Abstract
In the present research, the effect of morphine consumption during pregnancy on the development of the embryo's spinal cord was studied in Wistar rat.
Female Wistar rats (Wt: 250-300 g) were mated with males. The test group received morphine (0.01 mg/ml) in their drinking water. Pregnant rats were later killed with chloroform on the 12th, 13th and 14th days of pregnancy, and the embryos were taken out surgically. The embryos were fixed in formalin 10% for 2 weeks. Then, the weight of fixed embryos was calculated by a scale. In addition, several animals’ sizes including fronto-posterior and lateral length were measured by a caliper. Tissue processing, sectioning and hematoxylin and eosin (H&E) staining were applied for the embryos. The sections were examined for spinal cord development by light microscope and MOTIC software.
Significant decrease was observed in the fronto-posterior and lateral length and the weight of the embryos in the test groups. The thickness of the white matter layer decreased on the 12th, 13th and 14th embryonic days. The thickness of the spine's grey layer was also less than the control group, on the same days. Increase in the length of the ependimal duct observed as well. Number of grey substance cells decreased compared to the control group within the same days. Meanwhile, thickness of the germinal layer reduced in comparison to the control group on the mentioned days.
In conclusion, morphine consumption during pregnancy causes defects in growth and completion of the spinal cord.
Keywords: Spinal Cord, White Matter, Grey Matter, Epandimal Duct, Morphine, Rat
1. Introduction
Problems caused by addiction do not only end to the addict but the aftermath of addiction is also detrimental to the health of the children. Studies have shown that taking narcotics during pregnancy can delay fetal distinction and can also cause symptoms such as weight loss and neurological defects. For example, studies of the animal models have shown that daily morphine injection can lead to activity decline in chickens (Schmidt, 1983). Morphine administration can also reduce brain, kidney, and liver weight as well as a reduction of head-tail length in rat embryos (Erikson and Ronnback, 1989). Morphine consumption can change the ovary cycle and sexual periods of the female rats (Dohler, 1991). On the other hand, studies have shown that morphine can easily pass through biological barriers such as bloodplacenta barrier and affect the embryonic cells (Kazemi et al., 2011; Levitt, 1998; Behravan and Piduette-Miller, 2007).
In previous researches, the influence of the oral morphine on rats’ neural tube (Nasiraei, et al., 2005) and frontal cortex (Sadraei et al., 2008) completion has been tested and proved to be effective. Abnormal completion of the spinal cord may induce certain abnormalities in locomotion. On the other hand, opioid usage during pregnancy delays nervous system development (Orony et al., 1996; Ray et al., 1977; Kazemi and Sahraei, 2011). On the basis of these findings, this research has studied the effects of oral morphine consumption on development of spinal cord in Wistar rat embryo.
2. Experimental Procedures
2.1. Animals
In this study, Wistar rats with an average weight of 250-300g were used. Two rats were housed per cage at a temperature of 24 ± 1°C with natural light periods (12 hours light/dark). Enough food and water were available for rats during the experiment. Rats were kept in an animal house at Baqyiatallah Medical University. Animal experiments were carried out ethically.
2.2. Drugs
The morphine sulfate (TEMAD-Iran) was used in this research. The drug (0.01 mg/ml) was dissolved in tap water and was offered to the animals in a volume of 100 ml/rat/cage.
2.3. Procedure
The rats were divided in four groups each containing 6 rats (n = 6).
24 healthy rats were mated in dual groups with two adult male rats. Once the pregnancy was definite (observation of the vaginal plague and sperm observation in vaginal smear), they were separated from the male ones and kept in groups of two at embryonic day 0 (E0). The experimental group received 0.01 mg of morphine in each ml of their drinking water. In the previous research the suitable morphine dosage in order to induce addiction was determined to be 0.1 mg/ml (Nasiraei et al., 2005). But an important study has indicated that 0.01 of morphine has the highest impact in causing malfunctions in the embryo, therefore this dosage was used as the effective amount (Nasiraei et al., 2005). The amount of morphine in 100 ml of water was measured, but it was tried to provide animal with enough water. The first group was killed on E12, second group on E13 and third group on E14 (5,6), with chloroform and the embryos as well as the uterus were taken out surgically and transferred into 10% formalin solution for 2 weeks. At this stage the embryos were separated from the uterus and were weighed with digital scale (Sartorius, Germany) with 0.0001 g precision and their fronto-posterior length was measured with caliper with 0.05 mm precision. The embryos were then underwent tissue processing procedure and stained with Hematoxilin &vEozin (H&E) (Wilson and Gamble, 2002) and were examined by a light microscope and MOTIC software after staining. In this research, thickness of the grey and white matter layers and the epandimal duct were measured as three main factors in spinal cord evolution. Also, the thickness of the ventricular layer and number of the cells in the grey matter layer were counted. In order to count these cells, in each group, different areas were randomly chosen and were counted in a square area of 625 micrometer. Since the animals did not complete the morphine consumption period (Khalili et al., 1997), the morphine dependency test was not carried out.
2.4. Blood Sampling and Corticosterone Assessment
One ml blood was collected in an Eppendorf tube containing 5 µl heparin (5000 IU/ml) and centrifuged at 3000×g for 5 min. Plasma was removed and kept at –74 °C for measuring the corticosterone. Plasma corticosterone was analyzed by the corticosterone Eliza kit (Bio Activa, Germany).
2.5. Statistical Analysis
Data were reported as mean± SEM. The differences between means were assessed by one way analysis of variance (ANOVA) and unpaired sample T-test using SPSS (version 18.0). P-value <0.05 was considered as significant.
3. Results
3.1. Effects of Oral Morphine Consumption on Plasma Corticosterone
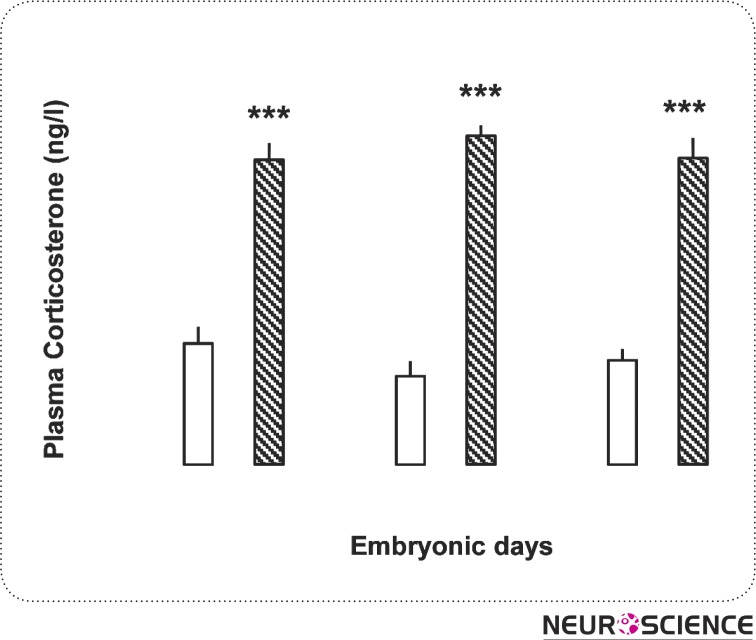
Plasma corticosterone level increased significantly in the experimental group (1850± 102.73 ng/l, day 12; 1995.85 ± 63.96 ng/l, day 13; and 1860. 23 ± 123 ng/l, day 14) compared to the control (735.48 ± 98.83 ng/l, day 12; 536.50 ± 89.27 ng/l, day 13; 632.67 ± 68.5 ng/l, day 14) (P < 0.001) (Fig. 1)
Figure 1.
It is suggested that micrograph of section of spinal cord of 12-days-old embryo in control group(A). W.M: White matter, G.M: Gray matter, Vent: Ventricular layer, E.C: and section of spinal cord of 12-days-old embryo in experimental group(B). W.M: White matter, G.M: Gray matter, Vent: Ventricular layer, E.C: Ependimal channel (×100).
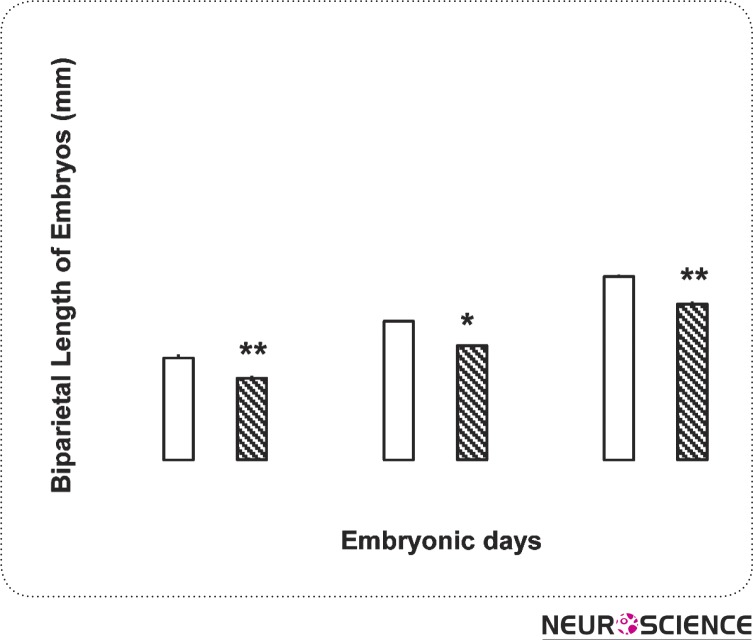
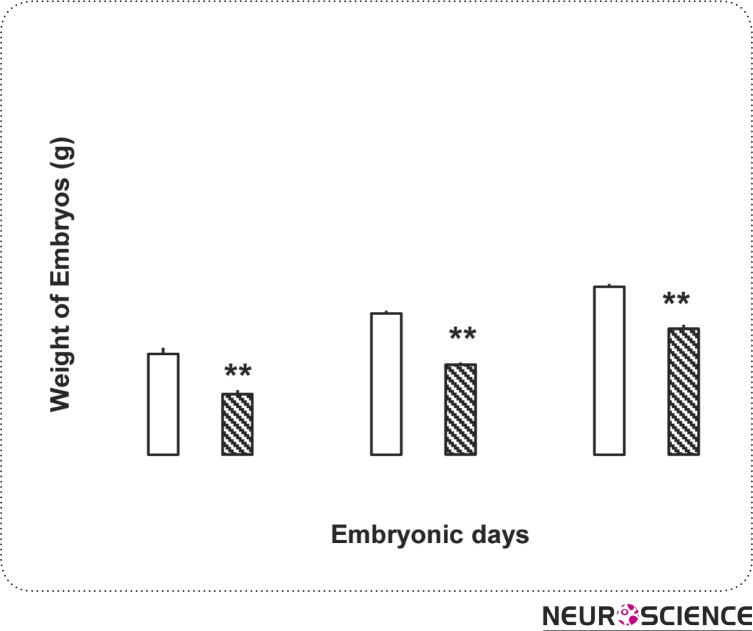
In this research, the fronto-occipital length of the embryos was measured as a pattern for their length and was measured in millimeter (Fig. 2). Examinations showed that morphine consumption in pregnant rats leads to decrease of embryos fronto-occipital length. The differences were statistically significant in all three days (P < 0.01) (Fig. 1). The weight of the embryos in control and test groups was measured in milligram and it was found that morphine can cause weight loss in embryo in all three test groups (P < 0.05) (Fig. 3).
Figure 2.
It is suggested that micrograph of section of spinal cord of 13-days-old embryo in control group(A). W.M: White matter, G.M: Gray matter, Vent: Ventricular layer, E.C: and section of spinal cord of 13-days-old embryo in experimental group(B). W.M: White matter, G.M: Gray matter, Vent: Ventricular layer, E.C: Ependimal channel (×100).
Figure 3.
It is suggested that Micrograph of section of spinal cord of 14-days-old embryo in control group(A). W.M: White matter, G.M: Gray matter, Vent: Ventricular layer, E.C: section of spinal cord of 12-days-old embryo in experimental group(B). W.M: White matter, G.M: Gray matter, Vent: Ventricular layer, E.C: Ependimal channel (×100).
3.2. Microscopic Observations
3.2.1. Differences Observed on 12th Embryonic Day (E12)
Observations showed that both grey and white layer were formed in control and test groups and theses layers can be differentiated. The thickness of all three posterior dorsal, abdominal ventral and lateral parts in the white substance, grey substance and ventricular layer in the control group was larger than the test group, whose variation was statistically significant. Also the length of the epandimal duct in the control group was less than the test group, which indicates better completion of the spinal cord in the control group. The number of grey cells was also more than the test group.
3.2.2. Differences Observed on 13th Embryonic Day (E13)
Both grey and white substances in control and test groups on this day have more thickness and a keratinous position was observable in both groups. The thickness of the white substance, grey substance and ventricular layer in control group is more than the test group. Also, the length of the epandimal duct in control group is less than the test group. The number of cells in the grey substance in the test group is less than the control group.
3.2.3. Differences Observed on 14th Embryonic Day (E12)
As shown in pictures 5 and 6, the thickness of white layer, grey layer and ventricular layer in the control group is more than the test group. Also, the length of epandimal duct in control group was less than test group which shows a more complete spine in control group. The number of grey cells was also more in comparison to the test group.
4. Discussion
This study showed that oral morphine consumption during pregnancy can delay spinal cord development. In addition, our findings show that oral morphine can excites corticosterone plasma level increment in pregnant rats. The results of this research are compatible with several other researches showing the effect of both oral and injection opioid prescription in inducing delay in embryonic development (Sadraei et al., 2008; Nasiraei et al., 2005; Ramazanyet al., 2009; Zagon and McLaughlin, 1985). The importance of these studies is that they all indicate that morphine consumption, even at the beginning of pregnancy, can cause defects in development of the embryos, a result which has been specifically shown in this research on spinal cord development. Since spinal development in rats begins on 12th embryonic day (E12) and is completed on the 14th day (E14), this period is chosen to examine the influence of morphine on spinal cord development.
Today, it is known that in certain periods of pregnancy, the embryos show more sensitivity to hexogen substances (such as narcotics) and these certain periods have been studied in previous researches (For review see: Ornoy et al., 1996). In one of the researches of Ornoy et al., it was realized that morphine shows its impact from the beginning of the formation of the fetus nervous system (Nasiraei et al., 2005; Kazemi and Sahraei, 2012; Sadraei et al., 2008; Kazemi and Sahraei, 2011). This research too, shows that one of the effects of morphine on the embryo is to control the development of the spinal cord. The importance of which, lies in the fact that the natural function of the spinal cord is essential for many of the sensory and movement activities and morphine delaying the spinal cord development can cause deficit in spinal cord function and thus the person may show extraordinary reactions in movement responses or sensory receptions.
The information gathered in this research stated that the thickness of the grey and white substances as well as the ventricular layer which is the germinal layer of the spinal cord decreased in the test groups. On the other hand, counting the number of cells in the gray substance showed that the number of these cells in test group in comparison with the control group is less (Kazemi and Sahraei, 2011). Meanwhile, the length of the Epandimal duct in the control group was more than the test group, which suggests that in morphine recipients the spinal cord is less complete. Thus, we can come to the conclusion that oral morphine consumption can delay spinal cord development in all morphine recipient groups. Such deficiency was observable in all test groups and no compensation was detected (Kazemi et al., 2011). In addition, embryos’ weight and height were reduced in the morphine treated group. Therefore, it seems that morphine prescription has destructive effects on embryo's development in general and spinal cord completion of the rats in particular. In this regard there are a few explanations: first, morphine affects all types of opioid receptors and the number of these opioid receptors is different both on embryo and placental tissues. Second, the opioid receptors on the placental tissue have different sensitivity in reaction to morphine (Kazemi et al., 2012). Therefore, it is possible that morphine affects one embryonic tissue more than the other.
The mechanism(s) by which morphine exerts its effects on the embryo is not well known yet. Previous studies have shown that morphine can easily pass through the placental barrier and reach the embryo (Levitt, 1998; Kazemi et al., 2011) and thus affect the embryonic cells. The presence of the kappa-opioid receptors on the villous and placental veins has also been shown (Ahmad et al., 1989). Once these receptors become active, the blood vessels contract and less blood is delivered to the fetus (Ahmad et al., 1989). Defect in oxygen delivery and loss of nutrition will delay the fetal growth (Ray et al., 1977; Fowden et al., 2006; Fowden et al., 2008). It is important to consider that certain opioid receptors have been found (Ray and Wadhwa, 1999; Leslie et al., 1998; Zhu and Barr, 2001) on embryonic tissue, whose function is not yet known. Such receptors might be responsible for morphine's delaying effects on embryonic cells. Therefore, future studies should examine morphine's impact zones.
On the other hand, our findings showed that corticosterone plasma level is also elevated in morphine treated pregnant rats. The effects of high plasma corticosterone level on fetal developmental delay are well known (For review see: Van den Bergh et al., 2005). Studies indicated that different stresses applied to the pregnant animals could lead to distinct nervous system developmental delay (Meaney et al., 2002). However, our finding is in consistence with the results that indicate single morphine injection can increases corticosterone plasma level in rats (Prinik et al., 2001; Kazemi et al., 2011; Buckingham and Cooper, 1984). Our results also indicated that chronic oral morphine consumption can increases plasma corticosterone level in pregnant rat. However, the source of corticosterone release in our study may be the mother or fetus adrenal and/or the placenta itself. The results of this research can open new horizons in behavioral complications of the human and livestock newborns whose parents have taken opioid during pregnancy. For example it is known that the rats, who have received heroin during embryonic period, are more active (Ramazany et al., 2009). Also, newborns of the rats who have received morphine are more sensitive against morphine (Erikson and Ronnback, 1989; Zagon and McLaughlin, 1985). In human subjects too, it has been realized that opioids during pregnancy can cause behavioral and movement abnormalities in newborns (Wilson et al., 1979; Kazemi et al., 2012), which could be due to the delay in spinal cord development.
In conclusion, the results show that oral morphine consumption can delay spinal cord completion in rats and the same can be true in humans as well. Such delay can also cause behavioral malfunctions in children, necessitating further studies.
Acknowledgment
This research was carried out with financial support of Neuroscience Research Center, Baqyiatallah (a.s.) Medical University, whose support is hereby highly appreciated.
References
- Ahmed, M.S., Timothy, S., Zhou, D.H., Quarles, C. (1989). Kappa opioid receptors of human placental villi modulate acetylcholine release. Life Sci, 45(25): 2383–93 [DOI] [PubMed] [Google Scholar]
- Behravan, J., Piduette-Miller, M. (2007). Drug transport across the placenta, role of the ABC drug efflux transporters. Expert. Opin. Drug. Metab. Toxicol, 3(6), 819–30 [DOI] [PubMed] [Google Scholar]
- Buckingham, J.C., Cooper, T.A. (1984). Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology, 38, 411–417 [DOI] [PubMed] [Google Scholar]
- Dohler, K.D. (1991). The pre-and postnatal influence of hormones and neurotransmitters on sexual differentiation of the mammalian hypothalamus. Int. Rev. Cytolo, 131, 1–75 [DOI] [PubMed] [Google Scholar]
- Eriksson, P.S., Ronnback, L. (1989). Effects of prenatal morphine treatment of rats on mortality, body weight and analgesic response in the offspring. Drug Alcohol Depenedence 24, 187–194 [DOI] [PubMed] [Google Scholar]
- Fowden, A.L., Forhead, A.J., Coan, P.M., Burton, G.J. (2008). The placenta and intrauterine programming. J. Neuroendocrinol, 20, 439–50 [DOI] [PubMed] [Google Scholar]
- Fowden, A.L., Ward, J.W., Woods, F.P.B., Forhead, A.J., Constancia, M. (2006). Programming placental nutrient transport capacity. J. Physiol, 572, 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezova, D., Vigas, M., Jurcovicova, J. (1982). ACTH and corticosterone response to naloxone and morphine in normal, hypophysectomized and dexamethasone-treated rats. Life Sci, 31, 307–314 [DOI] [PubMed] [Google Scholar]
- Kazemi, M., Sahraei, H., Azarnia, M., Dehghani, L., Bahadoran, H., Tekieh, E. (2011). The effect of morphine consumption on plasma corticosteron concentration and placenta development in pregnant rats. IJRM, 9: 71–76 [PMC free article] [PubMed] [Google Scholar]
- Kazemi, M., Sahraei, H., Dehghani, L. (2012). Identification of site of action of morphine in the pregnant Wistar rat's placenta: A [C]14-morphine study. Cell Journal, 14: 122–129 [PMC free article] [PubMed] [Google Scholar]
- Kazemi, M., Sahraei, H. (2011). The Effect of oral morphine consumption on Ependymal duct and Spinal cord development in Wistar rats embryos. Iranian South Medical Journal, 14: 16–9 [Google Scholar]
- Kazemi, M., Sahraei, H., Azarnia, M., Dehghani, L., Bahadoran, H. (2011). Effect of oral morphine consumption in female rats on development of brain cavities, central canal and choroid plexus of their embryos. Cell Journal, 12: 489–94 [Google Scholar]
- Kazemi, M., Tekieh, E., Sadeghi-Gharachdaghi, S., Ghoshoni, H., Zardooz, H., Sahraei, H., Rostamkhani, F., Bahadoran, H. (2012). Oral morphine consumption reduces lens development in rat embryos. Basic and Clinical Neurosciences, 3: 16–23 [Google Scholar]
- Kazemi, M., Sahraei, H. (2012). Effect oral morphine consumption on brain vesicles Prosencephslon and Rhombencephal development in Wistar rat embryos. Journal of Zanjan University of Medical Sciences and Health Service, 9: 24–33 [Google Scholar]
- Khalili, M., Semnanian, S., Fathollahi, Y. (2001). Caffeine increases paragigantocellularis neuronal firing rate and induces withdrawal signs in morphine-dependent rats. Eur. J. Pharmaco, 412, 239–245 [DOI] [PubMed] [Google Scholar]
- Kopcky, E.A., Simone, C., Knie, B., Koren, G. (1999). Transfer of morphine across the human placenta and it's interaction with naloxone. Life Science, 65, 2359–71 [DOI] [PubMed] [Google Scholar]
- Lasky, D.I., Zagon, I.S., McLaughlin, P.D. (1977). Effect of maternally administered heroin on the motor activity of rat offspring. Pharmacology Biochemistry and Behavior, 1, 281–284 [DOI] [PubMed] [Google Scholar]
- Leslie, F.M., Chen, Y., Winzer-Serhan, U.H. (1998). Opioid receptor and peptide mRNA expression in proliferative zones of fetal rat central nervous system. Can. J. Physiol. Pharmacol. 76, 284–293 [PubMed] [Google Scholar]
- Levitt, P. (1998). Prenatal effects of drugs of abuse on brain development. Drug and Alcohol Dependence, 51, 109–125 [DOI] [PubMed] [Google Scholar]
- Meaney, M.J., Brake, W., Gratton, A. (2002). Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology, 27, 127–138 [DOI] [PubMed] [Google Scholar]
- Michael, A.E., Papageorghiou, A.T. (2008). Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Human Reproduction Update, 14, 497–517 [DOI] [PubMed] [Google Scholar]
- Nasiraei-Moghadam, S., Sahraei, H., Bahadoran, H., Sadooghi, M., Salimi, S.H., Kaka, G.R., Imani, H., Mahdavi-Nasab, H., Dashtnavard, H. (2005). Effects of maternal oral morphine consumption on neural tube development in Wistar rats. Dev. Brain Res, 159, 12–17 [DOI] [PubMed] [Google Scholar]
- Ornoy, A., Michailevskaya, V., Lukooshov, I. (1996). The developmental outcome of children born to heroin-dependent mothers, raised at home or adopted. Child Abuse Negl, 20, 385–396 [DOI] [PubMed] [Google Scholar]
- Pirnik, Z., Schwendt, M., Jezova D. (2001). Single dose of morphine influences plasma corticosterone and gene expression of main NMDA receptor subunit in the adrenal gland but not in the hippocampus. Endocrine Regulations, 35, 187–193 [PubMed] [Google Scholar]
- Ramazany, M., Tekyeh, E., Zardooz, H., Bahadoran, H., Sahraei, H. (2009). Morphine delays fovea development in the eyes of Wistar rat embryos; Possible involvement of corticosterone. Physiology and Pharmacology, 13(3), 271–278 [Google Scholar]
- Ray, S.B., Wadhwa, S. (1999). Mu opioid receptors in developing humane spinal cord. J. Anat,195, 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, J.R., Dubinj, W., Blechner, J.N. (1977). Fetal growth retardation following maternal morphine administration: nutritional or drug effect? Biol. Neonal, 32, 222–8 [DOI] [PubMed] [Google Scholar]
- Sadraie, S.H., Kaka, G.R., Sahraei, H., Dashtnavard, H., Bahadoran, H., Mofid, M., Mahdavi Nasab, H., Jafari, F. (2008). Effects of maternal oral administration of morphine sulfate on developing rat fetal cerebrum: A morphometrical evaluation. Brain Res, 1245, 36–40 [DOI] [PubMed] [Google Scholar]
- Schmidt, M.B., Norton, S. (1983). Relationship of dose to morphine tolerance in the chick embryo. J. Pharmacol. Exp. Ther, 22, 376–382 [PubMed] [Google Scholar]
- Taylor, C.C., Wu, D., Soong, Y., Yee, J.S., Sseto, H.H. (1997). Opioid modulation of the fetal hypothalamic-pituitary-adrenal axis: The role of receptor subtypes and route of administration. JPET, 281, 129–135 [PubMed] [Google Scholar]
- Van den Bergh, B.V.H., Mulder, E.J.H., Mennes, M., Glover, V. (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms: A review. Neuroscience and Biobehavioral Reviews, 29, 237–258 [DOI] [PubMed] [Google Scholar]
- Williams, J.T., Christie, M.J., Manzoni, O. (2001). Cellular and synaptic adaptations mediating opioid dependence. Physiological Reviews, 81, 299–343 [DOI] [PubMed] [Google Scholar]
- Wilson, G.S., McCreay, R., Kean, J., Baxter, J.C. (1979). The development of pre-school children of heroin-addicted mothers: a controlled study. Pediatrics, 63, 135–141 [PubMed] [Google Scholar]
- Wilson, I., Gamble,M. (2002). The hematoxylins and eosin. In: Bancroft J.D. and Gamble M. (Eds.), Theory and practice of histological techniques, 5th Edition, Churchill Livingston, London, 125–138 [Google Scholar]
- Zagon, I.S., Mclaughlin, P.J. (1985). Naltrexone's influence on Neurobehavioral development. Pharmacol. Biochem. Behav, 22, 507–511 [DOI] [PubMed] [Google Scholar]
- Zhu, H., Barr, G.A. (2001). Opioid withdrawal during development: are NMDA receptors indispensable? Trends Pharmacol. Scim, 22, 404–408 [DOI] [PubMed] [Google Scholar]