Abstract
Background
The present study compares the protective properties of aqueous extracts of six medicinal plants, Phyllanthus emblica, Terminalia chebula (black and yellow), Terminalia arjuna, Balsamodendron Mukul and Alium sativum against lipid per-oxidation in mice brain.
Methods
The antioxidant activities were analyzed by lipid per-oxidation assay, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical assay, total antioxidant activity and metal chelation.
Results
The extracts (fruits and bark) showed inhibition against thiobarbituric acid reactive species (TBARS) induced by pro-oxidant (10 µM FeSO4) in mice brain. Moreover, the free radical scavenging activities of the extracts was evaluated by the scavenging of DPPH radical (IC50, 23.23±1.2 µg/ml (Phyllanthus emblica), 20.24±0.9 µg/ml (Terminalia chebula yellow) and 17.33±1.1 µg/ml (Terminalia chebula black), 19.44±0.45 µg/ml (Terminalia arjuna), 56.59±2.1 µg/ml (Balsamodendron Mukul) and < 200 µg/ml (Alium sativum).
Conclusion
The higher antioxidant and inhibitory effect of Terminalia chebula black in this study could be attributed to its significantly higher phenolic content, Fe(II) chelating ability, reducing ability and free radical scavenging activity. Therefore oxidative stress in brain could be potentially prevented by the intake of these plants.
Keywords: Antioxidant activity, Balb c mice, iron chelation, phenolics, oxidative stress, medicinal plants
Introduction
Reactive oxygen species (ROS) are generated spontaneously in cells during metabolism and are implicated in the aeitology of different degenerative diseases, such as heart diseases, stroke, rheumatoid arthritis, diabetes and cancer (Halliwell et al., 1992). Oxidative stress results from either a decrease of natural cell antioxidant capacity or an increased amount of reactive oxygen species (ROS) in organisms. It is well established, that free radicals are associated with processes that lead to cell degeneration, especially in the brain (Shulman et al., 2004). However, consumption of foods rich in antioxidant phytochemicals may help to fight against degenerative diseases caused by oxidative stress by improving body's antioxidant status. Studies have shown that the use of polyphenolic compounds found in medicinal plants, tea, fruits and vegetables is associated with low risk of these diseases (Hertog et al., 1993). Consequently, there is a great deal of interest in edible plants that contain antioxidants and health-promoting phytochemicals as potential therapeutic agents.
The brain and nervous system are particularly vulnerable to oxidative stress due to limited antioxidant capacity (Vega-Naredo et al., 2005). Most brain cells such as neurons, do not make glutathione, and instead rely on surrounding astrocytes to provide useable glutathione precursors, because the brain has limited access to the bulk of antioxidants produced by the body. Hence, neurons are the first cells to be affected by a shortage of antioxidants, and are most susceptible to oxidative stress (Perry et al., 2004). Recently, phenolic compounds have attracted researchers' interest owing to their antioxidant capacity; they can protect the human body from free radicals, whose formation is associated with the normal natural metabolism of aerobic cells. Polyphenols are common constituents of the human diet, present in most foods and beverages of plant origin. It is considered that they contribute to the prevention of various neurodegenerative diseases such as Alzheimer's diseases. The in vitro studies have shown the antioxidant properties of polyphenols and their ability to modulate the activity of various enzymes (Sabir et al., 2012).
Phyllanthus emblica L. (Euphorbiaceae) is a(n) euphorbiaceous plant of high medicinal value which is widely distributed in China, India, Indonesia, Malaysia and Pakistan. Emblica fruit is well accepted by consumers for its special taste. It has abundant amounts of vitamin C and superoxide dismutase (Verma and Gupta, 2004). The fruit is reported to have hypolipidemic and hypoglycemic activities, and acts as an important constituent of many hepato-protective formulations available (Antarkar et al., 1980; Panda and Kar, 2003).
The dried ripe fruit of Terminalia chebula (Combretaceae), which is a native plant in India and Southeast Asia, is commonly known as black myroblans in English and harad in Hindi and has traditionally been used for a popular folk medicine for homeostatic, antitussive, laxative, for diuretic and cardiotonic treatments (Bartakhar and Arnold, 1991). T. chebula exhibits in vitro antioxidant and free radical-scavenging activities (Cheng et al., 2003). Its antimicrobial, anticancer anti-anaphylaxis and anti-diabetic activities have been reported (Sabu et al., 2002). Chebulinic acid, ellagic acid and gallic acid are the most important phenolics of T. chebula (Saleem et al., 2002). Terminalia arjuna (Combretacea) bark is used as astringent, aphrodisiac, cardiotonic, antiulcer, anti-diabetic and used in diarrhea (Tripathi and Singh, 1996). Terminalia arjuna active constituents include tannins, cardenolide, triterpenoid saponins (arjunic acid, arjunolic acid, arjungenin, arjun glycosides), flavonoids (arjunone, arjunolone, luteolin), gallic acid, ellagic acid, oligomeric proanthocyanidins (OPCs) and phytosterol (Kapoor, 1990). Reported pharmacological activities of Balsamodendron Mukul are anti-hypercholesterolaemic, anti-inflammatory, antibacterial, anti-diabetic and anti-obesity etc (Darshan, 2012). A detailed chemical study of Balsamodendron Mukul revealed that it is a complex mixture of steroids, diterpenoids, aliphatic esters, carbohydrates and amino acids (Rout et al., 2012). Whereas, Alium sativum (Alliaceae) have been claimed to prevent everything from high cholesterol to cancer (Rahman et al., 2012). Garlic contains some sulphur-containing compounds such as alliin, ajoene, diallylsulphide, dithin, S-allylcysteine and enzymes as well as some non sulphur-containing compounds including vitamin B, proteins, minerals, saponins and flavonoids (Olusanmi and Amadi, 2009).
The reports from literature have shown that there is still limited information on potential use of these plants in the management of neurodegenerative diseases associated with oxidative stress. These plants were chosen as they are frequently used in the diet and also in the herbal medicine as antihyperchloesterolaemic drugs. As the antioxidant activity can vary greatly depending on the pro-oxidant used, we determined the effect of aqueous extracts against neurotoxic agents such as iron sulphate. Hence, the objective of this study is to investigate the antioxidant and inhibitory effect of yellow and black Terminalia chebula varieties, Terminalia arjuna, Phyllanthus emblica, Balsamodendron Mukul and Alium sativum on Fe(II) induced lipid per-oxidation in mice brain in vitro.
Materials and Methods
Chemicals
Thiobarbituric acid (TBA), malonaldehyde-bis-dimethyl acetal (MDA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), quercetin, gallic acid and 1,10-phenanthroline were purchased from Sigma Aldrich (St. Louis, MO, USA). Sodium nitroprusside (SNP) was obtained from Merck (Darmstadt, Germany) and ferrous sulphate from Biocehmicals (Lahore).
Preparation of the plant extracts
Fruits of Phyllanthus emblica, Terminalia chebula yellow, Terminalia chebula black, Balsamodendron Mukul, Alium sativum and bark of Terminalia arjuna were collected and authenticated by a botanist. The voucher specimens: Phyllanthus emblica (HU/FEM/RC/S01029), Terminalia chebula yellow (HU/FEM/RC/S01030), Terminalia chebula black (HU/FEM/RC/S01031), Balsamodendron Mukul (HU/FEM/RC/S01032) and Alium sativum (HU/FEM/RC/S01033) were deposited in Herbarium of Faculty of Eastern Medicine and Surgery, Hamdard University Karachi, Pakistan. Dried and finely ground plant material (5 g) was soaked in hot water (250 ml) for 30 minutes and filtered using whatman filter paper. The obtained residues were further extracted twice and then concentrated using a rotary evaporator at 50 °C, giving a percentage yield of 17–19.2%. Serial dilutions of these were made to obtain the desired concentration of plant extracts for the experiment. The aqueous extract was used as these are non toxic and suitable for their use in traditional medicine. All animal studies were used in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male balb c mice (2.0– 2.5 months and 24–30 g), were purchased from National Institute of Health Islamabad and used for in vitro studies. The animals were kept in separate cages with continuous access to food and water in a room with controlled temperature (22 ± 3 °C) and on a 12 h light/dark cycle with lights turned on at 7:00 a.m.
Production of TBARS from brain tissue
Production of TBARS was determined using a modified method (Ohkawa et al., 1979). The mice were anaesthetized with chloroform, sacrificed by decapitation and the brain was quickly removed and placed on ice. One gram of brain tissue were homogenized in cold 100 mM Tris buffer pH 7.4 (1:10 w/v) and centrifuged. The homogenates (100 µl) were incubated with or without 50 µl of the freshly prepared oxidant (iron) and different concentrations of the plant extracts together with an appropriate volume of de-ionized water to give a total volume of 300 µl at 37 °C for 1 hr. The color reaction was carried out by adding 200, 500 µl, 500 µl each of the 8.1% Sodium dodecyl sulphate (SDS), acetic acid (pH 3.4) and 0.6% TBA respectively. The reaction mixtures, including those of serial dilutions of 0.03 mM standard MDA were incubated at 97 °C for 1 h. The absorbance was read after cooling the tubes at 532 nm in a spectrophotometer.
Antioxidant activity by DPPH radical scavenging
The antioxidant activity of the plant extracts were measured using the stable DPPH radical according to the method of Hatano et al., (1998). Briefly 0.25 mM solution of DPPH radical (0.5 ml) was added to the sample solution in ethanol (1 ml) at different concentrations (25–200 µg/ml) of aqueous extracts. The mixture was shaken vigorously and left to stand for 30 minutes in the dark, and the absorbance was measured at 517 nm. The capacity to scavenge the DPPH radical was calculated using the following equation: (%) scavenging = [(Ao -A1)/Ao)] × 100, Where, Ao is the absorbance of the control reaction and A1 is the absorbance of the sample itself. The IC50 values (Extract concentration that cause 50% scavenging) were determined from the graph of scavenging effect percentage against the extract concentration. All determinations were carried out in triplicate.
Total antioxidant assay
The assay was based on the reduction of molybdenum, Mo (VI)-Mo (V) by the extracts and subsequent formation of a green phosphate/Mo (V) complex at acidic pH (Prieto et al., 1999). The extracts at different concentrations (25–200 µg/ml) were mixed with 3 ml of the reagent solution (0.6 M H2SO4, 28 mM sodium phosphate and 4 mM amonium molybdate). The tubes were incubated at 95 °C for 90 mins. The mixture was cooled to room temperature and the absorbance of the solution was measured at 695 nm.
Metal chelating activity
The Fe(II) chelating ability of the aqueous extracts were determined using a modified method of Puntel et al., (2005).
Determination of Phenolics content
The total phenolics content as gallic acid equivalent was determined by the method of Singleton et al., (1999). The aqueous extract (0.5 ml) was added to 2.5 ml, 10% Folin-Ciocalteau's reagent (v/v) and 2 ml of 7.5% sodium carbonate. The reaction mixture was incubated at 45 °C for 40 minutes and the absorbance was measured at 765 nm in the spectrophotometer. Gallic acid was used as a standard phenol. The mean of three readings was used and the total phenol content was expressed as milligrams of gallic acid equivalents/g extract.
Determination of Flavonoid content
The total flavonoids as quercetin equivalents were determined by the method of Kosalec et al., (2004). Quercetin was used to make the calibration curve [0.04, 0.02, 0.0025 and 0.00125 mg/ml in 80% ethanol (v/v)]. The standard solutions or extracts (0.5 ml) were mixed with 1.5 ml of 95% ethanol (v/v), 0.1 ml of 10% aluminium chloride (w/v), 0.1 ml of 1 mol/l sodium acetate and 2.8 ml of water. The volume of 10% aluminium chloride was substituted by the same volume of distilled water in the blank. After incubation at room temperature for 30 min, the absorbance of the reaction mixture was measured at 415 nm. The mean of three readings was used and the total flavonoid content was expressed as milligrams of quercetin equivalents/g of extract.
Statistical analysis
The results were expressed as means ± standard deviation. The data was analyzed by one way ANOVA and different group means were compared by Duncan multiple range (DMR) test where necessary. P < 0.05 was considered significant in all cases. The software Package Statistica was used for analysis of data.
Results
Lipid per-oxidation in mice brain
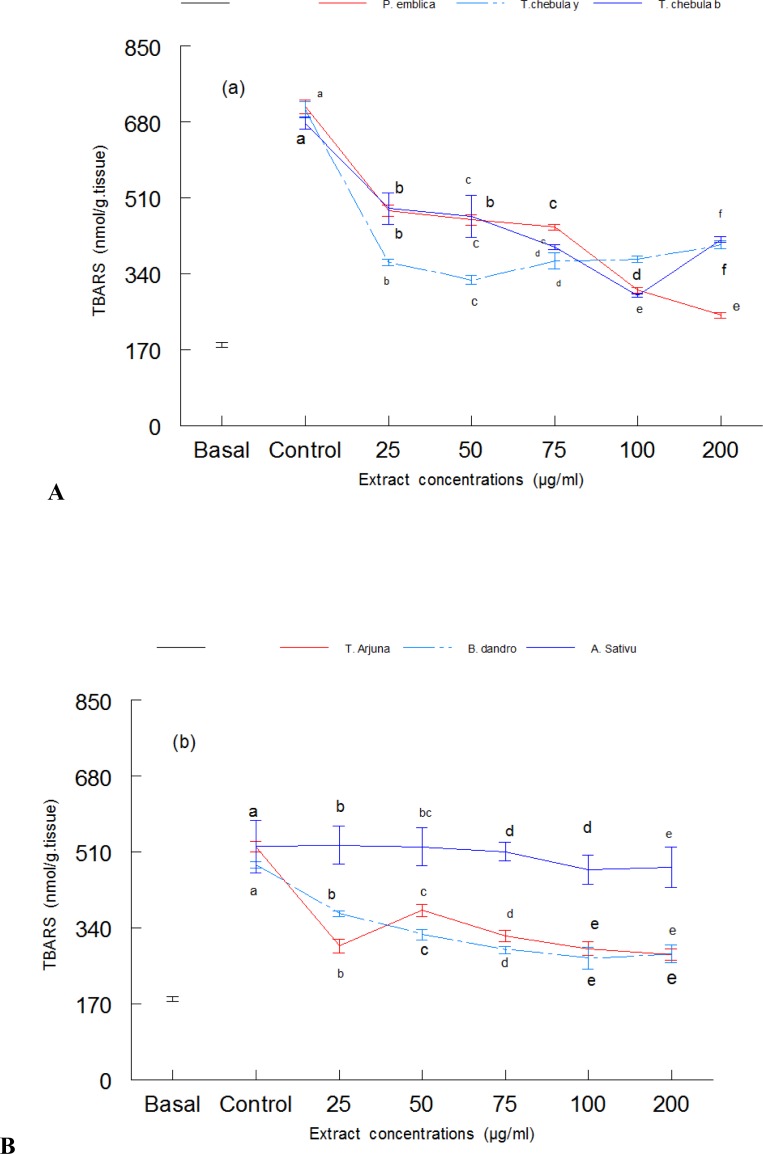
Lipid per-oxidation in mice brain homogenate was induced with iron and the potential antioxidant effect of aqueous extract of plants was determined. Figure 1(a) shows the antioxidant effect of Phyllanthus emblica, Terminalia chebula yellow and Terminalia chebula black in mice brain. Here, the TBARS was induced with 10 µM iron. The results revealed that treatment with Fe(II) caused a significant (P < 0.05) increase in thiobarbituric acid reactive substances (TBARS) compared to the basal. Three separate controls were used for Phyllanthus emblica, Terminalia chebula yellow and Terminalia chebula black (Figure 1). Treatment with different concentrations of Phyllanthus emblica, Terminalia chebula yellow and Terminalia chebula black caused a marked decrease in lipid per-oxidation (Figure 1). The antioxidant effect of Terminalia arjuna, Balsamodendron Mukul and Alium sativum in mice brain is shown in Figure 1(b). The results revealed that treatment with Fe(II) caused a significant (P < 0.05) increase in thiobarbituric acid reactive substances (TBARS) compared to the basal. However, treatment with different concentrations of extracts (25–200 µg/ml) caused a significant decrease in lipid per-oxidation (Figure 1b). Except Alium sativum all the extracts showed ability to reduce the lipid per-oxidation. Phyllanthus emblica and Terminalia chebula black demonstrated higher ability in decreasing lipid per-oxidation compared to the other plant species. Terminalia chebula yellow caused a decrease in lipid peroxidation up to 75 µg/ml, above which it showed the pro-oxidant effect by causing increase in lipid per-oxidation.
Figure 1.
The inhibitory effect of aqueous extracts of plants against lipid peroxidation in mice brain. (a). antioxidant activities of Phyllanthus emblica, Terminalia chebula yellow and Terminalia chebula black on iron sulphate induced lipid peroxidation in mice brain. (b). antioxidant activity of Terminalia arjuna, Balsamodendron Mukul and Alium sativum. The results are means of three separate experiments ± SD. Values in figures followed by different letters are significantly (P < 0.05) different from each other by DMR test.
DPPH radical scavenging activity
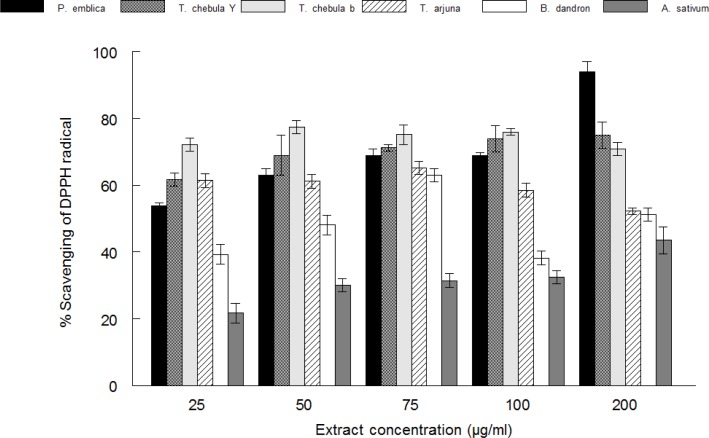
The radical scavenging activities of the extracts were analyzed by measuring the scavenging of stable DPPH radical (Figure 2). All the extracts possessed high antioxidant activity which is evident by their ability to scavenge DPPH radical to higher than 50% (Figure 3). On the basis of their IC50 values (extract concentration that cause 50% scavenging) their antioxidant activity can be ranked as Phyllanthus embilica > Terminalia chebula black > Terminalia chebula yellow > Terminalia arjuna > Balsamodendron Mukul > Alium sativum.
Figure 2.
DPPH radical scavenging activity of Phyllanthus emblica, Terminalia chebula yellow, Terminalia chebula black, Terminalia arjuna, Balsamodendron Mukul and Alium sativum. Values are means ± SD (n=3).
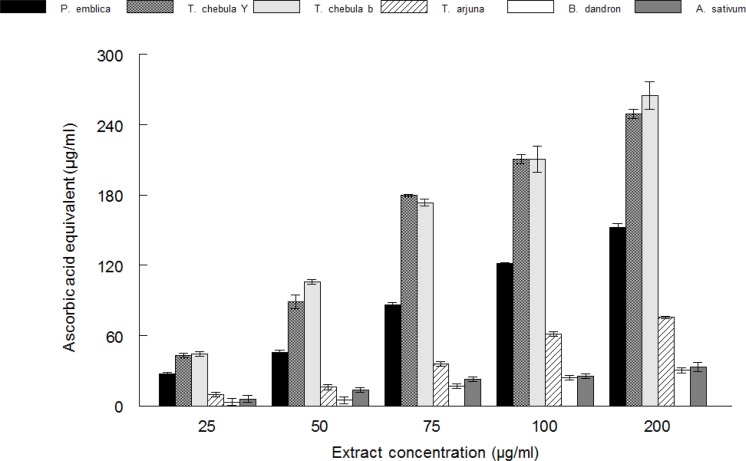
Figure 3.
Total antioxidant activity of Phyllanthus emblica, Terminalia chebula yellow, Terminalia chebula black, Terminalia arjuna, Balsamodendron Mukul and Alium sativum measured by phosphomolybdenum reduction assay. Values are means ± SD (n=3).
Total antioxidant activity by phosphomolybdenum assay
The total antioxidant activity of different plants extract was expressed as ascorbic acid equivalent (Figure 3). All the extracts possessed reducing activity. However, the order of their reactivity was Terminalia chebula black > Terminalia chebula yellow > Phyllanthus emblica > Alium sativum > Balsamodendron Mukul.
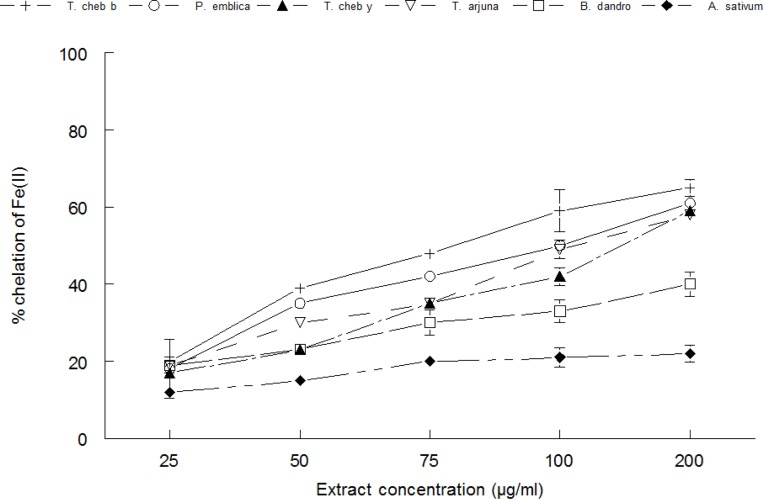
Iron chelating ability
The Fe(II) chelating ability of the extracts is shown in Figure 4. The order of chelation was Terminalia chebula black > Phyllanthus emblica > Terminalia chebula yellow > Terminalia arjuna > Balsamodendron Mukul.
Figure 4.
The Fe(II) chelating ability of Phyllanthus emblica, Terminalia chebula yellow, Terminalia chebula black, Terminalia arjuna, Balsamodendron Mukul and Alium sativum measured by reaction with 1,10-phenanthroline. Values are means ± SD (n=3).
Phytochemical analysis of plants
Phyto-chemical analysis of plants showed the high content of phenolics and flavonoids. Mean values of total phenolic and flavonoid content are shown in Table 1. The results revealed that the highest phenolic content was found in Terminalia chebula black (323.5±5.3 mg/g) followed by Terminalia chebula yellow (292.78±0.3 mg/g), Phyllanthus emblica (160.91± 1.12 mg/g) and Terminalia arjuna (96.3± 1.1 mg/g). Whereas, the highest flavonoids was found in Phyllanthus emblica (25.1± 0.8 mg/g), Terminalia arjuna (20.2 ± 0.42 mg/g), followed by Terminalia chebula black (18.1± 0.31mg/g) and Terminalia chebula yellow (17.1± 0.42 mg/g).
Table 1.
Total phenolic and flavonoid contents among aqueous extract of plants; Mean ± SD.
| Extracts | Phenolics (mg/g) | Flavonoids (mg/g) |
| Phyllanthus emblica | 160.91± 1.12 | 25.1± 0.8 |
| Terminalia chebula yellow | 292.78±0.3 | 17.1± 0.42 |
| Terminalia chebula black | 323.5±5.3 | 18.1± 0.31 |
| Terminalia arjuna | 96.3±1.1 | 20.2±0.42 |
| Balsamodnedron Mukul | 13.56±0.45 | 17.1±0.3 |
| Alium sativum | 4.3±0.21 | 1.2±0.13 |
Discussion
Oxidative stress is now recognized to be associated with more than 200 diseases, as well as with the normal aging process (Ghasanfari et al., 2006). There is a strong correlation between thiobarbituric acid-reactive substances (TBARS) as a marker of lipid per-oxidation and products that reflect oxidative damage to DNA (Chen et al., 2005). It is known that metal-catalyzed generation of ROS results in an attack not only on DNA and proteins, but also on other cellular components involving polyunsaturated fatty acid residues of phospholipids, which are extremely sensitive to oxidation (Shacter, 2000). Inhibition of lipid per-oxidation in rabbit brain homogenate is analogous to neuro-protection (Muralikrishnan et al., 2008). The brain is particularly vulnerable to oxidative damage because of its high oxygen utilization, its high content of oxidizable polyunsaturated fatty acids and the presence of redox-active metals (Cu, Fe). Increases in the formation of TBARS in iron(II) sulphate (10 µM)-induced oxidative stress compared to the normal, suggest possible damage of tissues with an overload of iron. Free iron in the cytosol and mitochondria can cause considerable oxidative damage by increasing superoxide production, which can react with Fe(III) to regenerate Fe(II) that participates in the Fenton reaction (Fraga and Oteiza, 2002). Iron overload results in the formation of lipid per-oxidation products, which have been demonstrated in a number of tissues, including the brain, liver and kidneys (Houglum et al., 1990, Sabir et al., 2012). Storage of iron in the liver leads to liver cirrhosis. Rats overloaded with iron showed toxic effects, such as hepatocellular hypertrophy, cardiomyopathy, pancreatic atrophy, splenic white pulp atrophy and hemosiderosis in the liver, heart, pancreas and endocrine glands, respectively (Whittaker et al., 1997). The possible mechanisms of iron toxicity include free radical-mediated per-oxidative reactions, which are readily catalyzed by iron. The protections offered by the aqueous extracts of Phyllanthus emblica, Terminalia chebula yellow Terminalia chebula black, Terminalia arjuna and Balsamodendron Mukul suggest that they may be useful to ameliorate the oxidative stress in the mice brain. The decrease in the Fe(II) induced lipid per-oxidation in the mice brain homogenates in the presence of the extracts could be as result of the ability of the extracts to chelate Fe(II) and/or scavenge free radicals produced by the Fe(II) catalyzed production of reactive oxygen species (ROS) in the mice's brain. However, the higher ability of the extracts to protect the mice's brain could be because of their higher extractable phytochemical content (Table 1).
Antioxidants are substances that neutralize free radicals and their negative effects. They act at different stages (prevention, interception and repair) and by different mechanisms: reducing agents by donating hydrogen, quenching singlet oxygen, acting as chelators and trapping free radicals (Devasagayam et al., 2004). DPPH• is considered to be a model of a stable lipophilic radical. A chain reaction of lipophilic radicals is initiated by lipid auto-oxidation. Antioxidants react with DPPH•, reducing the number of DPPH free radicals to the number of their available hydroxyl groups. Therefore, the absorption at 517 nm is proportional to the amount of residual DPPH•. It is visually noticeable as a discoloration from purple to yellow. The high DPPH radical scavenging activity of these plants suggests their use in diseases arising from free radical attack. In the phosphomolybdenum assay, which is a quantitative method to evaluate water-soluble and fat-soluble antioxidant capacity (total antioxidant capacity), the plant extracts demonstrated electron-donating capacity showing its ability to act as chain terminators, transforming relative free radical species into more stable nonreactive products (Dorman et al., 2003). Allhorn et al., (2005) reported that the reducing property can be a novel anti-oxidation defense mechanism, possibly through the ability of the antioxidant compound to reduce transition metals. Therefore, the higher reducing ability of the black Terminalia chebula extract may have contributed to the higher protective effect observed. The use of iron chelation is a popular therapy for the management of Fe(II)-associated oxidative stress in brain. The iron chelating ability of studied plants is again an indicator of the neuro-protective property of the plants as iron is involved in the pathogenesis of Alzheimer's disease and other diseases by multiple mechanisms.
Plant-derived polyphenolic flavonoids are well known to exhibit antioxidant activity through a variety of mechanisms including scavenging of ROS, inhibiting lipid per-oxidation and chelating metal ions (Shahidi et al., 1997). The high content of phenolics and flavonoids in the extracts of plants contributes to the antioxidant activity. In addition, Phyllanthus emblica and Terminalia chebula contained high amount of ascorbic acid which also contribute to the antioxidant activity (Naik et al., 2005; Barthakur et al., 2001). Our results are in agreement to the results of Xioli et al., (2007), where methanolic extract of Phyllanthus emblica has shown DPPH radical scavenging activity, hydroxyl radical scavenging, superoxide radical scavenging and metal chelation. The aqueous extracts of Emblica officinalis, Terminalia chebula and Terminalia belerica have shown their ability to scavenge DPPH and superoxide. They show greater efficacy in lipid per-oxidation and plasmid DNA assay (Naik et al., 2005). These results have indicated that these plants are potential candidates of antioxidant activities.
Finally the results of this study demonstrated the high efficacy of the crude aqueous extract of P. emblica, Terminalia chebula black, Terminalia chebula yellow, Terminalia arjuna and Balsamodendron Mukul growing in Pakistan, in free radical scavenging, inhibition of reactive oxygen species and lipid per-oxidation which may be associated with their high medicinal use as a functional food and effectiveness in treatment of diseases associated with oxidative stress in brain. Phyllanthus Emblica and Terminalia chebula fruit can be considered as a source of plant antioxidants, with a potential use in food, cosmetics and pharmaceutical fields. As part of the mechanisms through which the extractable phytochemicals in Terminalia chebula (black and yellow), Terminalia arjuna and Phyllanthus emblica protect the brain may be through their antioxidant properties, iron chelating, reducing abilities and radical scavenging activities. However, more detailed studies are required to evaluate the neuro-protective ability of these plants.
Conflict of interest
The authors declare that there is no conflict of interest to disclose.
References
- 1.Allhorn M, Klapyta A, Akerstrom B. Redox properties of the lipocalin alpha-1microglobulin: reduction of cytochrome c, hemoglobin, and free iron. Free Radical Biol Med. 2005;38:57–67. doi: 10.1016/j.freeradbiomed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Antarkar DS, Ashok BV, Doshi JC, Athavale AV, Vinchoo KS, Natekar MR, Barthakur NN, Arnold NP. Nutritive value of Chebulic myrobalan (Terminalia chebula Retz.) and its potential as food source. Food Chem. 1991;40:213–219. [Google Scholar]
- 3.Barthakur NN, Arnold NP. Nutritive value of chebulinic myrobalan (Terminalia chebula Retz.) and its potential as a food source. Food Chem. 1991;40:213–219. [Google Scholar]
- 4.Cheng HY, Lin TC, Yu KH, Yang CM, Lin CC. Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharm Bull. 2003;26:1331–1335. doi: 10.1248/bpb.26.1331. [DOI] [PubMed] [Google Scholar]
- 5.Chen HJ, Wu CF, Huang JL. Measurement of urinary excretion of 5- hydroxymethyluracil in human by GC/NICI/MS: Correlation with cigarette smoking, urinary TBARS and etheno DNA adduct. Toxicol Lett. 2005;155:403–410. doi: 10.1016/j.toxlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Darshan VS, Nitin M, Somshekhar SS, Sagar DK, Dhanajay AL, Yogesh SK. Antioxidant activity of Balsamdendron Mukul hook extract. Der Pharm Lett. 2012;4:1501–1504. [Google Scholar]
- 7.Dorman HJD, Kosar M, Kahlos K, Holm Y, Hiltunen R. ntioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem. 2003;51:4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- 8.Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. ree radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 9.Elise AM, James RC. The case of iron chelation and or antioxidant therapy in Alzheimer's disease. Drug Develop Res. 2002;56:520–526. [Google Scholar]
- 10.Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology. 2002;80:23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- 11.Ghasanfari G, Minaie B, Yasa N, Leilu AN, Azadeh M. Biochemical and histopathological evidences for beneficial effects of Satureja Khuzestanica Jamzad essential oil on the mouse model of inflammatory bowel diseases. Toxicol Mech Method. 2006;16:365–372. doi: 10.1080/15376520600620125. [DOI] [PubMed] [Google Scholar]
- 12.Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root; their relative astringency and radical scavenging effects. Chem Pharm Bull. 1998;36:2090–2097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants and human disease: Where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 14.Hertog MGL, Hollman PCH, Van PB. Content of potentially anticarcinogenic flavonoids of tea infusions, wines and fruit juices. J Agric Food Chem. 1993;41:1242–1246. [Google Scholar]
- 15.Houglum K, Filip M, Witztum JL, Chojkier M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J Clin Invest. 1990;86:1991–1998. doi: 10.1172/JCI114934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosalec I, Bakmaz M, Pepeliniak S, Vladimir-Knezevic S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharmaceut. 2004;54:65–72. [PubMed] [Google Scholar]
- 17.Kang HW, Lee CM, Lee KC, Park ST, Lee EJ, Lim JP, Kim HM, Lee YMJ. Inhibitory action of water soluble fraction of Terminalia chebula on systemic and local anaphylaxix. J Ethnopharm. 2001;74:133–140. doi: 10.1016/s0378-8741(00)00360-3. [DOI] [PubMed] [Google Scholar]
- 18.Kapoor LD. Handbook of Ayurvedic Medicinal Plants. Boca Raton, FL: CRC Press; 1990. pp. 319–320. [Google Scholar]
- 19.Muralikrishnan D, Binu T, Bala VM. Antiparkinson drug- Mucuna pruriens shows antioxidant and metal chelating activity. Phytother Res. 2008;22:6–11. doi: 10.1002/ptr.2109. [DOI] [PubMed] [Google Scholar]
- 20.Naik G, Priyadarsini K, Bhagirathi R, Mishra B, Mishra K, Banavalikar M, Mohan H. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother Res. 2005;19:582–586. doi: 10.1002/ptr.1515. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1997;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Olusanmi MJ, Amadi JE. Studies on antimicrobial properties and phytochemical screening of garlic (Allium sativum) extracts. Ethnobot Leaftlets. 2009;13:1186–1196. [Google Scholar]
- 23.Perry SW, Norman JP, Litzburg A, Gelbard HA. Antioxidants are required during the early critical period, but not later, for neuronal survival. J Neurosci Res. 2004;78:485–492. doi: 10.1002/jnr.20272. [DOI] [PubMed] [Google Scholar]
- 24.Puntel RL, Nogueira CW, Rocha JBT. Krebs cycle intermediates modulate tshiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res. 2005;30:225–235. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 25.Prieto P, Pineda M, Aiguel M. Spectrophotometer Quantization of antioxidant capacity through the formation of Phosphomolybdenum Complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 26.Rahman MM, Fazlic V, Saad NW. Antioxidant properties of raw garlic (Alium sativum) extract. Int Food Res J. 2012;19:589–591. [Google Scholar]
- 27.Rout OP, Achaya R, Mishra KM. Oleogum Resin Guggulu: A review of the medicinal evidence for its therapeutic properties. IRAP. 2012;3:15–21. [Google Scholar]
- 28.Sabir SM, Ahmad SD, Abdul H, Khan MQ, Athayde ML, Santos DB, Boligon AA, Rocha JBT. Antioxidant and Hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem. 2012;131:741–747. [Google Scholar]
- 29.Sabir SM, Syed M Salman, Rocha JBT. Antioxidant properties of β- selenoamines against lipid peroxidation in rat brain and liver. Environ Tox Pharm. 2012;34:446–453. doi: 10.1016/j.etap.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Shacter E. Protein oxidative damage. Method Enzymol. 2000;319:428–436. doi: 10.1016/s0076-6879(00)19040-8. [DOI] [PubMed] [Google Scholar]
- 31.Singh C. 2α-Hyroxymicromeric acid, a pentacyclic triterpene from Terminalia chebula. Phytochem. 2000;29:2348–2350. [Google Scholar]
- 32.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu,s reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 33.Saleem A, Husheem M, Harkonen P, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit J Ethnopharm. 2002;81:327–336. doi: 10.1016/s0378-8741(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 34.Shin TY, Jeong HJ, Kim DK, Kim SH, Lee JK, Kim DK, Chae BS, Kim JH, Sabu MC, Kuttan R. Antidiabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharm. 2001;81:155–160. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 35.Shahidi F. Natural antioxidants: An overview. In: Shahidi F, editor. Natural Antioxidants, Chemistry, Health Effects and Applications. Champaign: AOCS Press; 1997. pp. 1–11. [Google Scholar]
- 36.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Tripathi VK, Singh B. Terminalia arjuna- its present status (a review) Orient J Chem. 1996;12:1–16. [Google Scholar]
- 38.Verma RC, Gupta A. Effect of pre-treatments on quality of solar-dried amla. J Food Eng. 2004;65:397–402. [Google Scholar]
- 39.Vega-Naredo I, Poeggeler B, Sierra-Sanchez V, Caballero B, Tomas-Zapico C, Alvarez-Garcia O, Tolivia D, Rodriquez-Colunqa MJ, Coto-Montes A. Melatonin neutralizes neurotoxicity induced by quinolinic acid in brain tissue culture. J Pineal Res. 2005;39:266–275. doi: 10.1111/j.1600-079X.2005.00243.x. [DOI] [PubMed] [Google Scholar]
- 40.Whittaker BS, Berlett ER, Stadtman RR. Protein oxidation in aging diseases and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 41.Xiaoli L, Mouming Z, Jinshui W, Bao Y, Yueming J. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J Food Compos Anal. 2008;21:219–222. [Google Scholar]