Abstract
Background
Cactus polysaccharides are the active components of Opuntia dillenii which have been used extensively in folk medicine. In this study, we investigate the anti-tumor effect of cactus polysaccharides on lung squamous carcinoma cells SK-MES-1.
Materials and Methods
The inhibitory effect of Cactus polysaccharides on lung squamous carcinoma cells were detected by MTT assay. Cell cycle was determined by flow cytometry and cell apoptosis was determined by AnnexinV assay. Western-blotting was applied to detect P53 and PTEN protein expression in the cells treated with cactus polysaccharides.
Results
Results showed that different concentrations of wild cactus polysaccharides prevent SK-MES-1 cells growth and induces S phase arrest. The data also revealed that cactus polysaccharides cause apoptosis in SK-MES-1 cells determined by Annexin-V assay. Furthermore, cactus polysaccharides induced growth arrest and apoptosis may be due to the increase of P53 and phosphatase and tension homolog deleted on chromosome ten (PTEN) protein.
Conclusion
Cactus polysaccharides have anti-tumor activity on lung squamous carcinoma cells.
Keywords: Cactus polysaccharides, Lung squamous carcinoma, Anti-tumor effect, P53, PTEN
Introduction
Lung cancer is the most common cause of cancer-related death in men and women, and is responsible for 1.4 million deaths each year, and has a tremendous impact on human health (Liao et al.,2007; Jemal et al.,2006). Non-small-cell lung cancer (NSCLC) accounts for about 80–85% of all types of lung cancer, including large-cell carcinomas, squamous cell carcinoma and adenocarcinoma (D'Addario et al., 010). NSCLC is usually treated with surgery accompanied with chemotherapy or radiation therapy at early stage (Pil et al., 2013). However, there are no very effective therapies for lung cancer patients at late stage. It is urgent to find other novel therapy for lung cancer patients.
Opuntia dillenii Haw was introduced from Milpa region into China in 1997. Because its rhizome is an excellent herb medicine, it has been used extensively for treatments of wounds, burns, asthma and diabetes (Huang et al., 2009). This plant contains high levels of nutrients, such as polysaccharides, flavonoids (Zhao et al., 2011). Opuntia dillenii polysaccharides have been reported to display neuoprotective and antioxidative effects (Huang et al., 2009). However, whether cactus polysaccharides from Opuntia dillenii have anti-tumor activities and what kind of molecular mechanisms involved are still unclear. Therefore, this study was designed to examine the effect of cactus polysaccharides on lung squamous carcinoma cells SK-MES-1 to provide evidence for developing novel drug.
Materials and Methods
Cell culture, antibodies and reagents
Human lung squamous carcinoma cells SK-MES-1 was from American Type Culture Collection (ATCC) and cultured in RPMI 1640 medium (Thermo,USA) supplemented with 10% Fetal bovine serum (FBS) (Thermo,USA), 100µg/ml penicillin and 100µg/ml streptomycin. Antibodies to p53, PTEN and β-actin were from Boster Biological Technology Wuhan, China.3-(4,5-Dimethylthiazol-2-yl)-2,5 -diphenyltetrazolium bromide (MTT) was from Amresco, USA.
Preparation of cactus polysaccharides
Fresh Opuntia dillenii were collected from Lvshun, Dalian City, Liaoning Province, China. Extracts were prepared by removing the thorns and then soaking in distilled water 10 times at 80□ for 2h. The supernatant was collected and concentrated by a rotary evaporator at 50□ with pressure of 80 Mbar. Crude Cactus polysaccharides were precipitated by 95% ethanol at 4□ and collected by centrifugation (1810×g, 20min) and freeze-drying. Cactus polysaccharides were dissolved in 0.2 mol/L Phosphate buffered saline (PBS) (pH7.4) and stored at 4□.
In vitro cell proliferation assay
Cells were seeded into 96-well plates and treated with gradient concentration of cactus polysaccharides (1.44 mg/ml, 0.72 mg/ml, 0.36mg/ml, 0.18 mg/ml, 0.09 mg/ml, 0.045 mg/ml, 0.0225 mg/ml, 0.01125 mg/ml and 0.005625mg/ml). After 24h or 48h cactus polysaccharides treatment, 20µl MTT (5 mg/ml) was added to each well and incubated for 4 hours. Then 100µl Dim ethyl sulfoxide (DMSO) was then added into each well and the plates. The absorbance (Abs) of the individual wells was detected at the wavelength of 492 nm using microplate reader (Thermo, USA). The inhibiting rate of the cells was determined by the following equation: inhibiting rate (%) = (1-Abs test /Abs cont)×100%.
Morphology test
SK-MES-1 cells were seeded into each flask at appropriate densities (1×105 cells /ml) and treated with different concentrations of cactus polysaccharides (0.36 mg/ml, 0.18mg/ml) for 24h and 48h. The morphology changes were examined with phase contrast microscope (Nikon, Japan).
Cell cycle and apoptosis analysis
For cell cycle analysis, SK-MES-1 cells were treated with different final concentrations of cactus polysaccharides solution (0.18 or 0.36mg/ml) compared to the control group treated with PBS. All groups were cultured for 24h and the cell pellets were collected by centrifugation for 5min at 1000rpm. The pellet was washed twice with PBS and then added with 50µl (lmg/ml) propidium iodide (PI) and 5µl (10mg/ml) DNase-free RNase for 30min-60min at 40□ without light. The cell cycle was detected by flow cytometry and analyzed by the system of Macintosh (Apple Inc. USA). For Annexin V assay, cactus polysaccharides treated cells were stained using an AnnexinV-FITC/PI Apoptosis Detection Kit (BD Biosciences Pharmingen, SanDiego, California, USA) according to standard protocol. The Annexin positive cells apoptosis were quantified flow cytometry and analyzed by Cell Quest Pro software (BD Biosciences Pharmingen, SanDiego, California, USA).
Western-blotting analysis
Cells treated with 0.36, 0.18mg/ml of cactus polysaccharides for 24h were collected by centrifugation at 4000rpm for 5 min at 4°C. Protein extracts and Western blotting was carried out as described (Singhal et al., 2005). P53 was detected using rabbit polyclonal antibody (Boster, China, 1:500 dilution), PTEN was detected using rabbit polyclonal antibody (Boster, China, 1:1000 dilution). β-actin was used as the internal control (Boster, China, 1:1000 dilution). The protein bands detected by antibodies were visualized by Super signal West Pico ECL (Thermo Fisher Pierce, USA).
Statistical analysis
Data were expressed as mean ± SD. Statistical Package for the Social Sciences (SPSS) 11.5 software was used for all statistical analysis of data. One-way Analysis of Variance (ANOVA) analysis of variance was used to determine the significance in two comparisons. Statistical significance was set at P<0.05.
Results
Cactus polysaccharides inhibits proliferation of human lung squamous carcinoma cells
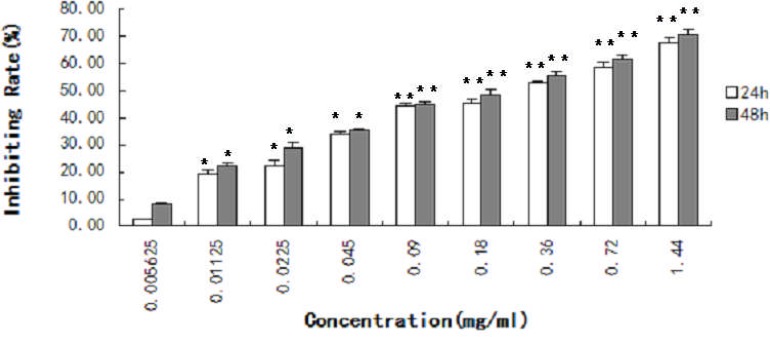
The inhibiting effect of cactus polysaccharides on the lung squamous carcinoma cells was detected by MTT assay for 24h and 48h. Cactus polysaccharides significantly inhibited cellular proliferation of the lung squamous carcinoma cells. The results showed that the inhibited rate of SK-MES-1 cells treated with cactus polysaccharides in a concentration and time dependent manner (P <0.01) (Fig. 1).
Figure 1.
Effect of cactus polysaccharides on proliferation of lung cancer cells SK-MES-1 cells were treated with cactus polysaccharides (1.44 mg/ml, 0.72 mg/ml, 0.36mg/ml, 0.18 mg/ml, 0.09 mg/ml, 0.045 mg/ml, 0.0225 mg/ml, 0.01125 mg/ml, 0.005625mg/ml) for 24h and 48h. The inhibiting rate of the cell growth was determined by MTT assay and expressed using the following equation: inhibiting rate (%) = (1-Abs test /Abs cont)×100%. *, P<0.05. **, P<0.01.
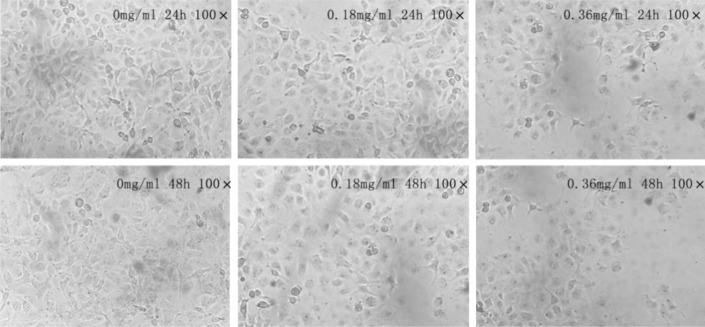
Morphological changes of SK-MES-1 cells
Cells were treated with different concentrations of cactus polysaccharides 0.36 mg/ml and 0.18mg/ml for 24h and 48h. The morphological changes of SK-MES-1 cells treated with cactus polysaccharides were detected by microscope. Compared to the control group, the cells were arranged from thickness to dispersion and fuzzy (Fig. 2).
Figure 2.
Effect of cactus polysaccharides on morphology of lung cancer cells SK-MES-1 cells were treated with cactus polysaccharides (0.18mg/ml and 0.36mg/ml) for 24h and 48h. The shape of cells was observed by phase contrast microscope. The cells treated with Cactus Polysaccharides were dispersion and fuzzy.
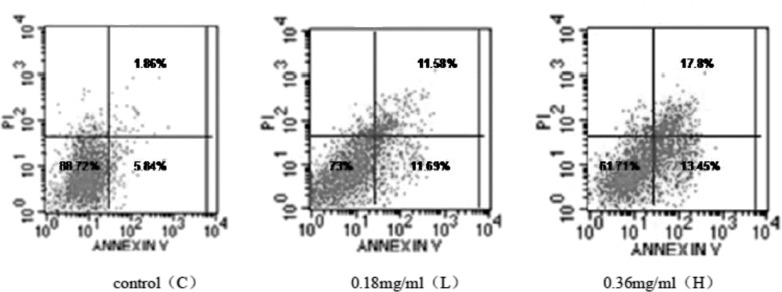
Cell cycle arrest and apoptosis induced by cactus polysaccharides treatment
We detected whether cactus polysaccharides could change the cell cycle and induce apoptosis. Therefore, we evaluated the effect of different dose of cactus polysaccharides exposure on the cell cycle progression of SK-MES-1 cells. The results showed that there was no significant difference in G1 phase among the control, low and high dose groups. However, cactus polysaccharides caused cells to accumulate in S phase and reduce in G2 phase (Table 1). It suggested that cactus polysaccharides blocked SK-MES-1 cells in S phase. We then detected apoptosis of cactus polysaccharides treated cells by Annexin V/PI staining. Our data showed that a steady increase in Annexin-positive cells to 31.15% in high dose group (Fig. 3).
Table 1.
Effect of cactus polysaccharides on the cell cycle.
| Dose (mg/ml) | Cell cycle (%) | ||
| G1 | S | G2 | |
| 0.00 | 46.11 | 41.50 | 12.38 |
| 0.18 | 46.13 | 44.68 | 9.18 |
| 0.36 | 47.98 | 51.65 | 0.38 |
Figure 3.
Effect of cactus polysaccharides on apoptosis of lung cancer cells SK-MES-1 cells were treated with 0, 0.18 and 0.36 mg/ml of cactus polysaccharides for 24h. Then they were stained with annexin-V-FITC and propidium iodide and analyzed by flow cytometry. Lower left quadrant area indicated that the survival cells. The early and late apoptosis cells were presented by the lower right and upper right quadrants, respectively.
After treated with Cactus Polysaccharides (0.18 mg/ml, 0.36 mg/ml) for 24h, the cells were harvested and stained with propidium iodide, and the proportion of cells in each phase of cell cycle was assayed.
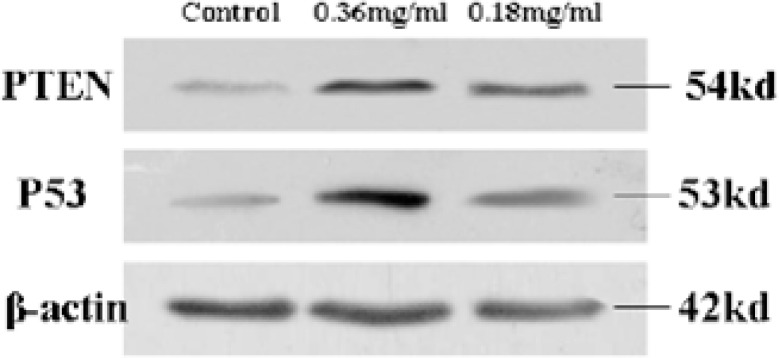
P53 and PTEN expression levels in SK-MES-1
The cell cycle is composed of many steps which are regulated by a lot of factors. P53 is one of most important negatively regulators of cell cycle. Mutation and alteration of P53 can lead to cancer (Levine et al., 1991). The study showed that P53 often inactivated in NSCLC. The increased expression of p53 can induce cell cycle arrest and cause apoptosis (Singhal et al., 2005). To further investigate the mechanisms underlying the cell cycle arrest and apoptosis induced by cactus polysaccharides, we detected the expression amount of P53 in the cells treated with cactus polysaccharides. The expression of p53 in the high dose cactus polysaccharides group (0.36mg/ml) and low dose cactus polysaccharides group (0.18mg/ml) were 3.83-fold and 1.99-fold increase than that of control group (Fig. 4).
Figure 4.
Effect of cactus polysaccharides on P53 and PTEN expression SK-MES-1 cells were treated with 0, 0.18 and 0.36mg/ml of Cactus Polysaccharides for 24h. Protein from the total cell lysate was subjected to western-blotting analysis for P53 and PTEN protein. PTEN and p53 were expressed relative to β-actin level and nomalized to control group. Representative results are shown from three independent experiments.
PTEN is a kind of tumor-suppressor gene which has the dual-specificity phosphatase and plays an important role in both regulating the cell growth and inducing the cell apoptosis (Weng et al.,2001). To further investigate the mechanisms underlying the cell cycle arrest and apoptosis induced by cactus polysaccharides, we detected PTEN expression in the cells treated with cactus polysaccharides as well. Our data showed that the expression of PTEN in high dose cactus polysaccharides group (0.36mg/ml) and low dose cactus polysaccharides group (0.18mg/ml) were 2.61-fold and 3.59-fold increase than that of control group (Fig. 4).
Discussion
Polysaccharide components of some fork medicine had anti-tumor effects and can stimulate immune response (Chen et al.,2014; Sun et al.,2011). Opuntia dillenii was a famous plant originating from Milpa characterized by high amounts of polysaccharides and water (Chang et al., 2008). Opuntia dillenii polysaccharides could enhance the immune function of immunosuppressed mice and had a good antiocidant activity (Zhao et al., 2012; Yang et al.,2013). However, there were no studies about anti-tumour effects of cactus polysaccharides on lung cancer. In this study, we detected the effects of cactus polysaccharides from Opuntia dillenii on lung squamous carcinoma cells in vitro.
The results revealed that cactus polysaccharides inhibited the proliferation of lung squamous carcinoma SK-MES-1 cells in a concentration and time dependent manner. Report had been indicated polysaccharides extracted from cactus pear fruit inhibited tumor growth in S180-bearing mice by inducing apoptosis, increasing anti-oxidation and promoting immune responses (Liang et al., 2008). Considering most of polysaccharides with non-toxic effects (Zong et al., 2012), the cactus polysaccharides could be an effective therapeutics without serious side-effects for lung cancer patients.
We further detected the mechanisms of inhibiting effect of cactus polysaccharides on the growth of lung cancer cells. Our data showed that the cactus polysaccharides inhibited proliferation of lung squamous carcinoma SK-MES-1 cells through inducing S phase arrest and triggering apoptosis. Insight into molecular mechanisms indicated that the cactus polysaccharides could significantly increased P53 and PTEN expression, which were negatively regulated cell cycle and induced apoptosis in the lung cancer cells.
In conclusion, our study is the first one to identify anti-tumor affects of cactus polysaccharides on lung squamous carcinoma cells. Our data generate evidences for further evaluation of cactus polysaccharides as a new drug for lung cancer. The molecular mechanisms of wild cactus polysaccharides preventing cell proliferation need further investigation.
Acknowledgments
This study was kindly supported by the National Natural Science Foundation of China (Grant No. 31070066).
Abbreviations
- PTEN
phosphatase and tension homolog deleted on chromosome ten
- NSCLC
Non-small-cell lung cancer
- FBS
Phosphate buffered saline
- MTT
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- PBS
Phosphate buffered saline
- DMSO
Dimethyl sulfoxide
- PI
Propidium iodide
References
- 1.Chang SF, Hsieh CL, Yen CG. The protective effect of Opuntia dillenii Haw fruit against low-density lipoprotein peroxidation and its active compounds. Food chemistry. 2008;106(2):569–575. [Google Scholar]
- 2.Chen JU, Chen JQ, Wang XT, Liu CB. Anti-tumor effects of polysaccharides isolated from Artemisia annual by inducing cell apoptosis and immunomodulatory anti-hepatoma effects of polysaccharides. African Journal of Traditional. 2014;1:15–22. [PMC free article] [PubMed] [Google Scholar]
- 3.D'Addario G, Früh M, Reck M, Baumann P, Klepetko W, Felip E. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(5):116–119. doi: 10.1093/annonc/mdq189. On behalf of the ESMO Guidelines Working Group. [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Li Q, Li H, Guo L. Neuroprotective and Antioxidative Effect of Cactus Polysaccharides In Vivo and In Vitro. Cell Mol Neurobiol. 2009;29(8):1211–1221. doi: 10.1007/s10571-009-9417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 6.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 7.Liang BB, Liu HG, Cao JT. Antitumor effect of polysaccharides from cactus pear fruit in S180-bearing mice. Ai Zheng. 2008;27(6):580–584. [PubMed] [Google Scholar]
- 8.Liao ML, Chen ZW, Zheng Y, Wu CX, Lu S, Yu YF, Jian H, Cheng BJ. Incidence, time trend, survival, and predictive factors of lung cancer in Shanghai populations. Zhonghua Yi Xue Za Zhi. 2007;87:1876–1880. [PubMed] [Google Scholar]
- 9.Zhao LY, Zhang SL, Yuan QX, Cheng J, Zeng FH. Immunomodulatory effects of Opuntia dillenii polysaccharides on specific immune function of mice. Zhong Yao Cai. 2012;35:98–102. [PubMed] [Google Scholar]
- 10.Yang Q, Chen H, Zhou X, Zhang J. Optimum extraction of polysaccharides from Opuntia dillenii and evaluation of its antioxidant activities. Carbohydr Polym. 2013;97(2):736–742. doi: 10.1016/j.carbpol.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 11.Pil JC, Sang SJ, Sung SY. Prognosis of Recurrence after Complete Resection in Early-Stage Non-Small Cell Lung Cancer. Korean J Thorac Cardiovasc Surg. 2013;46(6):449–456. doi: 10.5090/kjtcs.2013.46.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singhal S, Vachani A, Antin-Ozerkis D, Kaiser LR, Albelda SM. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: a review. Clin Cancer Res. 2005;11(11):3974–3986. doi: 10.1158/1078-0432.CCR-04-2661. [DOI] [PubMed] [Google Scholar]
- 13.Sun LX, Lin ZB, Li XJ, Li M, Lu J, Duan XS, Ge ZH, Song YX, Xing EH, Li WD. Promoting effects of Ganoderma lucidum polysaccharides on B16F10 cells to activatelymphocytes. Basic Clin Pharmacol Toxicol. 2011;108(3):149–154. doi: 10.1111/j.1742-7843.2010.00632.x. [DOI] [PubMed] [Google Scholar]
- 14.Weng L, Brown J, Eng C. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and?independent pathways. Hum Mol Genet. 2001;10(3):237–242. doi: 10.1093/hmg/10.3.237. [DOI] [PubMed] [Google Scholar]
- 15.Zhao LY, Lan QJ, Huang ZC, Ouyang LJ, Zeng FH. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine. 2011;18:661–668. doi: 10.1016/j.phymed.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Zong A, Cao H, Wang F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr Polym. 2012;90(4):1395–1410. doi: 10.1016/j.carbpol.2012.07.026. [DOI] [PubMed] [Google Scholar]