Abstract
Background
Combretum vendae A.E. van Wyk (Combretaceae) is used for the treatment of bacterial related infections and oxidative related diseases by indigenous people of South Africa. Dried leaves extracts of C. vendae were investigated for bioactivity against a variety of bacterial strains and their antioxidant potential evaluated.
Materials and methods
Constituents of leaf material were serially extracted using solvents of varying polarities, TLC chromatograms of the fractions were sprayed with 2,2 diphenyl-1-picrylhydrazyl (DPPH) to determine the presence of antioxidant compounds. Bio-autography was used to determine the number of antibacterial compounds active against Staphylococcus aureus, Enterococcus faecalis, Eschericha coli and Pseudomonas aeruginosa. Minimum inhibitory concentration (MIC) values were determined using serial microplate dilution method. The chloroform fraction was subjected to bio-assay guided column chromatography to isolate the active compound.
Results
The mass extracted by different solvents was below 10% dry weight. MIC values for different extracts against different pathogens ranges from 0.08 to 0.64 mg/ml. The compound isolated was identified as acacetin having an Rf value of 0.28 following elution in the Ethanol: Methanol: Water [E: M: W (10: 1.35: 1 v/v). Acacetin had MIC values ranging from 0.16 to 0.35 mg/ml.
Conclusion
We report for the first time the isolation of acacetin as the main antibacterial compound from the leaves of Combretum vendae.
Keywords: Combretum vendae, Minimum Inhibitory Concentration, Bio-autography, acacetin, Phytochemical analysis, Anti-oxidant
Introduction
The World Health Organization's (WHO) estimate indicate that 80% of more than 400 million inhabitants of the world rely chiefly on traditional medicines for their primary health care needs (WHO, 2008). A major part of traditional therapy involves the use of plant extracts or their active principles (Farnsworth et al., 1985). Approximately 25 to 50 % of currently used pharmaceuticals are derived from plants but only few are in use as conventional antimicrobials (Bansal et al., 2012). Western medicine is increasingly being receptive to the use of antimicrobials and other drugs derived from plants, as main stream antibiotics are becoming ineffective due to the emergence of resistant strains. The developments of resistant strains have made diseases intractable to conventional antimicrobials (Bansal et al., 2012). Another concern for the renewed interest in plant antimicrobials in recent years has been the rapid extinction of plant species (Lewis and Elvin-Lewis, 1995). These concerns have provoked renewed interest in the study of medicinal plants in term of conservation and as to whether their traditional uses are supported by actual pharmacological effects. Medicinal plants produce a variety of secondary metabolites with little or no toxic effect that can either inhibit the growth of pathogens or kill them (Cowan, 1995). Bioactive compounds with low toxicity are considered potential candidates for the development of new drugs.
The Combretaceae is a large family comprising at least 600 species (Hutchings et al., 1996). The two most common genera are Combretum with about 370 species and Terminalia, consisting of 250 species (Lawrence, 1951). These genera are widespread in parts of Africa and are easily characterized by the wing-shaped appendages of their fruits, and may be trees, shrubs or climbers (Rogers and Verotta, 1996). Combretum species are used throughout Africa for various medicinal purposes including several bacterial infections and oxidative stress related diseases (Eloff et al, 2008). As such these plants are considered to have antimicrobial compounds that may inhibit bacterial growth. The mechanism by which plants inhibit microbial growth may differ from presently used antibiotics and may have clinical value in treatment of resistant microbial strains. The leaves and bark of Combretum species are used in traditional medicines for treating a variety of conditions including, pneumonia, syphilis, colds, chest coughs, fever and mumps (Hutchings et al, 1996). The main objective of the study was to screen and isolate antibacterial and antioxidant compounds from C. vendae. We report for the first time the isolation of acacetin as the possible major antibacterial and antioxidant compound from the leaves of Combretum vendae. Although the compound has been previously isolated from other plants, including leaves of Premna odorata Blanco (Verbenaceae) (Pinzon et al., 2011) this is the first reported case of isolation, in high concentrations, from the leaves of C. vendae. We also show for the first time that leaves of C. vendae possess remarkable antibacterial activity.
Experimental
Plant collection
Leaves of C. vendae were collected from a tree at the University of Pretoria, (Onderstepoort Campus). The tree was identified by the plant label and Professor J. N. Eloff confirmed the identity (University of Pretoria, Phytomedicine Programme, Onderstepoort). A herbarium specimen (PRU0043278-0) is kept in the toxic plant herbarium at the University of Pretoria. The selection of the species was based on earlier work on the Combretaceae family (Eloff, 1997).
Plant storage
Leaves were separated from stems and dried at room temperature. Dried leaves were ground into a fine powder using a Junkel and Kunkel model A10 mill and stored at room temperature in a closed container in the dark until use.
Extraction procedure
Sequential exhaustive extraction
Finely ground leaves of C. vendae (200 g) were serially extracted using five solvents of varying polarities, viz., hexane (hex). Dichloromethane (DCM), chloroform (Chl), acetone (ACN) and methanol (MeOH), (technical grade; Merck, Johannesburg). In each case 2000 ml of extracting solvent was used. The container and its contents were vigorously shaken for 3–5 hrs on a Labotec model 20.2 shaking machine. The particulate matter was allowed to sediment and the supernatant was filtered using Whatman no. 1 filter papers (Grade 3:6 µm) and concentrated using a rotavaporator (R-11; Buchi, New Castle, USA). Samples were decanted into pre-weighed labeled beakers to determine the mass extracted by each solvent. The process was repeated three times to exhaustively extract the plant material, after which extracts of the same solvent were combined. This afforded five solvent fractions.
Analysis of extracts by Thin-layer chromatography (TLC)
Aliquots of 10 µL of 10 mg/ml of each of the extracts were loaded onto aluminum backed TLC plates (10 × 20 cm). The plates were developed in two mobile systems namely; Hexane: Ethyl acetate (H: E) (2: 1 v/v) and Ethyl acetate: Methanol: Water (E: M: W) (10: 1.35: 1 v/v). The developed plates were air dried in the fume cupboard and thereafter visualized under UV light (254 and 360 nm Camac Universal UV lamp). For further detection of phyto-chemical constituents, the plates were sprayed with either vanillin-sulphuric acid spray reagent (Sigma-Aldrich, Germany) (0.1g vanillin powder in 28 ml methanol and 1 ml sulphuric acid) or anisaldehyde spray reagent (5 % anisaldehyde in ethanol and 5 % sulphuric acid). The plates were then heated at 110 °C for optimal colour development.
Antibacterial activity
Bacterial test organisms
Four bacterial strains were obtained from the Bacteriology Laboratory, Faculty of Veterinary Science, University of Pretoria and used as test organisms. The four most important nosocomial microorganisms i.e. Staphylococcus aureus (ATCC 29213), Enterococcus faecalis (ATCC 21212), Escherichia coli (ATCC 27853) and Pseudomonas aeruginosa (ATCC 25922) were used. The organisms were maintained on Brain Heart Infusion (BHI) (Sigma®) agar.
Bio-autographic method
Developed TLC plates were air-dried overnight and sprayed with a suspension of actively grown test organisms, placed into a tank and incubated overnight at 37 °C in 100% relative humidity. After incubation, the TLC plates were sprayed with a solution of 2 mg/ml of p-iodonitrotetrazolium violet (INT) (Sigma®). Inhibition of growth was indicated by clear zones on the chromatogram (Begue and Kline, 1972).
Minimum inhibitory concentration (MIC)
The MIC of the crude (10 mg/ml) and pure compound (1 mg/ml) was determined by the serial dilution micro-plate assay method using INT solution as an indicator of bacteria growth as described by (Eloff, 1998). The MIC value was recorded as the lowest concentration of the extract that inhibited bacterial growth after 24 and 48 hrs.
Qualitative antioxidant activity (DPPH) assay on TLC
TLC was used to separate extracts as described earlier. The plates were dried in a fume hood. To detect antioxidant activity, chromatograms were sprayed with 0.2% 2-2-diphenyl-1-picryl-hydrazyl (DPPH) (Sigma®) in methanol, as an indicator. The presence of antioxidant compounds were detected by yellow spots against a purple background on TLC plates (Braca et al, 2002).
Isolation of an antibacterial compound
The most active fraction against the tested microorganisms on bio-autography was subjected to Silica gel 60 (63–200 mm) (Merck) in a column (15.5 cm × 10 cm), and eluted with varying mixtures of hexane/ethyl acetate, starting with (10:90) and followed with an increasing gradient of ethyl acetate up to 95%. About 60 fractions of 50 mL each were collected and analyzed by TLC. Fractions with similar composition were combined. Tubes with single spot were combined and concentrated on rotary evaporator, this afforded compound 1 (35mg).
Results and Discussion
Extraction yield was fairly low (below 10% of dry mass), with chloroform having the highest yield compared to other solvents (Table 1). The values found were substantially lower than those found with other Combretum species.
Table 1.
Mass extracted from 1 g and MIC values in mg/ml of C.vendae *leaf extracts against the test pathogens after 24 hours.
| Hex | DCM | Chl | MeOH | ACN | AMP | |
| Mass exctracted (mg) | ||||||
| 28 | 28 | 54 | 30.4 | 27 | ||
| MIC values (mg/ml) | ||||||
| S. aureus | 2.5 | 0.16 | 0.32 | 0.64 | 0.08 | 0.08 |
| E. faecalis | 0.64 | 0.08 | 0.32 | 0.64 | 0.08 | 0.16 |
| P. aeruginosa | 2.5 | 0.64 | 2.5 | 1.25 | 1.25 | 0.13 |
| E.coli | 1.25 | 0.08 | 0.32 | 0.16 | 0.32 | 0.16 |
| Average MIC | 1.72 | 0.24 | 0.86 | 0.55 | 0.45 | |
hexane (Hex), dichloromethane (DCM), chloroform (Chl), methanol (MeOH), acetone (ACN) and positive control ampicillin (AMP
Varieties of compounds were extracted by different solvents when viewed under UV light and thereafter sprayed with vanillin sulfuric acid (results not shown). The HE solvent system separated more components that are moderately polar whereas polar components were adequately revealed by EMW solvent system. Qualitative antioxidant activity was done to determine the free radical scavenging ability of the compounds present in the different extracts. DPPH is a stable free radical and on the interaction with DPPH, antioxidants either transfer electrons or hydrogen atoms to DPPH thus neutralizing its free radical character (Famakin et al., 2005). Some of the extracts showed antioxidant, evident of a yellow color against a purple background on TLC plates. The presence of antioxidant compounds varied with the type of solvent used for extraction (Figure 1). The acetone extract due to its intermediate polarity contained more antioxidant compounds when plates spotted with this extract were eluted in EMW, followed by methanol extract. However, the dichloromethane extract contained the most non-polar antioxidant compounds. This was evident by the fact that most of the compounds contained in the extract could move from the origin of TLC plates when eluted using EMW solvent system. Similar compounds contained in the chloroform extract, that showed antibacterial activity on bio-autography, also exhibited antioxidant activity. Antioxidant compounds are typically polyphenolic compounds such as flavonoids, proanthocyanidins and coumarins (Evans, 1989).
Figure 1.
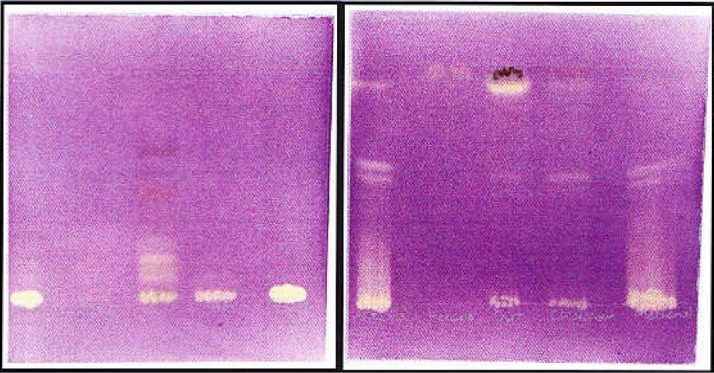
The profile of serial leaf extracts of C. vendae run side by side in HE (left) and EMW (right) solvent system and sprayed with 0.2 % DPPH in methanol. Lanes from left to right: Acetone, hexane, dichloromethane, chloroform and methanol.
To compare the sensitivity of the microorganisms to the extracts, the MIC values were determined. MIC is the lowest concentration of test sample expressed in mg/ml that leads to an inhibition of the growth of test pathogen. There was a substantial difference between the MIC values of different extracts (Table 1). The MIC values for the different extracts against S. aureus varied from 0.08 mg/ml for acetone extract to 2.5 mg/ml for hexane extract, P. aeruginosa (0.64 mg/ml for dichloromethane (DCM) extract to 2.5 mg/ml for chloroform and hexane extracts), E. faecalis (0.08 mg/ml for DCM and acetone extracts to 0.64 mg/ml for hexane and methanol extracts) and E.coli (0.08 mg/ml for dichloromethane extract to 1.25 mg/ml for hexane extract) (Table 1). Pseudonomas aeruginosa was generally more resistant to all the extracts. This may be due to the differences in the bacterial cell wall between Gram negative and Gram positive bacteria (Vlietinck et al., 1995) or the insensitivity of the pathogen to substances contained in the extracts. Since Gram negative bacteria have a thicker peptidoglycan cell wall layer, they tend to be more resistant to the uptake of antimicrobial as compared to their Gram positive counterparts with a thinner peptidoglycan layer. In practice, it is usually more difficult to treat infections resulting from Gram negative bacteria than those from Gram positive bacteria (Vlietinck et al., 1995).
The MIC results did not correlate well with those obtained from the bio-autography (results not shown). This may be due to the fact that some of the compounds that have antibacterial activity on MIC were acting synergistically and their activities were decreased when separated on TLC plates. It may also be possible that some of the active compounds were lost during the air-drying process prior to bio autography. With bioautography, the extracts showed more activity against the Gram-positive bacteria extracts compared to the extracts sprayed with Gram-negative bacteria. The MIC values obtained from C. vendae were compared with the MIC values from the leaf extracts of Combretum woodii obtained in a previous study (Eloff et al., 2005). The leaf extracts of C. vendae had higher activity than C. woodii extracts.
Total activity takes into account not only the MIC value, but also the potency of the active constituent extracted from a plant material (Eloff, 2004). The total activity was calculated by dividing the quantity in mg extracted from 1g of sample by the MIC in mg/ml. Total activity indicates the volume to which the bioactive compounds extracted from 1g of plant material can be diluted and still inhibits growth of bacteria (Table 2). The dichloromethane extract had the highest average total activity compared with the other extracts with the average value of 230 mL/g. Since the chloroform extract contained the highest number of antibacterial compounds, it was thus selected for further fractionation. The Gram-positive pathogens (S. aureus and E. faecalis) were more sensitive than the Gram-negative (P. aeruginosa and E.coli) for the different fractions.
Table 2.
Total activity in ml/g of C. vendae *leaf extracts against four test pathogens.
| Hex | DCM | MeOH | ACN | Chl | |
| E. coli | 22 | 350 | 190 | 84 | 169 |
| P. aeruginosa | 11 | 44 | 24 | 22 | 22 |
| E. faecalis | 44 | 350 | 190 | 169 | 17 |
| S. aureus | 11 | 175 | 48 | 338 | 169 |
| AVERAGE | 22 | 230 | 113 | 153 | 132 |
hexane (Hex), dichloromethane (DCM), methanol (MeOH), acetone (ACN) and chloroform (Chl)
The UV (MeOH) spectrum of the isolated compound showed two maxima at 328nm and 262nm for band I and II respectively. Bathochromic shift of 9nm in the presence of NaOAc indicated the presence of a free C-7 OH. The mass spectrum had a molecular ion peak at m/z = 285 corresponding to the molecular formula C16H12O5 and also an intense signal at 153 (M-C9H8O) indicating methoxy substitution at position 4′. The 1H NMR (DMSO-d6) spectrum had one methoxy signal at δ 3.84 (3H, s,OMe-4′). There was an AA′BB′ system due to ring B at δ6.97 (2H,d, J=8.7Hz, H-3′ H-5′) and δ 8.00 (2H, d, J=8.7, H-2′, H-6′). The presence of free 5-OH group was confirmed by chelated −OH signal at δ 13.0ppm. The 13C-NMR (75MHz) of the isolated compound was compared with the literature (Miyazawa and Hisama, 2003). The spectroscopic data are in good agreement with the literature. The compound was identified as acacetin (4-methoxy apigenin, 5,7-dihydroxy -4′methoxy flavone).
The isolated compound (acacetin) (figure 2) was more active against S. aureus in this experiment. However, the compound had a low activity against P. aeruginosa with an MIC of 0.35 mg/mL (Table 3). Overall activity was very low compared to that of the crude extracts, which had an MIC as low as 0.08 mg/ml for some dichloromethane extracts. This low activity observed maybe due to loss of synergism between components present in the crude.
Figure 2.
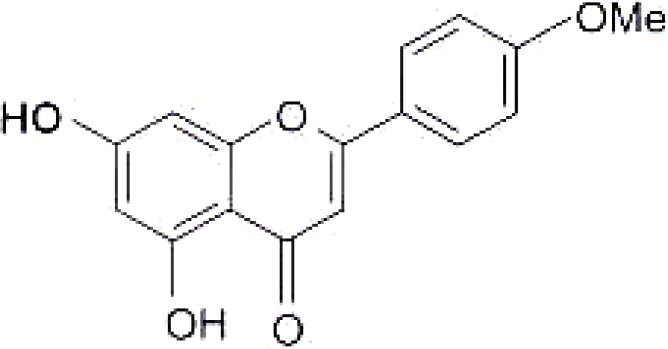
:Structure of acacetin isolated from C. vendae.
Table 3.
MIC values of acacetin in mg/mL
| Acacetin | Ampicillin | |
| S. aureus | 0.16 | 0.08 |
| E. faecalis | 0.32 | 0.16 |
| P. aeruginosa | 0.35 | 0.08 |
| E.coli | 0.32 | 0.16 |
Genkwanin (related to acacetin), isolated from C. erythrophyllum is shown in figure 3. The only difference between the genkwanin and acacetin is the substituent group on carbon 7. Acacetin has a hydroxyl group on carbon 7 whereas 5, hydroxyl-7,4 dimethoxy-flavone from C. erythrophyllum has a methoxy group. The minor difference in the two structures causes a major difference in the antibacterial activity of the two compounds. The MIC of genkwanin isolated from C. erythrophyllum was found to be 0.05 mg/mL (Martini et al., 2004) against E. faecalis, which had a higher activity compared to that of acacetin from C.vendae.
Figure 3.
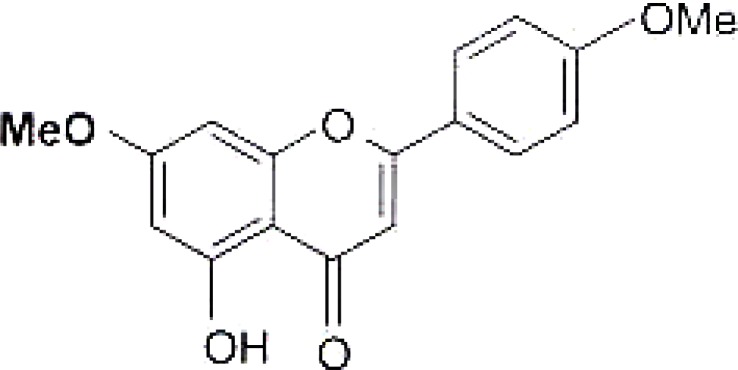
Genkwanin isolated compound from C. erythrophyllum
Acacetin is a flavonoid. Flavonoids are well documented for their biological effects, including antimicrobial and cardiovascular activity, which led to a belief that a diet rich in fruits and vegetables contributes to good health (Williamson et al., 2000).
Conclusion
Acacetin is reported for the first time from C. vendae, which is in the same genus of Angustimarginata with C. erythrophyllum. Although the isolated compound is known, this is the first report of antibacterial activity on acacetin in C. vendae. There are several antibacterial compounds that still need to be isolated from C. vendae.
Acknowledgements
The National Research Foundation (NRF) provided funding for the experiment.
Declaration of interest
There is no conflict of interest. The authors alone are responsible for the content and writing of the paper. All the authors have seen the manuscript.
References
- 1.Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. Journal of Ethnopharmacology. 2002;79:379–381. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 2.Bansal P, Paul P, Mudgal J, Nayak PG, Pannakal ST, Priyadarsini KI, Unnikrishnan MK. Antidiabetic, antihyperlipidermic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin- induced diabetes in mice. Experimental and Toxicologic Pathology. 2012;64(6):651–658. doi: 10.1016/j.etp.2010.12.009. 2012. [DOI] [PubMed] [Google Scholar]
- 3.Begue WJ, Kline R M. The use of tetrazolium salts in bioautographic procedures. Journal of Chromatography. 1972;64(1):182–184. doi: 10.1016/s0021-9673(00)92965-0. [DOI] [PubMed] [Google Scholar]
- 4.Cowan MM. Plant products as antimicrobial agents. Clinical Microbiology Review. 1999;12(1):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deby C, Margotteaux G. Relationship between essential fatty acid and tissue antioxidants levels in mice. CR Soc Biol. 1970;165:2675–2681. [PubMed] [Google Scholar]
- 6.Eloff JN. Monographs in Systematic Botany from the Missouri Garden plants Genes III: Conservation and utilisation of African plants. St Louis: Missouri Botanical Garden Press; 1998. Conservation of Medicinal Plants: Selecting Medicinal Plants for research and gene banking. [Google Scholar]
- 7.Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? Journal of Ethnopharmacology. 1998;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 8.Eloff JN. Quantifying the bioactivity of plant extracts during screening and bioassay guided fractionation. Phytomedicine. 2004;11:370–370. doi: 10.1078/0944711041495218. [DOI] [PubMed] [Google Scholar]
- 9.Eloff JN, Famakin JO, Katerere DRP. Isolation of an antibacterial stilbene from Combretum woodii leaves. African Journal of Biotechnology. 2005;4:1161–1166. [Google Scholar]
- 10.Eloff JN, Katerere DRP, McGaw LJ. The biological activity and chemistry of the Southern African Combretaceae. Journal of Ethnopharmacology. 2008;119:685–699. doi: 10.1016/j.jep.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 11.Evans WC. In Trease and Evans Pharmacognosy. 13th edition. London: Balliere Tindall; 1989. pp. 248–744. [Google Scholar]
- 12.Famakin JO, Eloff JN, Katerere DRP. Isolation of an antibacterial stilbene from Combretum woodii (combretaceae) leaves. African Journal of Biotechnology. 2005;4:1167–1171. [Google Scholar]
- 13.Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985;63(6):965–981. [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchings A, Scott AH, Lewis G, Cunningham AB. In Zulu Medicinal Plants-an Inventory. Pietermaritzburg, South Africa: University of Natal Press; 1996. [Google Scholar]
- 15.Lawrence GHM. In The Taxanomy of Vascular Plants. Macmillan, New York: 1951. [Google Scholar]
- 16.Lewis WH, Elvin-Lewis MP. Medicinal plants as source of new therapeutics. Annals of the Missouri Botanical Garden. 1995;82:16–24. [Google Scholar]
- 17.Martini N, Katerere DRP, Eloff JN. Seven flavonoids with antibacterial activity isolated from Combretum erythrophyllum (Burch) Sond (Combretaceae) S Afr J Bot. 2004;70(3):310–312. [Google Scholar]
- 18.Miyazawa M, Hisama M. Antimutagenic Activity of Phenylpropanoids from Clove (Syzygium aromaticum) Journal of Agricultural Food Chemistry. 2003;51:6413–6422. doi: 10.1021/jf030247q. [DOI] [PubMed] [Google Scholar]
- 19.Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, Mohan H. Comparative antioxidant activity of individual herbal components used in ayurvedic medicine. Phytochemistry. 2003;63:97–104. doi: 10.1016/s0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- 20.Pinzon LC, Uy MM, Sze KH, Wang M, Chu IK. Isolation and characterization of antimicrobial, anti-inflammatory and chemopreventive flavones from Premna odorata Blanco. Journal of Medicinal Plant Research. 2011;5(13):2729–2735. [Google Scholar]
- 21.Rencoret J, Gutiérrez A, Nieto L, Jimérez-Barbero J, Faulds CB, Kim H, Ralph J, Martinez AT, del Rio JC. Lignin composition and structure in Young versus Adult Eucalyptus globulus plants. Plant Physiology. 2011;155(2):667–682. doi: 10.1104/pp.110.167254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers CB, Verotta L. Chemistry and biological properties of the African Combretaceae. In: Hostettmann K, Chinyanganya F, Millard M, Wolfender JL, editors. Chemistry, Biological and pharmacological Properties of African Medicinal Plants. Zimbabwe: University of Zimbabwe Publications; 1996. [Google Scholar]
- 23.Vlietinck AJ, van Hoof L, Tottē J, Lasure A, van den Berghe D, Rwangwabo PC, Vukiyumwami J. Screening of hundred Rwandese medicina1 plants for antimicrobial and antiviral properties. Journal of Ethnopharmacology. 1995;46:31–47. doi: 10.1016/0378-8741(95)01226-4. [DOI] [PubMed] [Google Scholar]
- 24.Willson A, Day AJ, Plumb GW, Couteau D. Human metabolic pathways of dietary flavonoids and cinnamates. Biochemical Society Transactions. 2000;51:9–14. doi: 10.1042/bst0280016. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization, author. Traditional Medicine Fact sheet No 134. 2008. Retrieved from: www.who.int/mediacentre/factsheets/fs134/en/


