Abstract
Background
Postmenopausal osteoporosis (PMO) is an estrogen deficiency condition that causes severe loss of bone mass in the vertebrae and long bones. We explored the effect and the possible underlying mechanism of the extracts of Astragalus (AE) on the tooth alveolar bone rebuilding progress of postmenopausal osteoporosis of PMO animal models.
Material and methods
The PMO models were acquired by ovariectomy. After 8 weeks the first left maxillary molars of the rats were extracted and AE was administered orally for 8 weeks. Then the histological morphology and the transcription and expression of TGF-β1 and TNF-α in the tooth extracted socket were detected by HE dying, QRT-PCR and ELISA.
Results
The results showed that the new bone volume and TGF-β1 was significantly lower in PMO group than the control group and AE group at the observing period. At the same time TNF-α in PMO group was significantly higher than the other two groups. Moreover AE group had no significant differences with the control group in all data at the observing period.
Conclusion
AE has positive effect on alveolar bone rebuilding progress of tooth extracted socket of PMO rats. AE also has the potential to enhance the expression of TGF-β1 and decrease the expression of TNF-α of the rebuilding tooth extracted socket.
Keywords: PMO, TGF-β1, TNF-α, Astragalus
Introduction
Postmenopausal osteoporosis (PMO) is an estrogen deficiency condition that causes severe loss of bone mass in the vertebrae and long bones. The impact of PMO is expected to increase quickly (Johnell et al, 2006; Reginster et al, 2006). PMO not only leads to osteoporotic fractures of vertebrae and long bones, but also has a positive relationship with some jaw pathologic conditions, such as oral bone loss (Payne etal, 2000; Bollen etal, 2000; Steinberg et al, 1999; Southard et al, 1999), low mineral density in the mandible (Lee et al, 2005; Takaishi et al, 2005) and severity of residual ridge re-absorption (Hirai et al, 1993; Jonasson et al, 2005). The jaw bones are very important for oral functions such as mastication, swallowing, and pronunciation. In contrast to other bones, they are the base of preserving nature teeth and supporting artificial teeth after nature teeth losing, which are essential to raise the life quality of elderly people. Consequently, it is necessary for the dentists and researchers to study the correlation between PMO and jaw bones mass and find out effective and safe ways to prevent bone loss of alveolar and jaw.
Now the prescription drugs used to treat osteoporosis mainly include bisphosphonates?estrogen, selective estrogen receptor modulators, parathyroid hormone and so on. The drugs play an important part in preventing accelerated bone loss and decreasing age-related decreases in bone density, however, they have their own contraindications or adverse effects respectively (Rossouw et al, 2002; Woo et al, 2006; Beral et al, 2003; Barrett-Connor et al, 2006;). And all these drugs only have the single effect. Multiple traditional Chinese medicine formulas and their monomer ingredients were proved being effective on treating osteoporosis and the mechanisms were the focus of research too. The phytoestrogen was the mainly effective composition with relatively less side-effects comparing with HRT (Zhao et al, 2012). The extracts of AE are a kind of Chinese traditional medicine which includes polysaccharides, saponins, and flavonoids. These three major chemical classes contribute to the multiple bioactivities of Astragalus (Yang et al, 2013). And one of AE biological functions is anti-osteoporosis activity. (Kim et al, 2003). In this study, we investigated the effect of AE on alveolar bone rebuilding progress of teeth extracted socket on animal model of PMO.
Material and methods
Animal and groups
Sixty 3-month-old female Sprague-Dawley (SD) rats were randomly divided into two groups. 20 rats were brought into the control group, which underwent sham surgery and rest 40 rats were ovariectomized bilaterally. 8 weeks after operation the first left maxillary molar of all rats were extracted. The rats receiving ovariectomy then were randomly divided into non-treated group (PMO group, n=20) and AE treated group (AE group, n=20). Rats in the AE group received AE intra-gastric administration (10µl/kg) everyday. Rats in the control group and PMO group received the same volume distilled water intra-gastric administration. At 4w and 8w after extraction, 10 rats of each group were sacrificed respectively. The left maxillas were excised. The specimens were for histological observation, quantitative reverse transcription -polymerase chain reaction (QRT-PCR) and enzyme linked immunosorbent assay (ELISA).
Histological observation
The specimens were fixed in 4% paraform (Shanghai Suolaibao biotechnology, Shanghai, China) for 3 days, decalcified in 10% ethylene diamine tetraacetic acid (EDTA pH 7.4; Sigma, Steinheim, Germany) for 1 month, dehydrated with increasing concentrations of ethanol, and embedded in paraffin (Active motif, Carlsbad, CA, USA). The acquired blocks were unilaterally cut to the sagittal plane, which was perpendicular to occlusal plane and penetrated the wound of the first molar extracted. The specimens were then cut into 5µm sections by microtom. 5 sections of each specimen were stained by hematoxylin and eosin (HE) for histological observation.
Quantitative real-time polymerase chain reaction (QRT-PCR)
The specimens for QRT-PCR were taken and frozen at −80°C for 30mins and then transferred to liquid nitrogen until RNA extraction. For analysis frozen samples (100mg) were crushed in an achate mortar under liquid nitrogen and homogenized in 1ml precool Trisol (Invitrogen, Carlsbad, CA, USA) solution. After adding 200µl chloroform (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 5mins on the ice, in-soluble material was removed by centrifugation (12,000rpm, 15mins, 4 °C). 400µl supernatant were mixed with 400µl sopropanol(Aladdin, Shanghai, China) for 10mins on ice which then was under centrifugation (12,000rpm, 15mins, 4 °C). RNA was acquired after the in-soluble material was eluted by 1ml absolute alcohol followed by centrifugation (7500 rpm, 10 mins, 4 °C) and dissolution in 30 µl RNase-free H2O. Reverse-transcribed method accorded the manufacturer's instructions. QRT-PCR analysis was carried out in an Applied Biosystems 7500 Fast (Applied, BiosystemsCarlsbad, CA, USA). Primer sequences used to amplify TGF-β1 and TNF-α, and glyceraldehyde phosphate dehydrogenase (GAPDH), an internal reference, are listed in Table 1
Table 1.
Sequences of primer sets for quantitative real-time PCR
| Gene | Primers (5′–3′) | Product size (bp) | Accession no. |
| TGF-β1 | Sense:TGACAGCAAAGATAACACACTCC | ||
| Antisense:TCAATGGTGGCCAGATCA | 78 | X_99438.1 | |
| TNF-α | Sense:AGGAGCCACCACGCTCTT | 82 | NM_001081819 |
| Antisense: CTGGAAGGCATTCGGTAACT | |||
| GAPDH | Sense: CAGGGCTGCTTTTAACTCTGG | 102 | NM_002046 |
| Antisense: TGGGTGGAATCATATTGGAACA |
ELISA assays
To quantify the protein concentration of TGF-β1 and TNF-α, ELISA methods were used. The specimens for ELISA were taken and frozen at −80 °C for 30 mins and then transferred to liquid nitrogen until protein extraction. For extract preparation, frozen samples were ground in a prcooled sterile achate mortar and dissolved in 600-800µl lysis buffer, which consisted of 100 µg/ml bovine serum albumin (Sigma), 100 µg/ml Zwittergent-12 (Boehringer-Mannheim, Indianapolis, Ind., USA), 50 µg/ml Garamycin (Schering-Plough, Brussels, Belgium), 10 mM Hepes buffer (Life Technologies, Paisley, Scotland, UK), 1µg/ml aprotinin (Sigma), 1 µg/ml leupeptin (Sigma), 0.1 µM EDTA (Sigma), in RPMI-1640 (Sigma). The samples were then centrifuged at 12,000 rpm and the supernatant was stored at −80°C and then assayed for TGF-β1 and TNF-α with commercially available ELISA kits (R&D, Minneapolis, MN). The analyses were performed according to the instructions of the manufacturer. The concentration of TGF-β1 and TNF-α was calculated with reference to the standards provided by each kits. Results were expressed as pg cytokine/mg tissue.
Statistical analysis
One way ANOVA and Tukey'spost hoctests were used to evaluate statistical significance of the difference among 3 groups. The SPSS 16.0 version for Windows (SPSS Inc., Chicago, IL) was used for the statistical analysis. P values less than 0.01 were considered statistically significant.
Results
Bone alternation of the tooth extraction wound
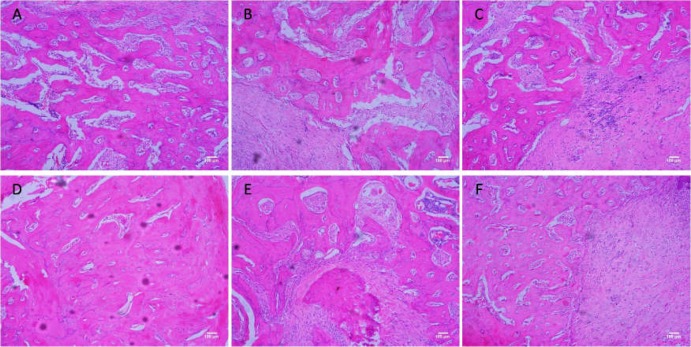
Bone formation was found in the tooth extraction wound at 4w and 8w by HE staining (Fig 1). In each group new bone volume was significantly lower at 4w than at 8w. At 4w, the trabecular number was significantly lower in the PMO group than in the control group. Accordingly, the trabecular separation was significantly higher in the PMO group than in the control group. At 8w, the trabecular of the PMO group was fragmented and seemed to form isolated islands in the large bone marrow. In contrast, the trabecular connected with each other and formed network structures with smaller bone marrow in the control group. And the histological characters in the AE group in every period were similar with control group.
Figure 1.
Bone formation in the tooth extraction wound in the control group (A and D at 4w and 8w)? PMO group (B and E at 4w and 8w) and AE group (C and F at 4w and 8w). Estrogen deprivation caused a decrease in new bone formation of healing progress of tooth extraction wound in 4w and 8w (A compared with B, D compared with E). There was no significant difference in new bone volume between the control group and the AE group (A compared with C, D compared with F), which proved that AE could decreased negative effect of estrogen to the new bone formation of the tooth extraction wound.
The alternation of TGF-β1 of the tooth extraction wound
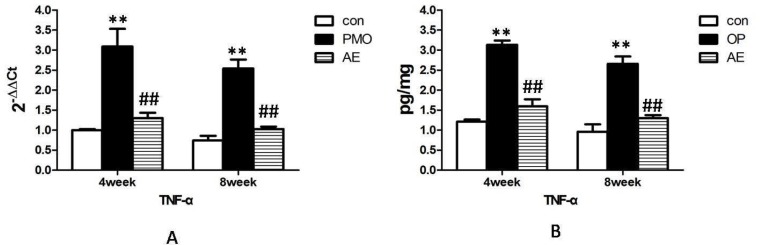
TGF-β1 mRNA was expressed in all the tooth extraction wound tissue in every period. The obvious increase of TGF-β1 mRNA was observed in control group and the AE group than in the PMO group at 4w. It decreased from 4w to 8w in all three groups and still significantly lower in the PMO group than in the other two groups at 8w. Meanwhile the expression of TGF-β1 mRNA had no significant difference between the control group and the AE group over the period of observation (Fig 2A).
Figure2.
mRNA and protein expression alternation of TGF-β1 in the control group, PMO group and AE group at 4w and 8w after tooth extraction. AE increased the mRNA expression (A) and protein expression (B) of TGF-β1 in the tooth alveolar bone rebuilding progress, which was down-regulated by estrogen deficiency in the PMO group. **, P<0.01, compared with the control group. ##, P<0.01, compared with PMO group.
The expression of TGF-β1 protein had the same tendency with its gene expression, which was significantly lower in the PMO group than in the other two groups at 4w. The AE group has no significant difference with the control group in the amounts of TGF-β1 protein. Although the TGF-β1 protein in the tooth extraction wound tissue showed a statistically significant decline at 8w in every group, the PMO group still expressed the least TGF-β1 protein in the three groups significantly, with no significant difference between the control group and the AE group (Fig 2B).
The alternation of TNF-α of the tooth extraction wound
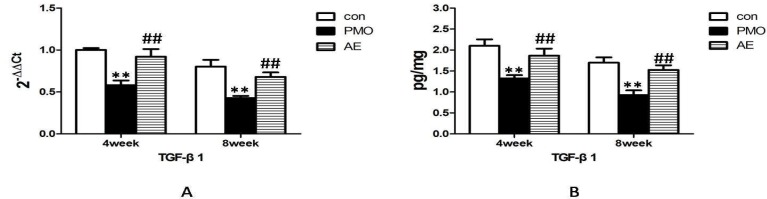
TNF-α mRNA was expressed in all the tooth extraction wound tissue. The mRNA for TNF-α was significantly higher in the PMO group than the control group and the AE group at 4w and 8w. Meanwhile no obvious difference was observed between the control group and the AE group at 4w and 8w (Fig 3A).
Figure 3.
mRNA and protein expression alternation of TNF-α in the control group, PMO group and AE group at 4w and 8w after tooth extraction. AE lowered the mRNA expression(A) and protein expression(B) of TNF-α in the tooth alveolar bone rebuilding progress, which was up-regulated by estrogen deficiency in the PMO group. **, P<0.01, compared with the control group. ##, P<0.01, compared with PMO group.
Fig 3B showed that the TNF-α protein in the PMO group were significantly higher than the control group and the AE group at 4w and 8w, despite an obvious decline from 4w to 8w in every group independently. No significant differences were observed between the control group and the AE group in the amounts of TNF-α protein at 4w and 8w. The expression of TNF-α protein had the same tendency with its gene expression.
Discussion
The ovariectomized (OVX) rat model is used widely as a preclinical model to evaluate bony changes that occurs with estrogen deficiency in postmenopausal osteoporosis (Kalu et al, 1991; Nishida etal, 2004; Boyd et al, 2006; Jeong-Hyun et al, 2010; Thompson et al, 1995). We used OVX rat model to examine the effects of AE on bony healing progress after tooth extraction. The normal metabolism of bone is composed by bone re-absorption and formation, called bone remodeling. Under the condition of osteoporosis the bone remodeling is unbalanced. The initiation of bone remodeling is induced rapidly and the resorptive and formative phases of remodeling are both enhanced, and the resorptive phases is faster than formative phases which results in the lost of bone. Shimizu reported that in comparison to sham-operated controls, new bone formation in OVX rats was slightly increased in the early phase but subsequently showed a marked decrease. Bone resorption in OVX rats was greatly stimulated and was comparatively long-lasting (Shimizu, 1998; Shimizu, 2000). Pereira reported the results with the similar tendency (Pereira et al, 2007). Our results showed that new bone volume of tooth extraction socket was lower in PMO group compared with the control group?which suggested that estrogen deprivation caused a decrease in new bone formation of healing progress of tooth extraction wound. While we did not see a significant difference in new bone volume between the control group and the AE group, which proved that the negative effect of estrogen deficiency to the new bone formation of the tooth extraction wound could been decreased by AE.
TGF-β1 is recognized as the powerful promoter of bone formation. It is produced by osteoblasts and other bone cells and affects osteoblast proliferation and differentiation. And it also incorporates into mineralized bone matrix. In most studies, it increased expression of markers of osteoblast differentiation, such as alkaline phosphatase, type I collagen, and osteonectin (Ingram et al, 1994; Stron et al, 1990; Wergedal et al, 1992). TGF-β1 is expressed at high levels in mature osteoblasts on bone surfaces during bone development and growth (Dodds et al, 1993; D‧ Souza et al, 1994) and in healing fracture callus (Joyce et al, 1990; Sandberg et al, 1993). The healing of the tooth extraction wound is similar to the bone fracture. Therefore TGF-β1 may play a pivotal role in bone wound healing progress especially in bone formation.
Ikeda's research demonstrated that three weeks after ovariectomy TGF-β1 mRNA levels from the tibiae of rats had 70%–80% decrease compared with the sham-operated group and ovariectomy caused 15% reduction in femoral bone mineral density as early as two weeks post-operation, which provide the evidence that expression of TGF-β1 was reciprocally regulated at the transcriptional level in estrogen deficient condition and suggests that TGF-β1 may play a role in estrogen-dependent maintenance of normal bone density (Ikeda et al, 1993). Therefore we can conclude from these studies that estrogen deficiency induced by overiectomy can lowered the expression of TGF-β1 in bone. Due to the important function of TGF-β1 in bone formation, it can be implied that one of mechanism that estrogen deficiency induce bone loss is from its negative effect on the expression of TGF-β1.
TNF-α plays a major role in osteoclastogenesis. It was reported to induce the formation of osteoclastic cells from bone marrow macrophages in vitro (Kobayashi et al, 2000; Azuma et al, 2000) and TNF-α-induced osteoclast recruitment is probably central to the pathogenesis of bone disorders (Wong et al, 2008). TNF-α causes osteoclast-induced bone destruction as well as the inhibition of osteoblast differentiation and apoptosis (Lu et al, 2006). TNF-α has an inhibitory effect during various stages of osteoblast differentiation and can act on osteoblast precursor cells during the early stages of differentiation to inhibit insulin-like growth factor 1, which increases the differentiation of osteoblast precursor cells from stem cells (Gilbert et al, 2002). TNF-α acts on osteoblasts to inhibit the transcription of runt-related transcription factor 2 (RUNX2), the main transcription factor for osteoblast differentiation, by inducing the degradation of RUNX2 mRNA (Gilbert et al, 2000). Therefore TNF-α may play a key role in bone metabolism and is important in patho-logical bone loss.
Many studies proved that a key mechanism by which estrogen deficiency induces bone loss is that estrogen deficiency enhances T cell production of TNF-α. Roggia found that ovariectomy increased the number of bone marrow T cell and induced more TNF-α further. Meanwhile the bone loss induced by ovariectomy was absent in T cell-deficient nude mice (Roggia et al, 2001). Simone got the similar result that ovariectomy failed to induce bone loss in T-cell deficient mice (Simone et al, 2000). Furthermore injection of TNF-α-binding protein that neutralizes TNF-α was shown to protect ovariectomized rat against bone loss by inhibiting osteoclast formation (Kimble et al, 1997). Roggia observed that TNF-α knock-out mice (TNF-/-), as well as transgenic mice without thymus (and therefore without mature T cell), did not lose bone after ovariectomy (Roggia et al, 2004). All These findings demonstrated the ability of estrogen to target T cells, suppressing their production of TNF-α, is a key mechanism by which estrogen prevents osteoclastic bone resorption and bone loss.
In this study the transcription and expression of TGF-β1 in the healing of extraction sockets were lower in the PMO group than the control group, while the transcription and expression of TNF-α were higher in the PMO group than the control group. All these results accorded with the previous studies. At the same time we founded that AE not only enhanced the transcription and expression of TGF-β1 in the healing of extraction sockets in ovariectomized rats significantly, but also lowered the transcription and expression of TNF-α under PMO condition. Many previous reports showed components of AE had effected the pathway of TGF-β1/Smad signaling and the production of TNF-α in different cells and tissues. (Hu etal, 2014; Qu etal, 2012; Jung etal, 2013). The findings of this study showed the positive effect of AE on TGF-β1 and negative effect on TNF-α in bone tissue under the condition of PMO, which provided insights on the mechanism of the stimulatory effect of AE on the bone healing progress of tooth extraction under PMO condition. AE increased the production of TGF-β1 which was down-regulated by estrogen deficiency as well as lowered the production of TNF-α which was up-regulated by estrogen deficiency. The bone resorption induced by TNF-α under the condition of PMO was decreased by AE and the increased TGF-β1 by AE enhanced the new bone formation under such condition.
Conclusion
Ovariectomy caused a decrease in new bone formation of healing progress of tooth extraction wound, which could be prevented by AE through enhancing the production of TGF-β1 which was limited by estrogen deficiency and lowering the secretion of TNF-α which was promoted by estrogen deficiency.
Acknowledgments
The present study was funded by Zhejiang Traditional Chinese Medicine Foundation (No. 2008YA023).
References
- 1.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. Journal of Biological Chemistry. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK, Raloxifene Use for The Heart (RUTH) Trial Investigators Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. New England Journal of Medicine. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 3.Beral V. Million Women Study Collaborators, Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;62:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 4.Bollen AM, Taguchi A, Hujoel PP, Hollender LG. Case-control study on self-reported osteoporotic fractures and mandibular cortical bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:518–524. doi: 10.1067/moe.2000.107802. [DOI] [PubMed] [Google Scholar]
- 5.Boyd SK, Davison P, Muller R, Gasser JA. Monitoring individual morphological changes overtime in ovariectomized rats by in vivo micro-computed tomography. Bone. 2006;39:854–862. doi: 10.1016/j.bone.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Dodds RA, Merry K, Littlewood A, Gowen M. Expression of mRNA for ILl, IL6 and TGF-β1 in developing human bone and cartilage. J Histochem Cytochem. 1994;42:733–744. doi: 10.1177/42.6.8189035. [DOI] [PubMed] [Google Scholar]
- 7.D' Souza RN, Niederreithe K, de-Crombrugghe B. Osteoblast-specific expression of the alpha 2(I) collagen promoter in transgenic mice: correlation with the distribution of TGF-beta1. J Bone Miner Res. 1993;8:1127–1136. doi: 10.1002/jbmr.5650080914. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-α. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS, Nanes MS. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2αA) is inhibited by tumor necrosis factor-α. Journal of Biological Chemistry. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 10.Hirai T, Ishijima T, Hashikawa Y, Yajima T. Osteoporosis and reduction of residual ridge in edentulous patients. J Prosthet Dent. 1993;69:49–56. doi: 10.1016/0022-3913(93)90240-o. [DOI] [PubMed] [Google Scholar]
- 11.Hu X, Rui W, Wu C, He S, Jiang J, Zhang X, Yang Y. Compound Astragalus and Salvia miltiorrhiza extracts suppress hepatocarcinogenesis by modulating transforming growth factor-β/Smad signaling. J Gastroenterol Hepato. 2014;2014:1284–1291. doi: 10.1111/jgh.12490. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda T, Shigeno C, Kasai R, Kohno H, Ohta S, Okumura H, Konishi J, Yamamuro T. Ovariectomy decreases the mRNA levels of transforming growth factor-beta 1 and increases the mRNA levels of osteocalcin in rat bone in vivo. Biochem Biophys Res Commun. 1993;194:1228–1233. doi: 10.1006/bbrc.1993.1954. [DOI] [PubMed] [Google Scholar]
- 13.Ingram RT, Bonde SK, Riggs BL, Fitzpatrick LA. Effects of transforming grouth factor beta(TGF beta ) and 1,25 dihydroxyvitamin D3 on the function, cytochemistry and morphology of normal human osteoblast-like cells. Differentiation. 1994;55:153–163. doi: 10.1046/j.1432-0436.1994.5520153.x. [DOI] [PubMed] [Google Scholar]
- 14.Jeong-Hyun Jee, Wan Lee, Byung Do Lee. The influence of alendronate on the healing of extraction sockets of ovariectomized rats assessed by in vivo micro-computed tomography. 2010;110:47–53. doi: 10.1016/j.tripleo.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 16.Jonasson G, Kiliaridis S. Changes in the bucco-lingual thickness of the mandibular alveolar process and skeletal bone mineral density in dentate women: a 5-yr prospective study. Eur J Oral Sci. 2005;113:114–120. doi: 10.1111/j.1600-0722.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 17.Jung, Koo H, Sohn EH, Kim YJ, Jang SA, Namkoong S, Chan Kang S. Effect of the combinatory mixture of Rubus coreanus Miquel and Astragalus membranaceus Bunge extracts on ovariectomy-induced osteoporosis in mice and anti-RANK signaling effect. J Ethnopharmacol. 2013;2014:951–959. doi: 10.1016/j.jep.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Joyce ME, Jingushi S, Bolander ME. Transforming growth factor-βin the regulation of fracture repair. Orthop Clin N Amer. 1990;21:199–209. [PubMed] [Google Scholar]
- 19.Kalu D N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 20.Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 21.Kim C, Ha H, Lee JH, Kim JS, Song K, Park SW. Herbal extract prevents bone loss in ovariectomized rats. Arch Pharm Res. 2003;26:917–924. doi: 10.1007/BF02980200. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. Journal of Experimental Medicine. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BD, White SC. Age and trabecular features of alveolar bone associated with osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:92–98. doi: 10.1016/j.tripleo.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, Graves DT, Rueger JM, Gerstenfeld LC, Einhorn TA. Tumor necrosis factor alpha (TNF-α) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36:300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Gilbert L, He X, Rubin J, Nanes MS. Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NFκB pathways. Journal of Biological Chemistry. 2006;281:6297–6306. doi: 10.1074/jbc.M507804200. [DOI] [PubMed] [Google Scholar]
- 26.Nishida A, Ito M, Uetani M, Nakayama T, Tanaka T. Effect of etidronate on three-dimensional trabecular structure in ovariec-tomized or sciatic neurectomized rats. J Bone Miner Metab. 2004;22:335–340. doi: 10.1007/s00774-003-0491-x. [DOI] [PubMed] [Google Scholar]
- 27.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 28.Pereira MC, Zecchin KG, Campagnoli EB, Jorge J. Ovariectomy delays alveolar wound healing after molar extractions in rats. J Oral Maxillofac Surg. 2007;65:2248–2253. doi: 10.1016/j.joms.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 29.Qu ZH, Yang ZC, Chen L, Lv ZD, Yi MJ, Ran N. Inhibition airway remodeling and transforming growth factor-β1/Smad signaling pathway by astragalus extract in asthmatic mice. Int J Mol Med. 2012;2012:564–568. doi: 10.3892/ijmm.2011.868. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone (NY) 2006;38:S4–S9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roggia C, Tamone C, Cenci S, Pacifici R, Isaia GC. Role of TNF-alpha producing T-cells in bone loss induced by estrogen deficiency. Minerva Med. 2004;95:125–132. [PubMed] [Google Scholar]
- 33.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 34.Sandberg MM, Aro HT, Vuorio EI. Gene expression during bone repair. Clin Orth Rel Res. 1993;289:292–312. [PubMed] [Google Scholar]
- 35.Shimizu M. Bone wound healing after maxillary molar extraction in ovariectomized aged rats. J Electron Microsc (Tokyo) 1998;47:517–526. doi: 10.1093/oxfordjournals.jmicro.a023623. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu M. Bone wound healing after maxillary molar extraction in ovariectomized aged rats: quantitative backscattered electron image analysis. Anat Rec. 2000;259:76–85. doi: 10.1002/(SICI)1097-0185(20000501)259:1<76::AID-AR9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Simone Cenci M, Weitzmann Neale, Roggia Cristiana, Namba Noriyuki, Novack Deborah, Woodring Jessica, Pacifici Roberto. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-α. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Southard KA, Southard TE, Schlechte JA, Meis PA. The relationship between the density of the alveolar processes and that of post-cranial bone. J Dent Res. 2000;79:964–969. doi: 10.1177/00220345000790041201. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg BJ. Women's oral health issues. J Dent Educ. 1999;63:271–275. [PubMed] [Google Scholar]
- 40.Stron DD, Beachler AL, Wergedal JW, Linkhart TA. Insulin like growth factor and transforming growth factor beta regulate Type procollagen message levels in human osteoblast-like cells in vitro. J Bone Min Res. 1990;6:15–23. doi: 10.1002/jbmr.5650060105. [DOI] [PubMed] [Google Scholar]
- 41.Takaishi Y, Okamoto Y, Ikeo T, Morii H, Takeda M, Hide K, Arai T, Nonaka K. Correlations between periodontitis and loss of mandibular bone in relation to systemic bone changes in postmenopausal Japanese women. Osteoporos Int. 2005;16:1875–1882. doi: 10.1007/s00198-005-1955-8. [DOI] [PubMed] [Google Scholar]
- 42.Thompson D, Simmons, Pirie CM, Ke HZ. FDA guidelines and animal models for osteoporosis. Bone 1 HA. 1995;7:125S–133S. doi: 10.1016/8756-3282(95)00285-l. [DOI] [PubMed] [Google Scholar]
- 43.Wergedal JE, Matsuyama T, Strone DD. Differentiation of normal human bone cells by transforming growth factor-β and 1,25(OH)2 Vitamin D3. Metabolism. 1992;41:42–48. doi: 10.1016/0026-0495(92)90189-h. [DOI] [PubMed] [Google Scholar]
- 44.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR. TNFα blockade in human diseases: mechanisms and future directions. Clinical Immunology. 2008;12:121–136. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo SB, Hellstein JW, Kalmar JR. Systemic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 46.Yang LP, Shen JG, Xu WC, Li J, Jiang JQ. Secondary metabolites of the genus Astragalus: structure and biological-activity update. Chem Biodivers. 2013;10:1004–1054. doi: 10.1002/cbdv.201100444. [DOI] [PubMed] [Google Scholar]
- 47.Zhao P, Niu J, David YW, Wang J, Sun Y, Li Y. Effect and mechanism of traditional Chinese medicine and their active constituents in postmenopausal osteoporosis. Zhongguo Zhong Yao Za Zhi. 2012;37:1693–1699. [PubMed] [Google Scholar]