Abstract
This study has examined the functional importance of nucleus accumbens (NAc)-ventral tegmental area (VTA) interactions. As it is known, this interaction is important in associative reward processes. Under urethane anesthesia, extracellular single unit recordings of the shell sub-region of the nucleus accumbens (NAcSh) neurons were employed to determine the functional contributions of the VTA to neuronal activity across NAcSh in rats. The baseline firing rate of NAcSh neurons varied between 0.42 and 11.44 spikes/sec and the average frequency of spontaneous activity over 45-minute period was 3.21±0.6 spikes/sec. The majority of NAcSh neurons responded excitatory in the first and second 15-min time blocks subsequent to the inactivation of VTA. In the next set of experiments, eight experimental rats received morphine (5 mg/kg; sc). Three patterns of neuronal activity were found. Among the recorded neurons only three had an increase followed by morphine administration. Whereas the other three neurons were attenuated following morphine administration; and there were no changes in the firing rates of the two neurons left. Finally, unilateral reversible inactivation of VTA attenuated the firing activity of the majority of ipsilateral NAcSh neuron in response to morphine, except for a single cell. These results suggest that transient inactivation of VTA reduces the ability of neurons in the NAcsh to respond to systemic morphine, and that NAcSh neuron activity depends on basal firing rate of VTA inputs.
Keywords: Nucleus Accumbens, Ventral Tegmental Area, Reversible Inactivation, Single Unit Recording, Morphine, Rat
1. Introduction
The mesolimbic dopaminergic system projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) is critical for the initiation of opioid reinforcement and reward-related behaviors. Related anatomical and physiological evidences implicate the NAc and its afferents in mediating behavioral effects of drugs abuse such as ethanol (Bunney, Appel, & Brodie, 2001), cocaine (Navailles, Moison, Cunningham, & Spampinato, 2007), nicotine (Mansvelder & McGehee, 2000) and morphine (Bunney et al., 2001; Olmstead & Franklin, 1997; Rezayof, Nazari-Serenjeh, Zarrindast, Sepehri, & Delphi, 2007). Based on histochemical (Jongen-Relo, Groenewegen, & Voorn, 1993) and connective (He, Wang, & Wei, 2007) differences, the NAc has been divided into core and shell regions, besides several reports indicating that both parts are involved in reward related behaviors (Ikemoto, Glazier, Murphy, & McBride, 1997; Mansvelder & McGehee, 2000; Moaddab, Haghparast, & Hassanpour-Ezatti, 2009; Pennartz, Groenewegen, & Da Silva, 1994). Various biochemical and physiological adaptations in the VTA neurons (both dopaminergic and GABAergic neurons) following chronic exposure to morphine have been reported, and neuroplastic changes within the VTA neurons are believed to contribute to drug addiction (Chu et al., 2008). Dopaminergic neurons in the VTA are the origins of mesolimbic/ mesocortical dopamine pathway and they also provide dopaminergic innervation to the NAc (Bunney et al., 2001; Moaddab et al., 2009; Oades & Halliday, 1987). The neural activity of NAc neurons is strongly modulated by various cortical and subcortical limbic inputs, and thus may represent a site in which the motivational component of behavior gains access the motor system (Esmaeili, Kermani, Parvishan, & Haghparast, 2012; He et al., 2007; Olmstead & Franklin, 1997; Winder, Egli, Schramm, & Matthews, 2002). These findings suggest that the NAc is well positioned to integrate a wide range of limbic and motor information (Esmaeili et al., 2012; Lisman & Grace, 2005; Meredith et al., 1999). This converging connectivity suggests that emotional motivational influences gain access to behavior through the limbicmotor interface of the NAc (Martin, 1991). Also, it has been shown that systemically administered opiates inhibit or excite spontaneously active single units of the nucleus accumbens septi (NAS) (Hakan, Eyl, & Henriksen, 1994). Moreover, in another research, single unit recordings of NAS neurons in halothane-anesthetized rats revealed that microinfusions of morphine into the VTA primarily inhibited spontaneously active NAS units. These inhibitory effects were reversed by subcutaneously administration of alpha-flupenthixol, a DA receptor antagonist, suggesting a role for dopamine (DA) in the observed opiate-induced effect. VTA opiate microinfusions also inhibited the evoked (driven) responses of silent cells (spontaneously inactive) in the NAS elicited by stimulation of hippocampal afferents to the NAS. In addition, this inhibition of driven response was reversed by naloxone but not by alpha-flupenthixol, implying a VTA-mediated non-DA mechanism (Hakan & Henriksen, 1989).
Morphine applied iontophoretically to cells within the NAS inhibited spontaneous activity but not fimbriadriven cellular activity, suggesting that the systemic effects of opiates on NAS activity can be mediated directly in the NAS as well as through VTA afferents. Moreover, since VTA-induced inhibition of fimbria-driven activity was reversed by systemic opiates, opiates also can exert effects through other, as yet unidentified NAS afferent systems (Gysling & Wang, 1983). Therefore, in this study, we tried to investigate the effects of reversible inactivation of the ventral tegmental area on the alteration in neural firing of shell sub-region of NAc neurons and the effects of morphine on this alteration in the rats.
2. Methods
2.1. Animals
Twenty nine adult male Wistar rats (Pasteur Institute, Tehran, Iran) weighing 240-300 g were used in these experiments. Animals were housed in groups of three per cage in a 12/12 h light/dark cycle (light on between 7:00 a.m. and 7:00 p.m.) with free access to chow and tap water. The animals were randomly allocated to different experimental groups. Each animal was used only once. Rats were habituated to their new environment and handled for 1 week before the experimental procedure started. All experiments were executed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 80-23, revised 1996) and were approved by the Research and Ethics Committee of Shahid Beheshti University of Medical Sciences.
2.2. Drugs
Morphine sulfate (Temad, Iran) and lidocaine, which was dissolved in sterile saline (0.9%) as a vehicle, were used in this study. Control animals received saline.
2.3. Experimental Procedures
Testing sessions were carried out in a quiet room, with the room temperature kept at 25°C. There were three groups as follows:
2.3.1. Effects of Intra-VTA Lidocaine Administration on the Firing Rate of NAcSh Neurons
In this set of experiments, after stabilization period (15 min) and baseline recording (30 min), lidocaine 2% (0.5 µl/rat) was administrated into the VTA, and 15 min later subcutaneous (sc) saline (1 ml/kg) was applied. Control animals received saline (0.5 µl/rat) instead of lidocaine 2%.
2.3.2. Effects of Systemic Administration of Morphine on the Firing Rate of NAcSh Neurons
In the next set of experiments, the effects of morphine on a total of eight single neurons recorded in the NAcSh were determined. After stabilization period (15 min) and baseline recording (30 min), saline (0.5 µl/rat) was applied into the VTA as a vehicle instead of lidocaine. 15 min later the administration of morphine (5 mg/kg; sc) was done. In control animals, saline (1 ml/kg) was administered instead of morphine.
2.3.3. Effects of Reversible Inactivation of VTA on NAcSh Neural Response After Morphine Administration
In this set of experiments, after stabilization period (15 min) and baseline recording (30 min), the animals received lidocaine 2% (0.5µl/rat) and after 15 min morphine (5 mg/kg; sc) was administrated. Control animals received saline (0.5 µl/rat) instead of lidocaine 2%.
In all aforementioned experimental groups, after the second injection, single unit recording was followed by 30 min period without any intervention. In this study, a total of 29 single NAcSh neurons were analyzed.
2.4. Electrical Recording and Data Acquisition
Animals were initially anesthetized with urethane intraperitoneally (1.2 g/kg) with additional doses (0.1 g/ kg) administered every 1 h as needed for maintaining a deep and constant level of anesthesia as determined by lack of movement in response to a strong tail pinch. Then tracheotomy was done in order to prevent suffocation and the animals were placed in a stereotaxic instrument (Stoelting; USA). Body temperature was maintained at 36-36.7°C with a thermistor-controlled heating pad. Two 2-mm diameter holes were drilled in the skull; one above the NAcSh (1.2 mm rostral to bregma, 0.8 mm lateral to the sagittal suture) and the other on top of the VTA (4.75- 5 mm caudal to bregma, and 0.8- 0.9 mm lateral to the sagittal suture) based on the rat brain atlas (Paxinos & Watson, 2007). The Dura was removed and the hole was covered by a drop of mineral oil, for facilitating the microelectrode entrance. The injector cannula was stereotaxically aimed to the VTA (8.2- 8.4 mm ventral to skull surface).
Extracellular recording from individual neurons was obtained with tungsten microelectrode (Harvad Apparatus, USA; Parylene Coated; 127-µm diameter shaft with extra fine tip; 5 MΩ impedance tip). Microelectrode was stereotaxically advanced into the NAcSh (7 mm ventral to skull surface). Spike signals which were received from neurons by a preamplifier, were amplified by a differential amplifier (DAM-80, WPI, USA; ×1000 gain; 300 Hz and 10 KHz for low and high filters, respectively) and were modified from analog to digital form by data acquisition, as signals. Then the signals were displayed continuously on the computer via a homemade software and were auditory monitored (Haghparast A, 2010; Haghparast A 2012). Only the single cells having a consistent spike amplitude and waveform during the experimental procedure were studied. The action potentials were categorized by the initial direction of the voltage deflection (positive or negative); amplitude (peak-to-peak), and duration were also determined. Action potentials were isolated from background activity with two windows which generated output pulses for signal units based on spike height (amplitude), and which counted the number of spikes per unit time (bin widths were 1000 ms). Sampling of extracellular recordings was done using an electrophysiological data acquisition (D3109; WSI; Iran) on an IBM Pentium computer for on-line data collection (Haghparast A, 2010; Haghparast A 2012). In this manner, the computer saves the number of output signals as spikes in time unit that is set manually (0.1-12000 sec). In these experiments, time setting for data collection was 4800 sec with 1000 ms bin size as a file which was saved continuously during experiment in hard disk, and unit activity was calculated by computer as an average frequency (spikes/sec). In the present study, the signal to noise ratio was considered at least 3 to 1. For data presentation, unit activity is shown at 1- and/or 5-min intervals.
2.5. Data Analysis
In this study, discharges of each neuron were counted in 60 s time bins using a data acquisition interface program to construct peri-stimulus time histograms (PSTHs), with a time range of 15 min (stability period) and 30 min (baseline recording) to 35 min after the injections of saline/lidocaine (intra-VTA microinjection) and saline/morphine (sc). The data were later analyzed off-line with the homemade analysis software for windows. In order to detect the neuronal response patterns to lidocaine and morphine administration over time, the whole period of observation was sectioned into 1-min time bins. An increase or decrease in firing rates over two-fold of the standard deviation of the baseline for at least 3 consecutive bins (i.e., 3 min) was considered as an excitatory or inhibitory response, respectively. A clustering analysis (K-means, SPSS) was performed to classify neuronal responses depending on the similarities in patterns of excitation or inhibition (both latency and duration of response) induced by injection of drugs (Haghparast et al. 2010, 2012). The number of neurons exhibiting excitatory or inhibitory response to lidocaine and morphine injections at each time bin was counted. To calculate the significance, the mean ± SEM values of the 1 min time blocks, representing the lidocaine and morphine responses of each of the groups, were compared with the control group. Statistical analysis of the data was done using one-way ANOVA and followed by Student-Newman-Keuls test for multiple comparisons. P-value < 0.05 was statistically considered significant.
2.6. Histological Verification
After completion of the recordings, subjects were overdosed with urethane and the electrode position was confirmed by electrolytical markings (50 µA of negative current for 10-15 sec) with signs of electrode penetration to confirm microelectrode placement within the NAcSh. Under deep anesthesia, the animals were perfused transcardially with 0.9% saline followed by 10% formalin. The brains were removed and placed in a 10% formalin solution for at least three days. The recording site was subsequently examined in coronal sections (150 µm) by light microscopy by an observer unfamiliar with the electrophysiological data. Recording site was histologically verified and plotted on standardized sections derived from the atlas of Paxinos and Watson (2007) and only those data that were histologically verified to be located in NAcSh were included in the data analysis.
3. Results
3.1. Electrophysiologic Profile of NAcSh Recording
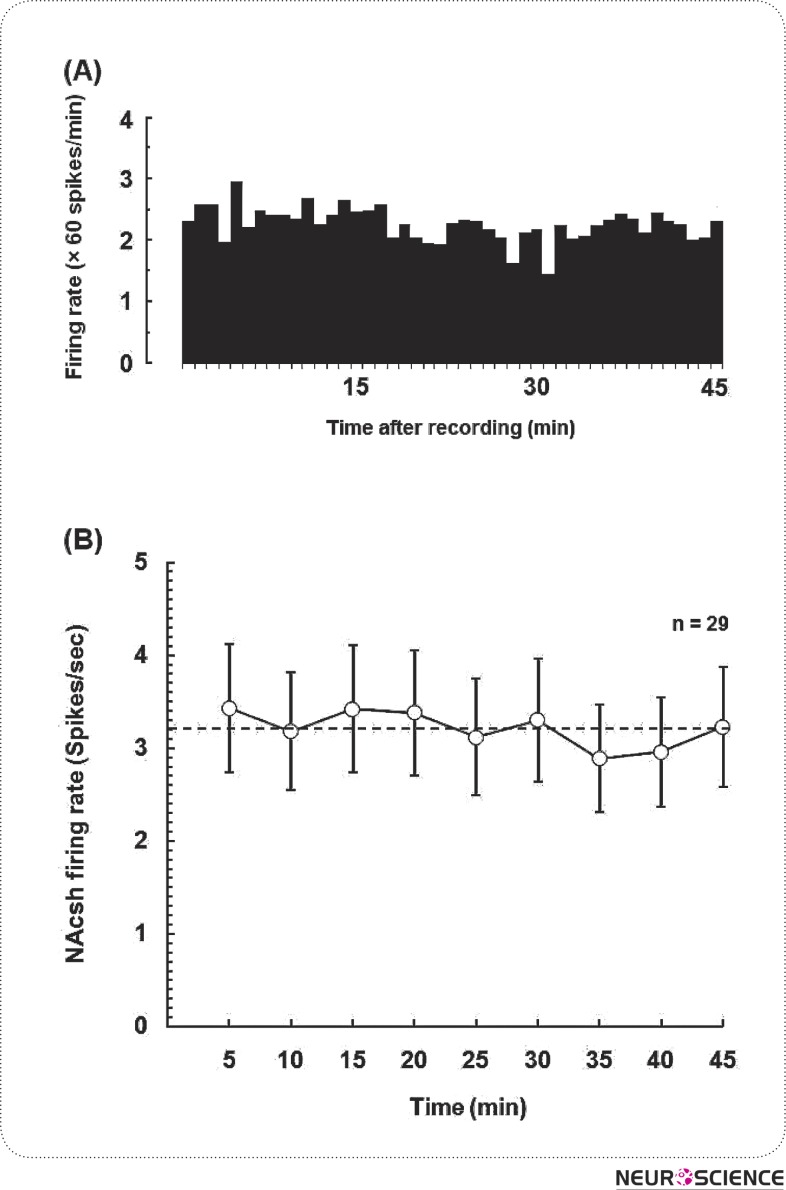
Histological evaluations revealed that electrophysiological recordings were obtained from 29 neurons located throughout the shell part of the nucleus accumbens. After isolating a unit and determining the stability of its firing rate (30–40 min), background activity is tested merely for determining spontaneous firing rate of NAcSh neurons during 45 min (Fig. 1A). The saline experiments served as control for the effect of the injection procedure and the volume administered on neural activity over the recording period. Data was subjected to one-way ANOVA and showed that there were no significant differences in any 5-min points of baseline firing rate of neurons in the NAcSh during 45-min recording time after the stabilization period [F(8,223) = 0.6833, P = 0.8711; Fig 1B]. Their baseline firing rate varied between 0.42 and 11.44 spikes/sec and the average frequency of spontaneous activity over the 45-min time period was 3.21 ± 0.6 spikes/sec as shown in Fig 1B. Neurons in the NAcSh exhibited mostly action potentials with biphasic waveforms (∼ 90%; 26 cells), a width of 1.3 to 2.8 ms in duration and an inflection in the initial positive component with different amplitudes (130-240 µV).
Figure 1.
(A) An example of spontaneous activity of neuron (2.16 ± 0.27 spikes/sec) recorded from the NAcSh in urethaneanesthetized rat. (B) Average firing rate of the NAcSh neurons in control (open circles), anesthetized rats (n = 18 to 29 neurons at each time point) at 5-min set intervals for the 45-min recording time period. Dash line shows the mean baseline activity (3.21±0.6 spikes/sec) in the NAcSh.
3.2. Response of NAcSh Neurons to VTA Lidocaine Administration
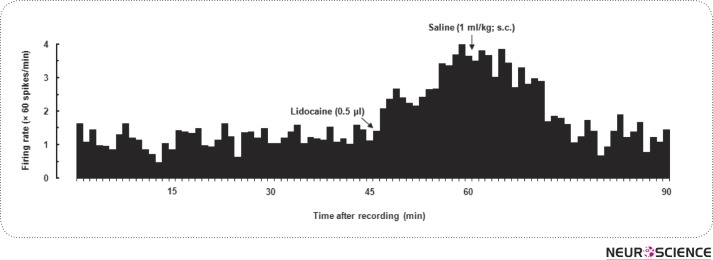
After stabilization period (15 min) and baseline recording (30 min), lidocaine 2% (0.5 µl/rat) was administrated into the VTA, and 15 min later, saline (1 ml/ kg; sc) was applied. In 5 out of 6 neurons, 1-7 min after injection of lidocaine, neural firing rate was increased in the NAcSh and it continued for 19-33 min (Fig. 2). The spontaneous activity of NAcSh was variable, between 0.58 and 8.92 spikes/sec and the average of firing rate was 3.27 ± 1.01 spikes/sec. The maximal percentage of lidocaine-induced activation of neural activity in the first 15-min time period (0-15) after lidocaine injection was 24.54 ± 9.16% and in the second 15-min (15-30) which was coincidence with the injection of saline (1 mg/kg; sc) was 47.57 ± 15.44%. In addition, in the case of the one remained neuron, lidocaine increased the neural activity only 8.1 ± 0.27%, hence it was considered as an ineffective neuron. Student's paired t-test (t5 = 2.88, P < 0.05) revealed that the average unit activities of NAcSh neurons after lidocaine administration into the VTA were significantly different from those of preinjection recording time.
Figure 2.
A typical effect of administration of 2%lidocaine (0.5µl) alone into the VTA on spontaneous activity of neurons in the NAcSh followed by saline (1 ml/kg; sc) injection. The firing rate of neuron continually recorded 90 min following injection of lidocaine at 45th-min of recording period.
3.3. Responses of NAcSh Neurons to Systemic Administration of Morphine
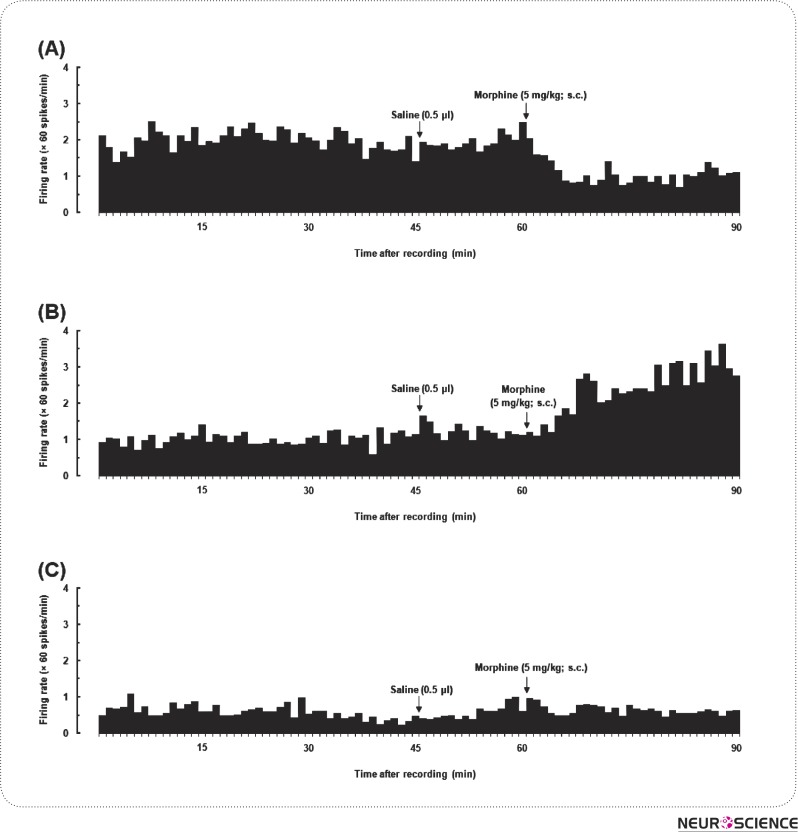
In the next set of experiments, the effects of morphine on a total of 8 single neurons recorded in the NAcSh were determined. After stabilization period (15 min) and baseline recording (30 min), saline (0.5 µl; one side) as a vehicle was applied into the VTA; and 15 min later the sc administration of morphine (5 mg/kg) was done. As it can be seen in Fig. 3 after morphine injection, the following neural activity profiles were seen in the NAcSh: in 3 neurons, 1-3 min after morphine injection, the neural activity significantly decreased and it continued till the 90th minute (Fig. 3A). Their baseline firing rate varied between 1.05 - 8.4 spikes/sec and the average frequency of spontaneous activity over the 90 min time period was 3.1 ± 1.15 spikes/sec while the mean percentage of the inhibition of these neurons after morphine injection was 49.43 ± 10.22%. In 3 out of 8 neurons, 3-7 min after morphine injection (5 mg/kg; sc) the neural activity of NAcSh neurons were significantly augmented which lasted until the 90th minute (Fig. 3B). The baseline firing rate was varied between 0.71- 8.02 spikes/sec and the average frequency of spontaneous activity over the 90-minute time period was 3.19 ± 2.22 spikes/sec. Moreover, the mean percentage of potentiation after morphine injection was 71.3 ± 7.61% in these neurons. Finally, in 2 out of 8 neurons, no effect was observed after morphine injection (Fig. 3C). Here, the baseline firing rate and the average frequency of spontaneous activity over the 90-minute time period was 1.11-5.43 spikes/sec and 2.96 ± 1.17 spikes/sec, respectively.
Figure 3.
Examples of the effect produced by morphine on NAc neurons recorded from anesthetized rats. The panel depicts the (A) decreasing firing rate and (B) increasing after morphine administration. In the bottom graph (c) neural firing rate didn't have any alteration.
3.4. Effect of VTA inactivation by lidocaine on NAcSh neural activity alteration caused by morphine administration
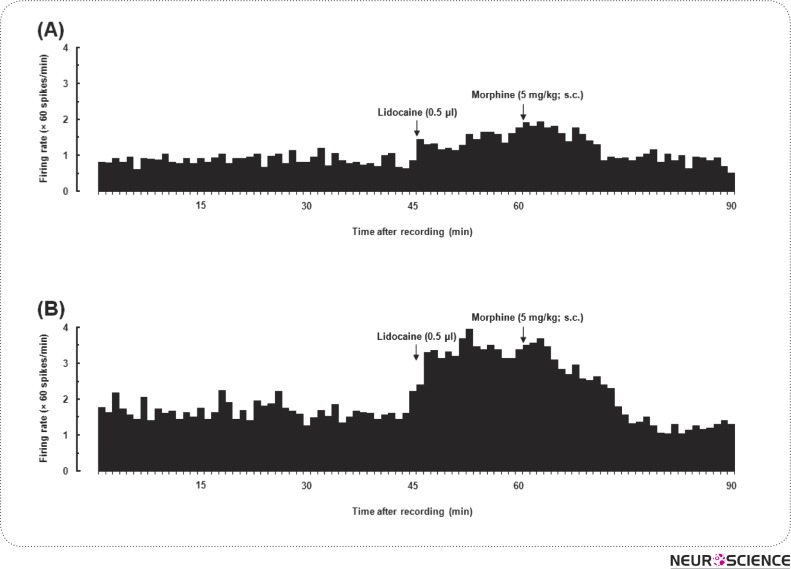
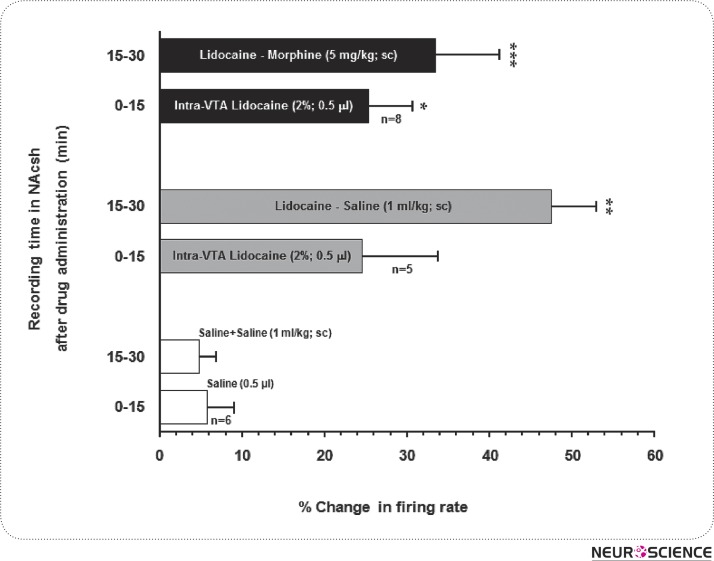
After stabilization period (15 min) and baseline recording (30 min), animals received 2% lidocaine (0.5 µl/rat in VTA), 15 min later morphine (5 mg/kg; sc) was administrated. Analysis of data in this experiment showed that there are two neural activity profiles for NAcSh neurons after morphine administration, as shown in Fig. 4A, B. In 8 out of 9 neurons, the mean activation of NAcSh neurons in 15-min after lidocaine administration was 25.4 ±5.23% while in the next 15- min (15-30), concurrent with morphine administration, was 13.53 ±17.69%. Meanwhile, the average frequency of spontaneous activity over this time was 2.57 ± 0.74 spikes/sec. In other words, student t-test revealed that the administration of lidocaine into the VTA suppressed the neural alteration followed by systemic administration of morphine (Fig. 5).
Figure 4.
Typical effects of intra-VTA administration of lidocaine followed by systemic injection of morphine. The upper figure (A) depicts neither lidocaine nor morphine didn't changed neural firing rate in NAsch. In the lower figure (B), lidocaine increased the NAcSh neural activity and firing rate decreased after morphine administration.
Figure 5.
The histogram presents the average changes in percentage of firing rate of neurons in baseline recording and after intra-VTA injection of 2%lidocaine and lidocaine + morphine. Values expressed as mean ± SEM.
*P < 0.05, **P < 0.01, ***P < 0.001 compared to saline respective group.
4. Discussion
The purpose of this study is to provide a conceptual framework for understanding the way the mesolimbic neurotransmission systems work in morphine-rewarding behaviors. To our knowledge, this is the first report of electrophysiological effects of VTA inhibition on provoked NAcSh neural activity through systemic morphine administration. A body of work indicates that impermanent inactivation of VTA reduced the effect of systemic administration of morphine on NAcSh. These finding is concurrent with our previous finding that support the necessity of projection from VTA to NAcSh for the formation of reward-related effects of abused drugs (Moaddab et al., 2009). It is well documented that the mesolimbic dopaminergic pathway that is projected from the VTA to the NAc is critical for the reinforcing effects of opioids and other abused drugs (Esmaeili et al., 2012; Ikemoto et al., 1997; Moaddab et al., 2009). These dopamine neurons are activated by systemic morphine injection or natural rewarding stimuli such as food or sex, resulting in increased dopamine release in the targeted brain regions particularly VTA and NAc (Leone, Pocock, & Wise, 1991). With this regard, previous studies revealed that opiates administered systemically increase the firing of VTA-dopamine neurons recorded in vivo (Matthews & German, 1984), moreover, microiontophoretic administration of morphine or enkephalin analogues significantly increases the spontaneous activity of the VTA and substantia nigra pars compacta (SNC) cells. Also, these effects were not reversed by neither iontophoretic nor intravenous naloxone, proposing that morphine- induced activation of the VTA dopamine cells could be indirectly mediated by non-dopaminergic cells (R. Hakan & Henriksen, 1989). The direct action of opioids on neurons elsewhere in the nervous system, including other catecholamine-containing cells, is inhibitory (Mihara & North, 1986; North & Tonini, 1977). This finding raises the possibility that the excitatory effect of the opioids on the principal, dopamine-containing cells, occurs indirectly; that is, opioids may inhibit non-dopamine neurons in the VTA (secondary neurons), specifically GABA-containing neurons that provide inhibitory synaptic input to the dopamine cells (Gysling & Wang, 1983). Our results in the second part shows that, approving those of similar works done before, transient inactivation of VTA by 2% lidocaine provokes neural firing rate in the NAcSh. It seems that the observed increase in neural activity followed by lidocaine injection was due to inhibition of dopaminergic and GABAergic projections.
The nucleus accumbens as a central part in the neural circuitry involved in drug addiction exhibits spontaneous neuronal activity as well as evoked (driven) neuronal responses to ipsilateral fimbria stimulation (Yang and Mogenson, 1984). Spontaneous active NAc single units are predominantly inhibited (but also excited or unaffected) by systemically administered opiate drugs (R. L. Hakan & Henriksen, 1987; R Yang & J Mogenson, 1984). Along with mentioned studies, we found out that the subcutaneous administration of morphine changed the NAcSh neural activity in opposite directions. Based on this phenomenon, it seems that the observed accretion in NAcSh neural activity following morphine injection was due to alterations in the neural substrates which is leading to the activation of dopaminergic neurons. In contrast, it can be suggested that GABA interneuron activation, following morphine administration caused inhibition in NAcSh neural activity. The shell portion of the accumbens appears to be more important than the core for drug reward. Ikemoto et al. showed that rats learn to self-administer psychomotor stimulants such as amphetamine or cocaine or dopamine receptor agonists into the accumbens shell, but not into the core (Ikemoto et al., 1997; Ikemoto, Qin, & Liu, 2005). To function, NAc is dependent on neurotransmitergic inputs from other brain areas involved in the reward circuitry specially the VTA.
Dopaminergic and GABAergic inputs from VTA typically converge on the NAc and there is considerable evidence suggesting that the NAc may have a pivotal role in the integration of limbic inputs relevant to motivated behaviors (Everitt & Wolf, 2002). Previous studies indicated that the lesion of dopaminergic projection from the VTA to the NAc or the blocking of dopaminergic transmission reduces the reinforcing effects of drugs in several experimental paradigms including conditioned place preference (Gholami, Zarrindast, Sahraei, & Haerri-Rohani, 2003; Moaddab et al., 2009). Our previous study revealed that reversible inactivation of VTA significantly decreased the acquisition and expression of morphine-induced CPP (Moaddab et al., 2009). These findings are of particular importance in light of the present report, showing that intraperitoneal administration of morphine was not able to change the firing of NAc neurons while VTA was provisionally inactivated. It can be concluded that observed alterations in neural activity followed by systemic morphine administration fit within the dopaminergic hypothesis of reward, and inhibition of neural activity of the VTA leads to the increase in neural activity of the NAc. Meanwhile, based on the two entrances of GABA and dopamine from the VTA to NAc, the augmentation of NAcSh neural activity could be due to the blockade of these neurotransmitters pathway. Finally, it could be concluded that the activated neuronal pathways by morphine, have to pass the VTA in order to get to the NAc, and ability of neurons in the NAcsh for responding to morphine depends on basal firing rate of VTA-NAc dopaminergic inputs.
Acknowledgements
This study was conducted as part of an MSc student thesis project. This work was supported by the grant from Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- Bunney, E.B. , Appel, S.B. , & Brodie, M.S. (2001). Electrophysiological effects of cocaethylene, cocaine, and ethanol on dopaminergic neurons of the ventral tegmental area. Journal of Pharmacology and Experimental Therapeutics, 297(2), 696–703 [PubMed] [Google Scholar]
- Chu, N. , Xia, W. , Yu, P. , Hu, L. , Zhang, R. , & Cui, C. (2008). PRECLINICAL STUDY: Chronic morphine-induced neuronal morphological changes in the ventral tegmental area in rats are reversed by electroacupuncture treatment. Addiction biology, 13(1), 47–51 [DOI] [PubMed] [Google Scholar]
- Esmaeili, M.H. , Kermani, M. , Parvishan, A. , & Haghparast, A. (2012). Role of D1/D2 dopamine receptors in the CA1 region of the rat hippocampus in the rewarding effects of morphine administered into the ventral tegmental area. Behavioural brain research, 231(1), 111–5 [DOI] [PubMed] [Google Scholar]
- Everitt, B.J. , & Wolf, M.E. (2002). Psychomotor stimulant addiction: a neural systems perspective. The Journal of neuroscience, 22(9), 3312–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami, A. , Zarrindast, M.R. , Sahraei, H. , & Haerri-Rohani, A. (2003). Nitric oxide within the ventral tegmental area is involved in mediating morphine reward. European journal of pharmacology, 458(1-2), 119–128 [DOI] [PubMed] [Google Scholar]
- Gysling, K. , & Wang, R.Y. (1983). Morphine-induced activation of A10 dopamine neurons in the rat. Brain research, 277(1), 119–127 [DOI] [PubMed] [Google Scholar]
- Haghparast A, Naderi N, Khani A, Lashgari R, Motamedi F. (2010). Formalin-induced differential activation of nucleus cuneiformis neurons in the rat: an electrophysiological study. J Pain 11(1), 32–43 [DOI] [PubMed] [Google Scholar]
- Haghparast A, Farzin D, Ordikhani-Seyedlar M, Motaman S,Kermani M , Azizi P. (2012). Effects of apomorphine and beta-carbolines on firing rate of neurons in the ventral pallidum in the rats. Behavioural brain research, 227(1), 109–15 [DOI] [PubMed] [Google Scholar]
- Hakan, R.L. , & Henriksen, S.J. (1987). Systemic opiate administration has heterogeneous effects on activity recorded from nucleus accumbens neurons in vivo. Neuroscience letters, 83(3), 307–312 [DOI] [PubMed] [Google Scholar]
- Hakan, RL , Eyl, C. , & Henriksen, SJ. (1994). Neuropharmacology of the nucleus accumbens: systemic morphine effects on single-unit responses evoked by ventral pallidum stimulation. Neuroscience, 63(1), 85–93 [DOI] [PubMed] [Google Scholar]
- Hakan, RL , & Henriksen, SJ. (1989). Opiate influences on nucleus accumbens neuronal electrophysiology: dopamine and non-dopamine mechanisms. The Journal of neuroscience, 9(10), 3538–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W. , Wang, T. , & Wei, X. (2007). Applied anatomy of nucleus accumbens in human brain [J]. Journal of China Medical University, 1 [Google Scholar]
- Ikemoto, S. , Glazier, B.S. , Murphy, J.M. , & McBride, W.J. (1997). Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. The Journal of neuroscience, 17(21), 8580–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto, S. , Qin, M. , & Liu, Z.H. (2005). The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: Is the division of the accumbens core, shell, and olfactory tubercle valid? The Journal of neuroscience, 25(20), 5061–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen Relo, A.L. , Groenewegen, H.J. , & Voorn, P. (1993). Evidence for a multi-compartmental histochemical organization of the nucleus accumbens in the rat. The Journal of comparative neurology, 337(2), 267–276 [DOI] [PubMed] [Google Scholar]
- Leone, P. , Pocock, D. , & Wise, RA. (1991). Morphine-dopamine interaction: ventral tegmental morphine increases nucleus accumbens dopamine release. Pharmacology Biochemistry and Behavior, 39(2), 469–472 [DOI] [PubMed] [Google Scholar]
- Lisman, J.E. , & Grace, A.A. (2005). The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron, 46(5), 703–713 [DOI] [PubMed] [Google Scholar]
- Mansvelder, H.D. , & McGehee, D.S. (2000). Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron, 27(2), 349–357 [DOI] [PubMed] [Google Scholar]
- Martin, J.H. (1991). Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neuroscience letters, 127(2), 160–164 [DOI] [PubMed] [Google Scholar]
- Matthews, RT , & German, DC. (1984). Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience, 11(3), 617–625 [DOI] [PubMed] [Google Scholar]
- Meredith, GE , Farrell, T. , Kellaghan, P. , Tan, Y. , Zahm, DS , & Totterdell, S. (1999). Immunocytochemical characterization of catecholaminergic neurons in the rat striatum following dopamine-depleting lesions. European Journal of Neuroscience, 11(10), 3585–3596 [DOI] [PubMed] [Google Scholar]
- Mihara, S. , & North, R.A. (1986). Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. British journal of pharmacology, 88(2), 315–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddab, M. , Haghparast, A. , & Hassanpour-Ezatti, M. (2009). Effects of reversible inactivation of the ventral tegmental area on the acquisition and expression of morphine-induced conditioned place preference in the rat. Behavioural brain research, 198(2), 466–471 [DOI] [PubMed] [Google Scholar]
- Navailles, S. , Moison, D. , Cunningham, K.A. , & Spampinato, U. (2007). Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology, 33(2), 237–246 [DOI] [PubMed] [Google Scholar]
- North, RA , & Tonini, M. (1977). The mechanism of action of narcotic analgesics in the guinea-pig ileum. British journal of pharmacology, 61(4), 541–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades, R.D. , & Halliday, G.M. (1987). Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Research Reviews, 12(2), 117–165 [DOI] [PubMed] [Google Scholar]
- Olmstead, M.C. , & Franklin, K.B.J. (1997). The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behavioral neuroscience, 111(6), 1324–30 [DOI] [PubMed] [Google Scholar]
- Paxinos, G. , & Watson, C. (2007). The rat brain in stereotaxic coordinates: Academic press; [DOI] [PubMed] [Google Scholar]
- Pennartz, CMA , Groenewegen, H.J. , & Da Silva, FH. (1994). The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Progress in Neurobiology, 42(6), 719-761. [DOI] [PubMed] [Google Scholar]
- R Yang, C. , & J Mogenson, G. (1984). Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain research, 324(1), 69–84 [DOI] [PubMed] [Google Scholar]
- Rezayof, A. , Nazari-Serenjeh, F. , Zarrindast, M.R. , Sepehri, H. , & Delphi, L. (2007). Morphine-induced place preference: Involvement of cholinergic receptors of the ventral tegmental area. European journal of pharmacology, 562(1-2), 92–102 [DOI] [PubMed] [Google Scholar]
- Winder, D.G. , Egli, R.E. , Schramm, N.L. , & Matthews, R.T. (2002). Synaptic plasticity in drug reward circuitry. Current Molecular Medicine, 2(7), 667–676 [DOI] [PubMed] [Google Scholar]