Abstract
Background
Plants are the natural source of antioxidants as well as antimicrobial compounds that has great potentials in pharmaceutical industry. In the present study, two medicinal plants Atropa belladonna and Matricaria chamomilla were collected from Northern areas of Pakistan.
Materials and Methods
The extracts of the collected plants were obtained by microwave assisted extraction (MAE) with changing parameters, power level and time; methanol and ethanol were solvents used during extraction. The extracts of plants were tested against different bacterial strains.
Results
It was observed that ethanolic extracts of Atropa belladonna has more significant antimicrobial activity against S.aureus than E.coli. In parallel, methanolic extract of Matricaria chamomilla showed greater significant antibacterial activity against S.aureus when compared with E.coli. In comparison, ethanolic extracts of Matricaria chamomilla has shown more significant results against S. aureus than E.coli (p≤0.05). Both plants had no antibacterial activity against S.typhi. The free radical scavenging activity observed by DPPH assay, indicate that both plants have antioxidant activity at all levels of concentrations in solvent tested during the present work. However, methanolic extracts had greater antioxidant activity when compared with ethanolic extracts.
Conclusion
Present study is thus helpful in highlighting present potentials for antioxidant and antimicrobial properties in the selected plants.
Keywords: Antimicrobial, antioxidant, Atropa belladonna, Matricaria chamomilla
Introduction
The extracts of medicinal plants are used directly or indirectly for the treatment of many diseases. Especially, in most developing countries, the use of medicinal plants in traditional medicine has been observed for maintaining good health (Edward, 2001). Around the world, scientists are trying to investigate the benefits of medicinal plants to assist the sufferings of humanity. Plants are used in more than 30% of the world's pharmaceutical preparations (Shinwari and Khan, 1998). Importance of the antioxidant compounds of the plants manifests in maintaining healthy conditions, prevention of cancer and heart disease (coronary) with increasing interest in scientific research, food production and consumption (Lo-Liger, 1991). Atropa belladonna, which is commonly known as deadly nightshade or belladonna belongs to the family Solanaceae. This plant is of great interest because it is a commercial source for the pharmaceutical bioactive tropane alkaloids like scopolamine and hyoscyamine which are broadly used as antagonists of acetylcholine in both central nervous system and autonomic system (Guggisberg and Hesse, 1983). Atropa belladonna is a perennial plant, widely distributed over central and Southern Europe and its cultivation global. Tropane alkaloids present within the plant have spasmolytic and anti-cholinergic properties (Tyler et al., 1988).
Matricaria chamomila (Chamomile) is herbal medicinal plant of 15 to 50 cm high and from planting stage it flowers, after a few weeks. This plant is distributed in east and south Europe, United States of America and Mediterranean White Sea beaches (Chakravarty, 1976). Chamomile flowers contain phenolic compounds e.g flavonoides such as a glycogen, apigenin, flavon glycoside and lutoline. The flowers contain glycosides such as anthamic acid, anthamedine and matricarin (Haslam, 1989). Matricaria chamomila is used to relief various body pain, calm headaches and tooth aches, to relieve menstruation pains. It is an anti-inflammatory drug that softens eyelids and eyes (Shivananda et al., 2007; Owlia et al., 2007). Aqueous and alcoholic extract of (chamomilla) flowers powder is used for therapeutics of skin infections caused by pathogenic bacteria, therapy for mouth injuries, therapeutics of respiratory system infection and digestive disorders treatment (Ribereau-Gayon, 1979). Medicinal plants are important source of antimicrobial compounds. In different countries, these plants are vital sources of numerous powerful medicines (Srivastava et al., 1996). Hundreds of medicinal plants have been tested for their antimicrobial potentials. (Balandrian et al., 1985) Medicinal plants have variety of secondary metabolites such as alkaloids, flavonoids, saponins and sterol superior in their antimicrobial potential. (Cowan, 1999) Alkaloids are nitrogenous compounds that contain heterocyclic ring and they have the therapeutics importance because of their great antimicrobial properties and their ability to bind with the nucleic acids (Jayasurriya et al., 1991). All the living organisms have a natural defense mechanism in the form of antioxidant compounds as well as enzymes that protect the organism from the damaging effects of oxidative stress (Ali et al., 2001). Plants are the primary source of naturally occurring antioxidants for human beings. It has been well-known for many years that plant extracts and essential oil have antioxidant and antimicrobial effects (Ozer et al., 2007.
Material and Method
Sample Collection
Plant samples Atropa belladonna and Matricaria chamomilla were collected from Northern areas of Pakistan. After collection plant material were weighed before drying
Drying and grinding of plants
The roots were collected from collected plants and dried in shady places in LCWU. Then powder of roots was kept in incubation over night at 37°C to remove complete moisture.
Microwave assisted extraction
Plant materials were extracted using microwave assisted extraction technique. Methanol and ethanol were used as solvents.
%age Yield: After extraction the percentage yield of plant extract was found out (by) using the formula
Weight of sample = (weight of china dish + extracts) − (Weight of china dish)
Content of plant in the extracted sample determined by the weight difference:
Plant sample content (%) = amount of sample extracted (g)/weight of original sample (g) × 100
Assessment of antibacterial potential
Bacterial strains
ATCC bacterial strains E. coli, S. aureus and S. typhi were used and confirmed by QOL, UVAS.
Preparation of Media
Firstly, weighed nutrient agar was added to distilled water (In a concentration of two grams per 100 ml) in autoclave enabled conical flask. Then media was autoclaved at 121°C and 15 lb pressure for 15–20 minutes. When the temperature was nearly (about), 50 to 55°C, from the Master Suspension bacterial suspension was taken and then mixed with media in such amount that each milliliter of media consist of 106 colony forming unit.
Sensitivity Testing
Antibacterial activity of plant extracts was determined through the method used by Shan et al. (2007). Briefly, Nutrient Agar media was prepared and 30 ml was poured in the each Petri plate. Three wells of diameter 0.7 centimeter cut in each plate with the help of cork borer and then sealed with Nutrient Agar. In each prepared plate, extracts of same concentration were poured in all wells using micro-pipette and four concentrations were made of each plant extract e.g. Concentration of ethanolic extract of Matricaria chamomilla were (2.5, 5, 10 and 20µg/ml) and concentration for its methanolic extracts were (1.5, 3, 6 and 12 µg/ml). Concentration of ethanolic extracts of Atropa belladonna were (1.5, 3, 6 and 12µg/ml) and concentration for its methanolic extracts were (1.25, 2, 4 and 10 µg/ml). In separate nutrient agar plates, discs of ampicillin, ciprofloxacin and Chloramphenicol were used as a positive control. In another plate, respective solvent without plant extract were used as negative control. The plates were left open for 20 minutes in Laminar Flow Hood, allowing organic solvents to evaporate and then the plates were closed and incubated at 37° C for 24 hrs. The diameter of inhibition zones were calculated in millimeters using a scale. Each test was performed in duplicates.
Assessment of Antioxidant potential
Antioxidant activity of extracts of both plants and standard was evaluated by DPPH radical scavenging activity with modified method (Braca et al., 2002). Diluted solutions of sample extracts were made in methanol upto 1 ml with different concentrations like Concentration of Atropa belladonna methanolic extracts were (8, 4, 2 and 1 µg/ml) and concentration for Atropa belladonna ethanolic extracts were (1.5, 3, 6, 12 µg/ml) concentration of Matricaria chamomilla ethanolic extract were (2.5, 5, 10, 20 µg/ml) and concentration for Matricaria methanolic extracts were (1.5, 3, 6, 12 µg/ml). Standard used was ascorbic acid in 1-100 µg/ml solution. In methanol prepared the solution of 0.001% DPPH and from this solution 1ml was mixed separately with the 1ml of the sample solution and also with standard solution. All these mixtures of solutions were kept in the dark for 30 minutes and O.D was measured at 517 nm by using Spectrophotometer. For blank solution 1 ml of methanol with 1 ml of 0.001% DPPH solution was used.
The O.D was recorded and percentage inhibition was calculated from the formula:
% inhibition of DPPH activity =A−B /A×100
Where A = O.D of blank
B = O.D of sample.
Statistical Analysis
All the data generated in the study are the mean ± SD of three replicates. Data were subjected to t-test with significance level of p ≤ 0.05 using SPSS statistics 17 Software.
Results and Discussion
The in vitro antibacterial activity of Atropa and Matricaria extracts were determined by measuring diameters of inhibition zone of these extracts. Two medicinal plants Matricaria and Atropa were tested against ATCC bacterial cultures Staphylococcus aureus, Escherchia coli and Salmonella typhi to determine and investigate their antibacterial potentials. Antibiotics such ampicillin, ciprofolxin and chloramphenicol were used against E. coli, S. aureus and S. typhi respectively as positive control. Solvents such as methanol and ethanol were used as negative control. It has been reported that the medicinal plants have antimicrobial effect. (Valero and Salmeron, 2003) In previous studies, it has been reported that the medicinal plants have great antibacterial potential; even they also have potentials against a few antibiotic resistant strains (Kone et al., 2004). The antiviral and antibacterial effects of Atropa belladonna and Matricaria chamomilla have been well documented (Aggag and Yousef, 1972; Mann and Staba, 1986).
Both ethanolic and methanolic extract of plants showed positive results against E.coli and S.aureus and negative result was shown against S.typhi.
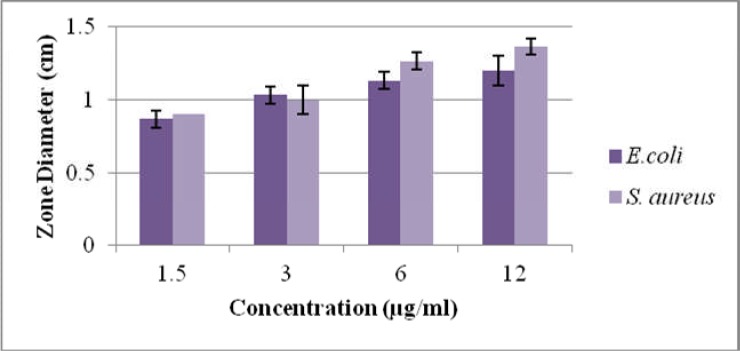
Ethanolic extract of Atropa belladonna showed maximum inhibitory zone (1.2cm) against E.coli and 1.23cm against S.aureus at 12 µg/ml concentration. It was concluded from results that S.aureus had more significant (p≤0.05) antibacterial potential as compared to E.coli as shown in Table. 1. In a previous study, Eftekhar et al. (2005) reported that antibacterial activity of the methanolic extracts of the D. stramonium and D. innoxia of Solanaceae family extracts has shown antibacterial activity against gram positive bacteria in a dose dependent way and very little or no antibacterial activity was found against E. coli. In contrast, present study revealed that ethanolic extract show activity against gram positive S.aureus and gram negative E.coli while no activity against salmonella. Furthermore methanolic extract showed no antibacterial activity against any of strain. The difference between results is may be due to use of different solvent and extraction method.
Table 1.
Antibacterial activity of ethanolic extract of Atropa belladonna against E. coli and S. aureus
| Strain | Plant used |
Solvent used |
concentration used (µg/ml) |
Mean inhibitory zone (cm) |
%age antibacterial activity |
| Escherichia coli |
Atropa belladona |
Ethanol | 1.5 | 0.83±0.05* | 18.44 |
| 3 | 0.96±0.05* | 21.33 | |||
| 6 | 1.03±0.05* | 22.88 | |||
| 12 | 1.2±0.1* | 26.66 | |||
|
Staphylococcus aureus |
Atropa belladona |
Ethanol | 1.5 | 0.86±0.05* | 26.87 |
| 3 | 1.06±0.05* | 33.12 | |||
| 6 | 1.1±0.1* | 34.37 | |||
| 12 | 1.23±0.05* | 38.43 |
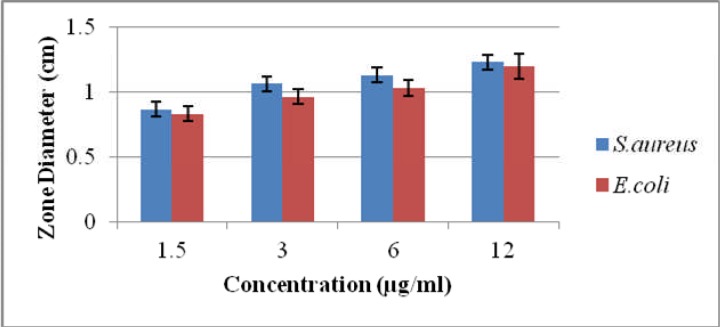
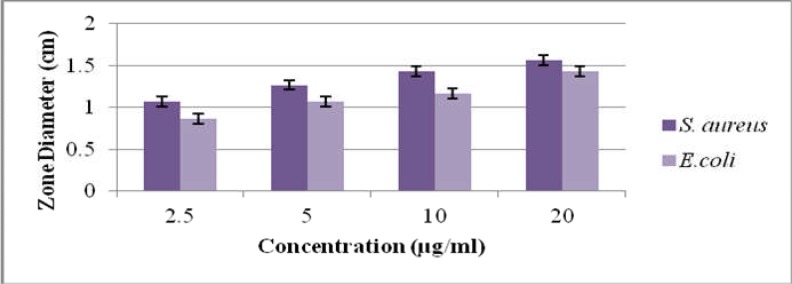
Ethanolic extract of Matricaria chamomilla showed maximum inhibitory zone (1.43cm) against E.coli and 1.56cm against S.aureus at 20 µg/ml concentration. It was concluded from results that S.aureus had more antibacterial potential as compared to E.coli as shown in Table.2. In correlation with ethanolic extracts, methanolic extracts of Matricaria chamomilla showed maximum inhibitory zone (1.2cm) against E.coli and 1.36cm against S.aureus at 12µg/ml concentration as shown in Table.3. It was concluded from results that S.aureus had more significant (p≤0.05) antibacterial potential as compared to E.coli as shown in (Table.2,3). In this study gram positive bacteria like S. aureus were more vulnerable than Gram-negative bacteria like E.coli to the antimicrobial activity of Matricaria chamomilla which is in accordance with previous study in which gram positive bacteria were also more susceptible than gram negative bacteria (Yu et al., 2012). Other workers have also reported that extracted compounds of M. chamomilla are more efficient against gram positive bacteria as compared to gram negative bacteria (Burt, 2004; Al-Bayati, 2008; Izadi et al., 2010).
Table 2.
Antibacterial activity of ethanolic extract of Matricaria chamomilla against E. coli and S. aureus
| Strain | Plant used |
Solvent used |
Concentration used (µg/ml) |
Mean inhibitory zone(cm) |
%age antibacterial activity |
| Escherichia coli |
Matricaria Chamomilla |
Ethanol | 2.5 | 0.9±0.1* | 20 |
| 5 | 1.06±0.05* | 23.55 | |||
| 10 | 1.16±0.05* | 25.77 | |||
| 20 | 1.43±0.05* | 31.77 | |||
|
Staphylococcus aureus |
Matricaria Chamomilla |
Ethanol | 2.5 | 1.06±0.05* | 33.12 |
| 5 | 1.26±0.05* | 39.37 | |||
| 10 | 1.43±0.05* | 44.68 | |||
| 20 | 1.56±0.05* | 48.75 |
Table 3.
Antibacterial activity of methanolic extract of Matricaria chamomilla against E. coli and S. aureus
| Strain | Plant used |
Solvent used |
Concentration used (µg/ml) |
Mean inhibitory zone(cm) |
%age antibacterial activity |
| Escherichia coli |
Matricaria Chamomilla |
Methanol | 1.5 | 0.86±0.05* | 19.11 |
| 3 | 1.03±0.05* | 22.88 | |||
| 6 | 1.13±0.05* | 25.11 | |||
| 12 | 1.2±0.1* | 26.66 | |||
|
Staphylococcus aureus |
Matricaria Chamomilla |
Methanol | 1.5 | 0.86±0.05* | 26.87 |
| 3 | 1.00±0.1* | 31.25 | |||
| 6 | 1.26±0.05* | 39.37 | |||
| 12 | 1.36±0.05* | 42.5 |
Antioxidant potential
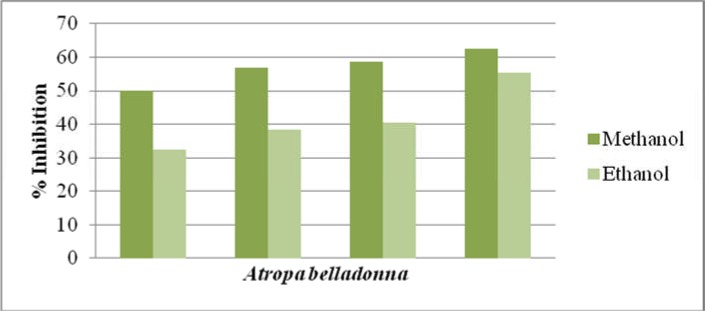
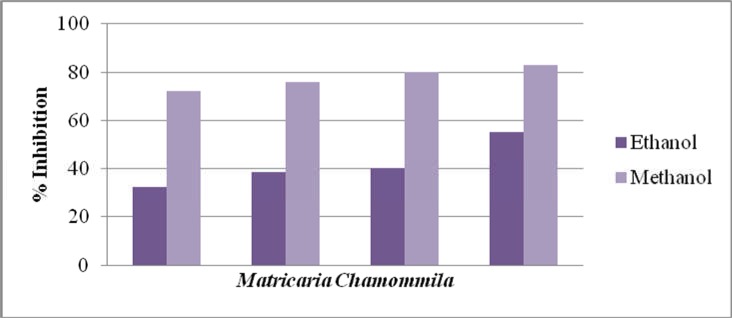
Antioxidant activity of methanolic and ethanolic extracts of two plant species Matricaria chamomilla and Atropa belladonna were measured using a radical scavenging method during the present wok. The stable free DPPH was oxidized and de-colorized differently on different extract concentrations using ascorbic acid as a standard. IC50 value described how stronger plant extract has antioxidant activity. IC50 value for Ascorbic acid was 10.12 on the minimum concentration so that ascorbic acid has more antioxidant activity. Methanolic extracts of Atropa belladonna showed the maximum % inhibition (62.64) on the highest concentration (8µg/ml). Ethanolic extracts of Atropa belladonna showed the maximum % inhibition 55.23 on the highest concentration 12 µg/ml. IC50 value for methanolic extract of Atropa belladonna range from 57.05 and for ethanolic extract its value was 41.76 (Table 4). Methanolic extracts of Matricaria chamomilla showed the maximum % inhibition 82.8 the concentration of the extract was 20µg/ml. Ethanolic extracts of Matricaria chamomilla showed the maximum % inhibition 55.23 when 12µg/ml concentration was used. IC50 value for methanolic Matricaria chamomilla extract was 41.69 and for ethanolic extract its value was 77.76 (Table 5).
Table 4.
Percentage inhibition of methanolic and ethanolic extract of Atropa belladonna
| Test Compound |
Concentration used (µg/ml) |
%Inihibition Mean ±S.D |
Mean IC50 (Y=mx+c) |
|
Atropa methanolic Extract |
1 | 50.14±0.44* | 57.05 |
| 2 | 56.78±0.58* | ||
| 4 | 58.64±0.64* | ||
| 8 | 62.64±0.46* | ||
|
Ascorbic acid as Standard |
1 | 19.14±0.52* | 22.14 |
| 2 | 21.51±0.54* | ||
| 4 | 23.34±0.80* | ||
| 8 | 24.58±0.48* | ||
|
Atropa ethanolic Extract |
1.5 | 32.29±1.17* | 41.76 |
| 3 | 38.31±0.71* | ||
| 6 | 40.38±0.63* | ||
| 12 | 55.23±0.66* | ||
|
Ascorbic acid as Standard |
1.5 | 16.06±0.45* | 25.78 |
| 3 | 18.19±1.20* | ||
| 6 | 28.29±1.14* | ||
| 12 | 30.95±0.81* |
Table 5.
Percentage inhibition of methanolic and ethanolic extract of Matricaria chamomilla
| Concentration used (µg/ml) |
% Inihibition Mean ± S.D |
Mean IC50 (Y=mx+c) |
| 2.5 | 72.15±0.48* | 77.76 |
| 5 | 75.86±0.36* | |
| 10 | 80.23±0.57* | |
| 20 | 82.8±0.57* | |
| 2.5 | 7.62±0.27* | 22.48 |
| 5 | 11.97±0.56* | |
| 10 | 32.69±0.48* | |
| 20 | 37.67±0.55* | |
| 1.5 | 32.2±0.31* | 41.6 |
| 3 | 38.65±0.41* | |
| 6 | 40.38±0.63* | |
| 12 | 55.23±0.66* | |
| 1.5 | 6.85±0.69* | 20.55 |
| 3 | 11.9±0.54* | |
| 6 | 30.26±0.59* | |
| 12 | 33.20±0.58* |
The other plants of family Solanaceae have also been reported to have high antioxidant activity. Khalighi-Sigaroodi et al. (2012) reported that by using DPPH free radical scavenging activity four species presented high antioxidant activity including S. dulcamara, D. innoxia, S. nigrum and S. incanum whereas the ascorbic acid (positive control) showed an IC50 value which is almost close to the IC50 value of ascorbic acid which was used as standard compound during the present study. Hence it is evident from the present study that Atropa belladonna has a strong antioxidant potential like the other plants of family Solanaceae. In Roby et al. (2013) findings antioxidant activity in extract of plants was evaluated by using the DPPH method. Plant extracts showed brilliant radical scavenging activity. The antioxidant activities of methanolic and ethanolic extracts were greater than other solvents. Furthermore, methanolic extracts showed more scavenging activity than ethanolic extracts. Similarly, the results of present work that methanolic extracts has more antioxidant activity than ethanolic extracts of Matricaria chamomilla depending on their IC50 values. Methanol is more polar than ethanol due to that it showed higher antioxidant potential.
In the previous study, methanolic extract of M. Chamomilla was used to determine the antioxidant activity by DPPH free radical scavenging. In the DPPH assay, methanolic extract showed good antioxidant activity. (Abdoul-Latif et al. 2011).Though the study highlights the importance of the selected plants however, on the basis of the results it can be suggested that there is great potential of these plants in utilizing them as a natural source of compounds having pharmaceutical importance. Hence, there is great need to further analyze the compounds present in these plants so that they can be used in the pharmaceutical industry.
Figure 1.
Comparison of antibacterial activity of ethanolic Atropa bellandonna against S. aureus and E. coli
Figure 2.
Comparison of antibacterial activity of ethanolic Matricaria chamomilla against S. aureus and E. coli
Figure 3.
Comparison of antibacterial activity of methanolic Matricaria chamomilla against S. aureus and E.coli
Figure 4.
Comparison of % inhibition of Atropa belladonna methanolic and ethanolic extracts
Figure 5.
Comparison of % inhibition of Matricaria chamomilla methanolic and ethanolic extracts
References
- 1.Abdoul-Latif F M, Mohamed N, Edou P, Ali A A, Djama S O, Obame L C, Bassole I H N, Dicko M H. Antimicrobial and antioxidant activities of essential oil and methanol extract of Matricaria chamomilla L. from Djibouti. Int J Med Plant Res. 2011;5:1512–1517. [Google Scholar]
- 2.Aggag M E, Yousef R T. Study of antimicrobial activity of chamomile oil. Planta Med. 1972;22:140–144. doi: 10.1055/s-0028-1099596. [DOI] [PubMed] [Google Scholar]
- 3.Al-Bayati F A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J Ethnopharmacol. 2008;116:403–406. doi: 10.1016/j.jep.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Ali Y, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extract. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 5.Balandrian M F J, Kjoke A, Wuretle E. Natural plant chemicals: source of industrial and medicinal materials. J Sci. 1985;228:1154–1160. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- 6.Braca A, Sortino C, Politi Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379–381. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 7.Burt S. Essential oil: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Cowan M M. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edward A. New, Old and Forgotten remedies. 2001. Pathogenesis Justicia adhatoda; pp. 210–220. [Google Scholar]
- 10.Eftekhar F, Yousefzadi M, Tafakori V. Antimicrobial activity of Datura innoxia and Datura stramonium. Fitoterapia. 2005;76(118):15–20. doi: 10.1016/j.fitote.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Guggisberg A, Hesse M. Putrescine, spermidine, spermine, and related polyamine alkaloids. In: Brossi A, editor. The Alkaloids. New York: Academic Press; 1983. pp. 85–188. [DOI] [PubMed] [Google Scholar]
- 12.Haslam E. Traditional herbal medicines -The role of polyphenols. Planta Med. 1989;55:1–8. doi: 10.1055/s-2006-961764. [DOI] [PubMed] [Google Scholar]
- 13.Izadi Z, Esna-Ashari M, Piri K, Davoodi P. Chemical Composition and Antimicrobial Activity of Feverfew (Tanacetum parthenium) Essential Oil. Int J Agri Bio. 2010;12:759–763. [Google Scholar]
- 14.Jayasurriya H, Nuphavan M K, Geahlen R L, Mclanghlin J L, Chang C J. Emodine, a proteinkinase inhibitor from polygonum Cusp. J Nat Pro. 1991;55(5):696. doi: 10.1021/np50083a026. [DOI] [PubMed] [Google Scholar]
- 15.Khalighi-Sigaroodi F, Ahvazi M, Yazdani D, Kashefi M. Cytotoxicity and Antioxidant Activity of Five Plant Species of Solanaceae Family from Iran. J Med Plants. 2012;11(43):41–53. [PMC free article] [PubMed] [Google Scholar]
- 16.Kone W M, Atindehou K K, Terreaux C, Hostettmann K, Traore D, Dosso M. Traditional medicine in North Cote-d'Ivoire: screening of 50 medicinal plants for antibacterial activity. J Ethnopharmacol. 2004;93:43–49. doi: 10.1016/j.jep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Lo-liger J. Use of antioxidants in food. In: Aruoma OI, Halliwell B, editors. Free radicals and food additives London. Taylor and Francis; 1991. pp. 129–150. [Google Scholar]
- 18.Mann C, Staba E. The chemistry, pharmacology, and commercial formuations of chamomile. J Herbs Spices Med Plants. 1986;1:235–280. [Google Scholar]
- 19.Owlia P, Rassooli I, Saderi H. Antistreptococcal and antioxidant activity of Essential oil from Matricaria chamomilla. Res J Bio Sci. 2007;2(2):237–239. [Google Scholar]
- 20.Ozer B C, Ozyoruk H, Çelebi S S, Yildiz A. Amperometric enzyme electrode for free cholesterol determination prepared with cholesterol oxidase immobilized in poly (vinylferrocenium) film. Enzyme Microb Technol. 2007;40(2):262–265. [Google Scholar]
- 21.Ribereau-Gayon P. Plant Phenol, Oliver and Boyd. Edinburgh. UK: Company Press; 1979. [Google Scholar]
- 22.Roby M H H, Sarhan M H, Selim K A H, Khalel K I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.) Ind Crop Prod. 2013;44:437–445. [Google Scholar]
- 23.Shan B, Cai Y, Brooks J D, Corke H. The in vitro antibacterial activity of dietry spice and medicinal herbs extracts. Int J Food Microbiol. 2007;117:112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Shinwari M I, Khan M A. Indigenous use of medicinal trees and shrubs of Margalla Hills National Park, Islamabad. Pak J Forest. 1998;48(1–4):63–90. [Google Scholar]
- 25.Shivananda B, Sivachandra R S, Rao AV. Wound healing activity of matricaria recutita L. extract. J Wound Care. 2007;16(7) doi: 10.12968/jowc.2007.16.7.27061. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava J, Lambert J, Vietmeyer N. Medicinal Plants: An Expanding Role in Development. World Bank Technical; 1996. p. 320. [Google Scholar]
- 27.Tyler V E, Brady L R, Robbers J E. Pharmacognosy. Philadelphia: Lea and Febiger; 1988. [Google Scholar]
- 28.Valero M, Salmeron M C. Antibacterial activity of 11 essential oils against Bacillus cereus in tyndallized carrot broth. Int J Food Microbiol. 2003;85:73–81. doi: 10.1016/s0168-1605(02)00484-1. [DOI] [PubMed] [Google Scholar]
- 29.Yu J S, Zhu L, Tian J Y. Chemical Composition and Antimicrobial Activities of Essential Oil of Matricaria songarica. Int J Agri Bio. 2012;14(1):107. [Google Scholar]