50 | N/A | 77.62 | 82.52 | |
| Etoposide | GI50 | 4.88 | 0.74 | 0.57 |
| TGI | 36.77 | 16.41 | N/A | |
| LC50 | 85.38 | 84.58 | N/A | |
All the tested compounds exhibited dose-responsive inhibition up to 100 µg/mL, with compounds 2, 3 and 5 being most active against the melanoma (UACC-62) cell line with GI50 values of 31.01, 40.14 and 27.35 µ/ml. Compounds 2 and 5 were also active against the breast (MCF-7) cell line with GI50 values of 45.51 and 31.92 µ/ml. From all the compounds tested, compound 5 seemed to have the best overall activity in all three cell lines having the lowest GI50 in each at 45.81, 27.35 and 31.92 µ/ml and was the only compound of those tested to show total growth inhibition (TGI) for all three cancer cell lines at below 100 µ/ml and a TGI for the melanoma (UACC-62) cell line of 52.49 µ/ml. However, the compounds were not as active as the control, etoposide.
Structurally, compounds 3–5 are all methoxylated at both the 8-and 4′-positions with compound 4 being acetylated at C-7 whereas the others are hydroxylated at C-7. Compound 5 also contains an extra hydroxyl group at the 3′ position on the phenyl ring and this added hydroxyl group resulted in improved anticancer activity. Acetylation at C-7 however led to a loss of activity as seen in compound 4, which was the most inactive of all the compounds tested. Compound 2 had a unique substitution pattern to the other three compounds tested, in that it was the only isoflavone not to be methoxylated at C-8 and C-4′. Nevertheless, hydroxylation at C-7 and methoxylation and hydroxylation, at each of the meta positions on the phenyl ring resulted in better activity than all the other isoflavones tested with the exception of compound 5.
It is reported that isoflavones from soybean also have preventive anticancer activity and that the methylated isoflavones (glycitein, biochanin A and formononetin) have much greater anticancer activity than those without methyl groups (Walle et al., 2007). Studies suggest that isoflavones with methoxy groups appear to have more beneficial qualities than their non-methylated counterparts and have been shown to be more bioavailable and biologically stable than the hydroxylated isoflavones (Wen et al., 2006). Although the isolated isoflavones isolated in this work is not as active as the control, etoposide, it is worth noting that they do show moderate activity against the cancer cell lines tested.
Conclusion
The stem and bark of C. aurea was investigated phytochemically for the first time and yielded a quinolizidine alkaloid, calpurnine found in other parts of the plant as well five isoflavones and a pterocarpan. Isoflavones were only found in the seed of C. aurea previously and all the isoflavones isolated in this work as well as the pterocarpan were isolated for the first time from this source. Furthermore, the isoflavones were shown to have moderate activity against the renal, melanoma and breast cancer cell lines tested against, with the 7-hydroxy-8-methoxy substitution on the chromone ring and 3′-hydroxy-4′-methoxy substitution on the phenyl ring as in compound 5 showing the best activity. This moderate activity shows that the plant extracts have the potential to be used as moderate anticancer therapy, preferably to enhance the effect of other known anticancer drugs.
Figure 1.
Structures of isoflavones isolated from Calpurnia aurea
Acknowledgements
The authors are grateful to the NRF for a grant holders bursary for funds used throughout the duration of this project.
References
- 1.Abebe W. A survey of prescriptions used in traditional medicine in Gonda region, Northwest Ethiopia: general pharmaceutical practice. J Ethnopharmacol. 1986;18:147–165. doi: 10.1016/0378-8741(86)90027-9. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ani H A M, Dewick P M. Isoflavonoid Biosynthesis: Concerning the aryl migration. J Chem Soc Perkin Trans I. 1984;12:2831–2838. [Google Scholar]
- 3.Albuquerque F B, Braz R F, Gottlieb O R, Magalhaes M T, Maia J G S, de Oliveira A B, de Oliveira G G, Wilberg V C. Isoflavone evolution in Monopteryx. Phytochemistr. 1981;20:235–236. [Google Scholar]
- 4.An R B, Jeong G S, Kim Y C. Flavonoids from the heartwood of Dalbergia odorifera and their protective effect on glutamate-induced oxidative injury in HT22 cells. Chem Pharm Bull. 2008;56:1722–1724. doi: 10.1248/cpb.56.1722. [DOI] [PubMed] [Google Scholar]
- 5.Adedapo A A, Jimoh F O, Koduru S, Afolayan A J, Masika P J. Antibacterial and antioxidant properties of the methanol extracts of the leaves and stems of Calpurnia aurea. BMC Complement Altern Med. 2008;8:53–61. doi: 10.1186/1472-6882-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asres K, Gibbons W A, Phillipson J D, Mascagni P. Alkaloids of Ethiopian Calpurnia aurea subsp. aurea. Phytochemistry. 1986a;25:1443–1447. [Google Scholar]
- 7.Asres K, Phillipson J D, Mascagni P. Two novel minor alkaloids from Ethiopian Calpurnia aurea ssp. Aurea. Planta Med. 1986b:302–304. doi: 10.1055/s-2007-969159. [DOI] [PubMed] [Google Scholar]
- 8.Asres K, Bucar F, Kartnig T, Witvrouw M, Pannecouque C, De Clercq E. Antiviral activity against human immunodeficiency virus type 1 (HIV1) and type 2 (HIV 2) of ethnobotanically selected Ethiopian medicinal plants. Phytother Res. 2001;15:62–69. doi: 10.1002/1099-1573(200102)15:1<62::aid-ptr956>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Barnes S. The chemopreventive properties of soy isoflavonoids in animal models of breast cancer. Breast Cancer Res Treat. 1997;46:169–179. doi: 10.1023/a:1005956326155. [DOI] [PubMed] [Google Scholar]
- 10.Beaumont A J, Beckett R P, Edwards T J, Stirton C H. Revision of the genus Calpurnia (Sophoreae: Leguminosae) Bothalia. 1999;29:5–233. [Google Scholar]
- 11.Bezuidenhout S C, Bezuidenhout B C B, Ferreira D. α-Hydroxydihydrochalcones and related 1,3-diarylpropan-2-ones from Xanthocercis zambesiaca. Phytochemistry. 1988;27:2329–2334. [Google Scholar]
- 12.Bird G. Hemagglutinins in Calpurnia aurea. Nature. 1957;180:657. doi: 10.1038/180657a0. [DOI] [PubMed] [Google Scholar]
- 13.Bruneton J. Pharmacognosy, phytochemistry, medicinal plants. Andover, UK: Intercept Limited; 1995. [Google Scholar]
- 14.Chen C C, Chen Y L, Chen Y P, Hsu H Y. A study on the constituents of Millettia reticulata Benth. Taiwan Yaoxue Zazhi. 1983;35:89–93. [Google Scholar]
- 15.Dixon R A, Ferreira D. Genistein. Phytochemistry. 2002;60:205–211. doi: 10.1016/s0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- 16.Desta B. Ethiopian traditional herbal drugs. Part III: anti-fertility activity of 70 medicinal plants. J Ethnopharmacol. 1994;44:199–209. doi: 10.1016/0378-8741(94)01187-7. [DOI] [PubMed] [Google Scholar]
- 17.Fouche G, Cragg G M, Pillay P, Kolesnikova N, Maharaj V J, Senabe J. In vitro anticancer screening of South African plants. J Ethnopharmacol. 2008;119:455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Fouche G, Khorombi E, Kolesnikova N, Maharaj V J, van der Merwe M, Nthambeleni R. Investigation of South African plants for anticancer activity. Pharmacol online. 2006;3:494–500. [Google Scholar]
- 19.Giday M, Teklehaymanot T, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110:516–525. doi: 10.1016/j.jep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Goosen A. The alkaloids of the Leguminosae. Part I. The structure of calpurnine from Calpurnia subdecandra. J Chem Soc. 1963:3067–3068. [Google Scholar]
- 21.Harper S H, Shirley D B, Taylor D A. Isoflavones from Xanthocercis zambesiaca. Phytochemistry. 1976;15:1019–1023. [Google Scholar]
- 22.Hayashi T, Thomson R H. Isoflavones from Dipteryx odorata. Phytochemistry. 1974;13:1943–1946. [Google Scholar]
- 23.Jansen P C M. Agricultural Research Reports 906. Wageningen: Centre for Agricultural Publishing and Documentation; 1981. Spices, condiments and medicinal plants in Ethiopia, their taxonomy and agricultural significance. [Google Scholar]
- 24.Jurd L, Stevens K, Manners G. Isoflavones from the heartwood of Dalbergia retusa. Phytochemistry. 1972;11:2535–2540. [Google Scholar]
- 25.Kokwaro J O. Medicinal plants of East Africa. Nairobi: East African Literature Bureau; 1976. [Google Scholar]
- 26.Kubo I, Matsumoto T, Kozuka M, Chapya A, Naoki H. Quinolizidine alkaloids from the African medicinal plant Calpurnia aurea: molluscicidal activity and structural study by 2D NMR. Agric Biol Chem. 1984;48:2839–2841. [Google Scholar]
- 27.Li X, Li J, Wang D, Wang W, Cui Z. Chromone and flavonoids from Maackia amurensis. Asian J Trad Med. 2009;4:98–103. [Google Scholar]
- 28.Louis S, Delobel B, Gressent F, Duport G, Diol O, Rahioui I, Charles H, Rahbé Y. Broad screening of the Legume family for variability in seed insecticidal activities and for the occurrence of the A1b-like knottin peptide entomotoxins. Phytochemistry. 2007;68:521–535. doi: 10.1016/j.phytochem.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 30.Nana P, Maniania N K, Maranga R O, Kutima H L, Boga H I, Nchu F, Eloff J N. Attraction response of adult Rhipicephalus appendiculatus and Rhipicephalus pulchellus Acari: Ixodidae) ticks to extracts from Calpurnia aurea (Fabaceae) Vet Parasitol. 2010;174:124–130. doi: 10.1016/j.vetpar.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 31.de Nysschen A-M, van Wyk B-E, van Heerden F R. Seed flavonoids of the Podalyrieae and Liparieae. Pl Syst Evol. 1998;212:1–11. [Google Scholar]
- 32.de Oliveira A B, Iracema M, Madruga L M, Gottlieb O R. Isoflavonoids from Myroxylon balsamum. Phytochemistry. 1978;17:593–595. [Google Scholar]
- 33.Palmer E, Pitman N. Trees of southern Africa. Vol. 2. Cape Town: A.A Balkema; 1972. [Google Scholar]
- 34.Polhill R M. Sophoreae. In: Polhill R M, Raven P H, editors. Advances in legume systematics, part 1. Royal Botanic Gardens, Kew: 1981. pp. 213–230. [Google Scholar]
- 35.Polhill R M. Classification of the Leguminosae. In: Bisby F A, Buckingham J, Harborne J B, editors. Phytochemical dictionary of the Leguminosae. New York, NY: Chapman and Hall; 1994. pp. xxxv–lvii. [Google Scholar]
- 36.Potapov M I. Plant agglutinins to human antigen A. Izvestiya Akademii Nauk SSSR, Seriya Biologicheskaya. 1968;1:59–66. [PubMed] [Google Scholar]
- 37.Radema M H, van Eijk J L, Vermin W, de Kok A J, Romers C. Alkaloids of South African samples of Calpurnia aurea. Phytochemistry. 1979;18:2063–2064. [Google Scholar]
- 38.Tadeg H, Mohammed E, Asres K, Gebre-Mariam T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol. 2005;100:168–175. doi: 10.1016/j.jep.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Teklehaymanot T, Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3:12–23. doi: 10.1186/1746-4269-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Wyk B-E. Tribe Podalyrieae. In: Lewis G, Schrire B, MacKinder B, Lock M, editors. Legumes of the world. Royal Botanic Gardens, Kew: 2005. pp. 267–271. [Google Scholar]
- 41.Van Wyk B-E, Schutte A L. Phylogenetic relationships in the tribes Podalyrieae, Liparieae and Crotalarieae. In: Crisp M D, Doyle J J, editors. Advances in legume systematics, part 7: phylogeny. Royal Botanic Gardens, Kew: 1995. pp. 283–308. [Google Scholar]
- 42.van Eijk J L, Radema M H. Some alkaloids of Ethiopian Calpurnia aurea and Cadia purpurea. Planta Med. 1977;32:275–279. doi: 10.1055/s-0028-1097600. [DOI] [PubMed] [Google Scholar]
- 43.Waka E M, Hopkins R J, Curtis C. Ethnobotanical survey and testing of plants traditionally used against hematophagous insects in Eritrea. J Ethnopharmacol. 2004;95:95–101. doi: 10.1016/j.jep.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Walle T, Ta N, Kawamori T, Wen X, Tsuji P A, Walle U K. Cancer chemopreventive properties of orally bioavailable flavonoids-methylated versus unmethylated flavones. Biochem Pharmacol. 2007;73:1288–1296. doi: 10.1016/j.bcp.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Nair M G, Strasburg G M, Booren A M, Gray J I. Antioxidant Polyphenols from Tart Cherries (Prunus cerasus) J Agric Food Chem. 1999;47:840–844. doi: 10.1021/jf980936f. [DOI] [PubMed] [Google Scholar]
- 46.Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metabolism and Disposition. 2006;34:1786–1792. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 47.Zorloni A, Penzhorn B L, Eloff J N. Extracts of Calpurnia aurea leaves from southern Ethiopia attract and immobilize or kill ticks. Vet Parasitol. 2010;168:160–164. doi: 10.1016/j.vetpar.2009.10.026. [DOI] [PubMed] [Google Scholar]