Abstract
Introduction
Anxiety is among the most common and treatable mental disorders. Adrenergic and cannabinoid systems have an important role in the neurobiology of anxiety. The elevated plus-maze (EPM) has broadly been used to investigate anxiolytic and anxiogenic compounds. The present study investigated the effects of intraperitoneal (IP) injection of cannabinoid CB1 receptor antagonist (AM251) in the presence of alpha-1 adrenergic antagonist (Prazosin) on rat behavior in the EPM.
Methods
In this study, the data were obtained from male Wistar rat, which weighing 200- 250 g. Animal behavior in EPM were videotaped and saved in computer for 10 min after IP injection of saline, AM251 (0.3 mg/kg), Prazosin (0.3 mg/kg) and AM251 + Prazosin, subsequently scored for conventional indices of anxiety. During the test period, the number of open and closed arms entries, the percentage of entries into the open arms of the EPM, and the spent time in open and closed arms were recorded. Diazepam was considered as a positive control drug with anxiolytic effect (0.3, 0.6, 1.2 mg/kg).
Results
Diazepam increased the number of open arm entries and the percentage of spent time on the open arms. IP injection of AM251 before EPM trial decreased open arms exploration and open arm entry. Whereas, Prazosin increased open arms exploration and open arm entry. This study showed that both substances in simultaneous injection have conflicting effects on the responses of each of these two compounds in a single injection.
Discussion
Injection of CB1 receptor antagonist may have an anxiogenic profile in rat, whereas adrenergic antagonist has an anxiolytic effect. Further investigations are essential for better understanding of anxiolytic and anxiogenic properties and neurobiological mechanisms of action and probable interactions of the two systems.
Keywords: Cannabinoid Receptor Antagonist, Adrenergic Antagonist, Elevated Plus-maze, Diazepam, Rat, Anxiety
1. Introduction
Anxiety affects one-eighth of the world's population and is the main issue of research in psychopharmacology during the recent decades (Eisenberg et al., 1998). Pharmacological studies, clinical investigations and, in recent years, analyses of genetically-modified mice implicated a remarkable diversity of mechanisms in the etiology, modulation and treatment of anxiety (Millan, 2003). The neurobiological underpinnings of anxiety disorder has been studied in both animal and human models, and it is widely accepted that dysregulation of brain regions and structures are associated with anxiety (Edenfield & Saeed, 2012).
Endocannabinoids and their receptors play a modulatory function in several physiological processes mainly in the brain (Fernandez-Ruiz, Hernandez& Ramos, 2010). Several evidences suggest that the endocannabinoid system plays a role in the regulation of mood or anxiety (Rubino et al., 2008). Consistent with this, the cannabinoid system can be seen as one of the key regulatory elements of anxiety behavior (Ruehle, Rey, Remmers & Lutz, 2012). Cannabinoids are produced throughout the brain and CB1 receptors are particularly well-represented in the cortex (entorhinal and cingulate), hippocampus, lateral septum, nucleus accumbens, amygdala and Peri-Aaqueductal Gray area (PAG) (Millan, 2003). Cannabinoid receptor agonists/antagonists have been shown to exert anxiolytic effects in some studies (Saito,Wotjak & Moreira 2010) but anxiogenic effects in the others (Carvalho et al., 2010b; Degroot, 2008; Haller et al., 2004; Moreira & Lutz, 2008). Furthermore, CB1 receptor agonists are reported to induce biphasic effects, with lower doses being anxiolytic and higher doses being anxiogenic (Rey, Purrio, Viveros & Lutz2012). Using CB1 receptor to knockout mice, several studies reported anxiogenic responses in classical anxiety paradigms such as elevated plus-maze (Ruehle et al., 2012).
Symptoms and physiological changes potentially associated with adrenergic dysfunction have been reported in people with anxiety disorders (Cameron et al., 1990). Brain noradrenergic systems regulate many of the same behavioral dimensions that are affected in depression and anxiety disorders (Oropeza, Mackie & Bockstaele, 2007).
Functional interactions between cannabinoids and central noradrenergic systems have been well described (Carvalho et al., 2010a, 2010b; Gobbi et al., 2005; Mendiguren & Pineda, 2006; Muntoni et al., 2006; Oropeza et al., 2007; Page, Oropeza&Bockstaele, 2008). Manipulation of the endocannabinoid system affects the mood and cognition that share similarities with the noradrenergic system (Carvalho & Bockstaele, 2012). Briefly, increasing endocannabinoid tone has been shown to improve mood similar to increasing noradrenergic tone with antidepressants. It has been shown in preclinical studies, where the antidepressant effects of chronic CB1 receptor agonist administration implicate a role for norepinephrine (NE) (Morrish, Hill, Riebe & Gorzalka, 2009). Furthermore, systemic administration of WIN 55,212-2, a synthetic cannabinoid agonist, increases the indices of noradrenergic activity (Oropeza et al., 2007; Page et al., 2008).
Localization of CB1 receptors on noradrenergic axon terminals in the some cortical areas (Oropeza et al., 2007), provide evidences for an anatomical and functional link between CB1 receptors and NE output that could affect neurobehavioral changes associated with cannabinoid use (Reyes et al., 2012). Based on previous studies, it is shown that the endocannabinoid and noradrenergic systems play a role in modulating stress and anxiety responses (Duncko, Brtko, Kvetnanský & Jezová, 2001; Fride, Bregman & Kirkham, 2005; Hill & Gorzalka, 2004; Martin et al., 2002; Millan, 2003; Miller & Walker, 1996; Sands et al., 2000). Although there are available data about effect of cannabinoid system on anxiety and also the data related to adrenergic system role on anxiety phenomenon, but there is no study about simultaneous stimulation effect and contemporary inactive of these two systems on anxiety. Also, this question that whether the effect of cannabinoid system on anxiety is the result of its effect on adrenergic synaptic transmission is still an open question. Therefore in this study, contemporary inhibitory effect of cannabinoid and adrenergic system on anxiety was studied.
2. Methods
2.1. Animals
Adult male Wistar rats, weighing 200–250 g, were used in the present study. The rats were randomly divided into seven groups (10 animals in each group) at the beginning of the study on a 12-h light schedule (lights on at 0700) in a temperature-controlled (22±2°C) colony room. They were allowed ad libitum access to standard rat chow and water. All procedures of research and animal care were approved by the Veterinary Ethics Committee of the Hamadan University of Medical Science (VECHUMS) and performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
2.2. Drugs
AM251 (Sigma, USA) was dissolved in 8% dimethylsulphoxide (DMSO; Sigma, USA) and used at dose of 0.3 mg/kg. Prazosin (Sigma, USA) was dissolved in DMSO and used at dose of 0.3 mg/kg. Diazepam (Kimidaru, Iran) was considered as a positive control drug with anxiolytic effect (0.3, 0.6, 1.2 mg/kg).
2.3. Groups
A: Control: Saline were given 30 min before test, B: AM251 (CB1 receptor antagonist) were given 30 min before test (0.3 mg/kg i. p.) (Wing &Shoaib 2010), C: Prazosin (α1-adrenergic antagonist) was given 30 min before test (0.3 mg/kg i.p.) (Bernardi, Ryabinin, Berger & Lattal 2009), D: Prazosin + AM251 (both 0.3 mg/kg i.p.) were given 30 min before the test, E, F, G: Diazepam (is a benzodiazepine) were given 30 min before test (in three doses 0.3, 0.6, 1.2 mg/kg i. p.) ( Davis 1979; Braida, Limonta, Malabarba, Zani & Sala, 2007).
2.4. Elevated Plus-Maze
Anxiolytic activity of extract was measured using the elevated plus maze (EPM) test. This test is widely validated to measure anxiety in rodents (Carobrez & Bertoglio 2005; Lister 1987; Pellow et al 1985). Briefly, for rats, the apparatus consisted of the two open arms (50 × 10 cm each), two enclosed arms (50 × 10 × 50 cm each) and a central platform (10× 10 cm), arranged in such a way that the two arms of each type were opposite to each other. The maze was elevated 100 cm above the floor. EPM is based on the natural conflict of rodents to explore a novel environment and their innate aversion to open, elevated and brightly lit spaces. As a consequence of the aversive properties of the open arms, subjects spend a greater amount of time on the closed arms and the proportion of total exploration in the open arms provides a measure of anxiety, so that the increase of percent time spent on the open arms is considered to be indicative of anxiolytic drug action (Handley & Mithani, 1984; Pellow & File, 1986). Conversely, the decrease of percent time spent on open arms reflects an anxiogenic effect of the drug (Carvalho & Bockstaele, 2012).
The animals were tested 30 min after i.p. infusion of AM251, prazosin and diazepam and 1 h after contemporary infusion of AM251 + Prazosin (Wing & Shoaib, 2010; Pitkänen, Mathiesen, Rønn, Møller & Nissinen, 2007). Behavioral parameters comprised both conventional spatiotemporal and ethological measures. Conventional measures were the frequencies of total, open and closed arm entries (arm entry = all 4 paws into an arm) and the time spent in open, closed and central parts of the maze (Rubino et al., 2008; Zarrindast, Eslahi, Rezayof, Rostami & Zahmatkesh, 2012; Youet al., 2012). After the test, the maze was carefully cleaned with a wet tissue paper (10% ethanol solution).
2.5. Statistical Analysis
The results were expressed as the mean + S.E.M from groups of 10 animals as indicated in the text and legends. The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test (t-test) for multiple comparisons. Differences were considered significant at p < 0.05.
3. Results
3.1. Effect of Diazepam on Anxiety
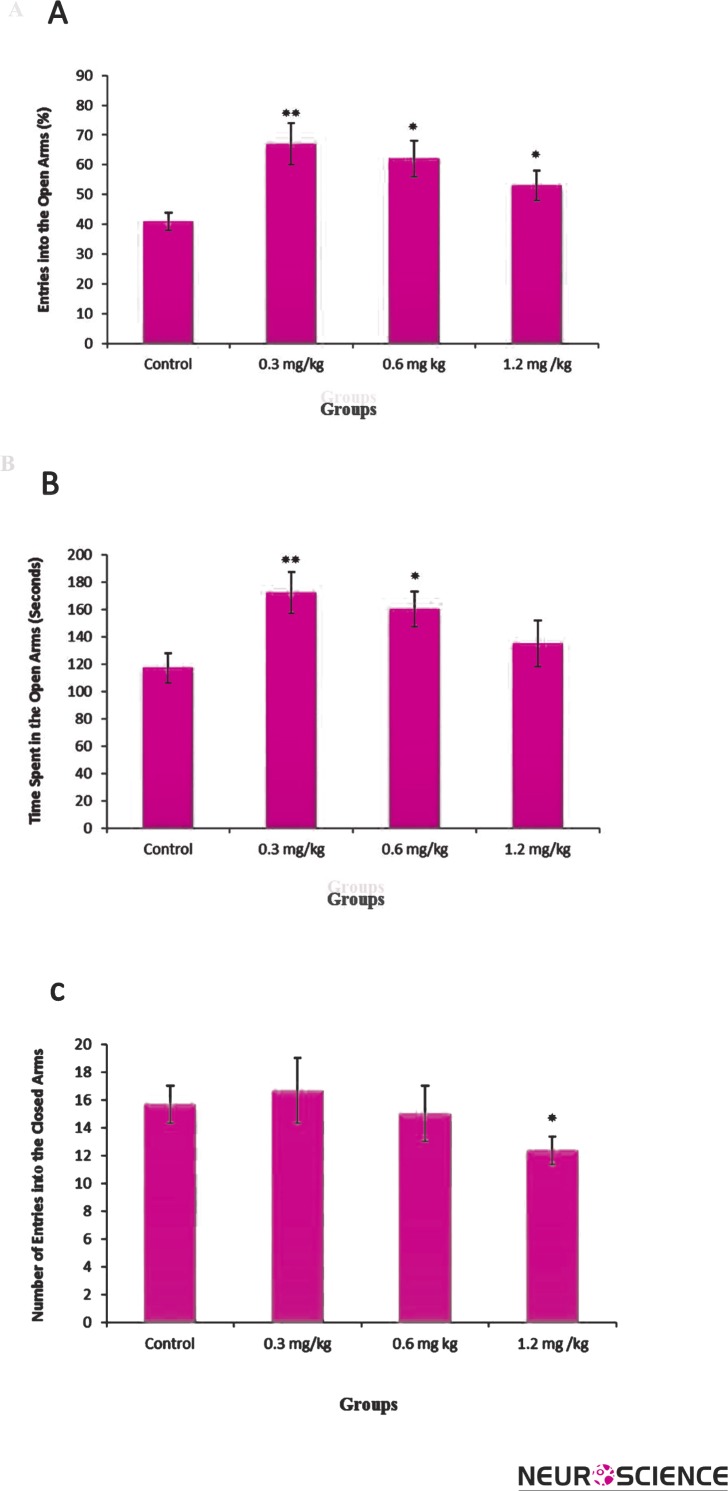
In this study, at first the effect of diazepam on rat behavior was surveyed to estimate the plus-maze set usage correction. Diazepam was considered as a positive control drug with anxiolytic effect)Souto-Maior et al., 2011; Gomes et al., 2010). This anxiolytic drug with low dose (0.3 mg/kg) showed significant increase in the percent of open arms entry (OE) (Figure 1-A), and the spent time on open arms (OT) (Figure 1-B) [F (3, 36) = 5.81, p < 0.01]. Diazepam showed significant increase in the open arms exploration in concentrations of 0.3 [F (3, 36) = 5.06, p <0.01] and 0.6 mg/kg [F (3, 36) = 3.33, p < 0.05], but interestingly not at 1.2 mg/kg (Figure 1-A, B). The number of closed arms entries was significantly [F (3, 36) = 2.89, p <0.05] different for the group that received 1.2 mg/kg of diazepam. But, the number of closed arms entries was not significantly different for the groups that received 0.3 or 0.6 mg/kg of diazepam (Fig. 1C).
Figure 1.
The effects of diazepam (0.3, 0.6, 1.2 mg/kg i.p.) on the percentage of entries in open arms (A), spent time in open arms (B) and number of closed arms entry (C) during the 10-min test session in EPM. Data represent means±SEM.
*: P < 0.05, **: P < 0.01 in comparison with control group.
3.2. Effects of AM251 and Prazosin
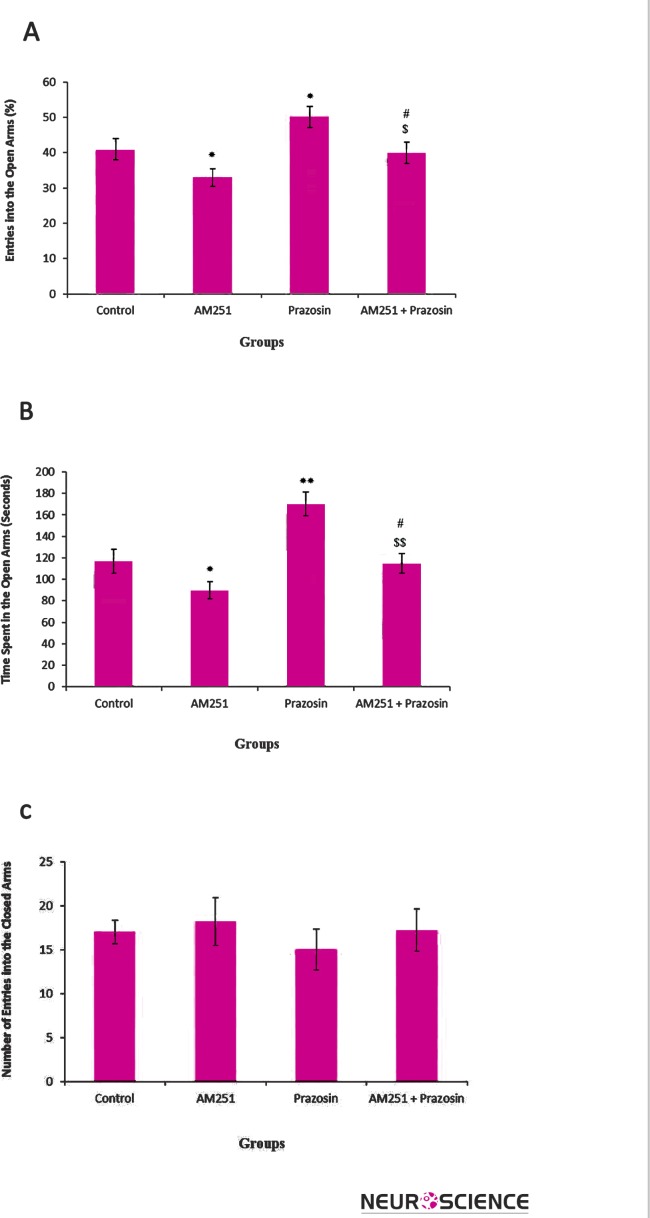
AM251 significantly decreased the percentage of entries to open arms (OE) [F (3, 36) = 3.974, p< .05] (Figure 2-A), as well as the spent time on these arms (OT) [F (3, 36) = 2.743, p< .05] (Figure 2-B), indicating an anxiogenic effect. While Prazosin injection increased the percent of open arms entry (OE) [F (3, 36) = 3.685, p< .05] (Figure 2-A) and the spent time in open arms (OT) [F (3, 36) = 4.134, p< .05] (Figure 2-B), indicating an anxiolytic effect.
Figure 2.
The effects of AM251(0.3 mg/kg i.p.), Prazosin (0.3 mg/kg i.p.) and AM251+ Prazosin on the percentage of entries in open arms (A), spent time in open arms (B) and the number of closed arms entry (C) during the 10-min test session in EPM. Data represent means±SEM. *: P < 0.05, **: P < 0.01 compared with control group. #: P < 0.05 compared to AM251 group
$: P < 0.05 in contrast with Prazosin group
$$: P < 0.05 in contrast with Prazosin group
The effects of contemporary injection of AM251 and Prazosin on open arms entry, as well as on the spent time in open arms were between AM251 and Prazosin effects. In the other word, contemporary injection of AM251 and Prazosin increased the percentage of entries to open arms [F(3,36) = 2.786, p< .05] (Figure 2-A), as well as the spent time on these arms compared with AM241 effects [F(3,36) = 4.156, p< .05] (Figure 2-B), decreasing these parameters compared with Prazosin effects [F (3, 36) = 4.465, p< .01]. The number of entries into the closed arms was not significantly different between the AM251, Prazosin and AM251 + Prazosin treated groups versus control group (Fig. 2C).
4. Discussion
The present study investigated the behavioral effects of the IP injection of cannabinoid receptor antagonist and adrenergic receptor antagonist on EPM. Our results demonstrated that the adrenergic receptor antagonist was able to produce anxiolytic effect in rats, whereas cannabinoid receptor antagonist produces anxiogenic effect. Also, our study showed both antagonists did not any significant effect on locomotor activity in EPM test. For comparison, the behavioral effects of diazepam, a typical anxiolytic compound, were also examined. It was observed that diazepam- treated rats, displayed anxiolytic behavior. Diazepam was applied as anxiolytic positive control drug (Souto-Maior.et al., 2011; Gomeset al., 2010(. In this method, high dose of diazepam could not induce anxiolytic effect because it reduces animal locomotion activity. Number of closed arm entery indicates the animal locomotion activity (Takahashi, Berton, Mormède & Chaouloff, 2001; Bradley, Starkey, Browna & Lea, 2007). Increase of the time and the proportion of the entrances into the open arms lacking a changed locomotors activity that are confirmed as a potent sign for an anxiolytic substance effect (Pellow et al., 1985). In this study, high dose of diazepam reduced locomotions of animals due to severe sedation effect. Therefore, EPM is not suitable to assess anxiolytic activity of the high dose of diazepam, therefore, the other anxiety survey methods, like Shuttle Box should be used.
The effects of Prazosin and AM251+ Prazosin on time ratio and entry ratio were not related to the effect of these compounds on locomotion activity of the animals but were related to the effects of these compounds on anxiety, because AM251, Prazosin and AM251 + Prazosin did not affect the number of closed arms entry. In this study, antagonist of cannabinoid system increased anxiety of the animals, while adrenergic system antagonist could decrease it. There are controversies about the effects of cannabinoids on anxiety. For instance, administration of CB1 receptor antagonists was found to exert antidepressant, anxiolytic, anxiogenic or null effects (Hill & Gorzalka, 2009). Also, it is reported that CB1 receptor antagonist, AM251, increased anxiety-like behavior in wild-type mice but had no effect on the CB1 receptor knockout mice (Pacher, Batkai & Kunos, 2006). This variance may be due to several factors including species, strain, testing conditions of the animal or off-target effects of the employed agents (Hill & Gorzalka, 2009), prior drug use, doses, basal anxiety levels and regional endocannabinoid basal tone (Degroot, 2008). Regarding CB1 antagonists, it seems that the preponderance of the data suggest that these compounds are anxiolytic. On the other hand, agonists seem to have biphasic effects. Low doses seem to be anxiolytic, while high doses are anxiogenic (Musty, 2005).
Functional interactions between cannabinoids and central noradrenergic systems have been well described (Carvalho et al., 2010a, 2010b; Gobbi et al., 2005; Mendiguren & Pineda, 2006; Muntoni et al., 2006; Oropeza et al., 2007; Page et al., 2008). CB1 receptors are located at noradrenergic presynaptic terminals (López-Moreno, González-Cuevas, Moren o& Navarro, 2008). Many studies on the interactions between the cannabinoid and adrenergic systems focused on inhibition of noradrenergic neurotransmission by presynaptic CB1 receptors (Hudson et al., 2010). In this regard, it is shown that CB1 receptors are localized to noradrenergic axon terminals in the prefrontal cortex (PFC) (Oropeza et al., 2007) contributed in regulating norepinephrine release. On the other hand, it is reported that idazoxan (a selective α2 adrenergic receptor antagonist) caused a decrease in CB1 receptor density in the PFC, suggesting that high extracellular level of norepinephrine down regulates CB1 receptors (Hardy, 2012). Some studies using dual immunohistochemical detection of dopamine-β-hydroxylase (or tyrosine hydroxylase) and CB1 receptors showed that some of the CB1 receptors-positive neurons in the locus coreleous (LC) (Scavone, Mackie & Bockstaele, 2010) and nucleus of the solitary tract (NTS) (Carvalho et al., 2010a) are noradrenergic. Also, it has been shown that anxiety-like behavior and NE levels return to control levels following chronic WIN 55, 212-2 exposure followed by a period of drug discontinuation (Page et al., 2008). This effect was blocked by administration of the CB1 receptor antagonist SR141716A. Interestingly, administration of SR141716A alone caused a significant reduction of LC spontaneous firing, suggesting that LC is under the control of an endogenous cannabinoid tone (Carvalho & Bockstaele, 2013).
α1 receptor are predominantly localized postsynaptically to noradrenergic neurons in diverse corticolimbic territories. Nevertheless, activation of α1 receptors in the amygdala is implicated in the induction of anxiety by stress and several reports of anxiolytic actions of α1 receptor antagonists have been appeared (Millan, 2003). In recognition of this issue, α1 receptors, which mediate many typical postsynaptic effects, are characterized by much greater affinity for the antagonist prazosin than for yohimbine (an antagonist of α2 receptors) (Hoffman et al., 1980). In this regard, it is reported that stress-induced cognitive deficits were blocked by infusion of the α1 receptor antagonist (Ramos & Arnsten, 2007).
5. Conclusion
Taken together, the effects of manipulating the endocannabinoid system and modulating noradrenergic transmission suggest that these two systems may interact or share some common signaling pathways. It seems that antagonist of cannabinoid system modulate noradrenergic output leading to increase of anxiety, while in contemporary consumption of two antagonist, 1 α receptor antagonist can inhibit anxiety which is due to inactivation of noradrenergic receptors. Also it seems that these two systems show antonym role in anxiety creation. The findings of this study can be useful for more effective treatment of anxiety.
Acknowledgments
This study was supported by Neurophysiology Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
References
- Bradley B. F., Starkey N. J., Browna S. L., & Lea R. W. (2007). Anxiolytic effects of Lavandulaangustifoliaodour on the Mongolian gerbil elevated plus maze. Journal of Ethnopharmacology, 111, 517–525. [DOI] [PubMed] [Google Scholar]
- Bernardi R. E., Ryabinin A. E., Berger S. P., & Lattal K. M. (2009). Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learn Mem, 25, 16(12), 777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D., Limonta V., Malabarba L., Zani A., & Sala M. (2007). 5-HT1A receptors are involved in the anxiolytic effect of Delta9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawleyrats. European Journal of Pharmacology, 555(2-3), 156–63. [DOI] [PubMed] [Google Scholar]
- Cameron O. G., Smith C. B., Lee M. A., Hollingsworth P. J., Hill E. M., & Curtis G. C. (1990). Adrenergic status in anxiety disorders: platelet Alpha2-Adrenergic receptor binding, blood pressure, pulse, and plasma catecholamines in panic and generalized anxiety disorder patients and in normal subjects. Biological Psychiatry, 28, 3–20. [DOI] [PubMed] [Google Scholar]
- Carobrez A. P., & Bertoglio L. J. (2005). Ethological and temporal analyses of anxietylike behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev, 29, 1193–1205. [DOI] [PubMed] [Google Scholar]
- Carvalho A. F., & Bockstaele E. J. V. (2012). Cannabinoid modulation of noradrenergic circuits: implications for psychiatric disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2,38(1), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. F. & Bockstaele E. J. V. (2013). Anatomical, Biochemical, and Behavioral Evidence for Cannabinoid Modulation of Noradrenergic Circuits: Role of Norepinephrine in Cannabinoid-Induced Aversion, Endocannabinoid Regulation of Monoamines in Psychiatric and Neurological Disorders (pp. 135–156). [Google Scholar]
- Carvalho A. F., Mackie K., & Bockstaele E. J. V. (2010a). Cannabinoid modulation of limbic forebrain noradrenergic circuitry. European Journal of Neuroscience, 31(2), 286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. F., Reyes A. S., Sterling R. C., Unterwald E., & Bockstaele E. J. V. (2010b). Contribution of Limbic Norepinephrine to Cannabinoid-induced Aversion. Psychopharmacology (Berl), 211(4), 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B., Siniscalco D., Trovato A. E., Comelli F., Sotgiu M. L., Colleoni M., Maione S., Rossi F., Giagnoni G. (2006). AM404, an inhibitor of anandamide uptake, prevents pain behaviour and modulates cytokine and apoptotic pathways in a rat model of neuropathic pain. British Journal of Pharmacology, 148, 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. (1979). Diazepam and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology (Berl), 29, 62(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Degroot A. (2008). Role of cannabinoid receptors in anxiety disorders In K. A, Cannabinoids and the brain (p. 559–72). USA: Springer. [Google Scholar]
- Duncko R., Brtko J., Kvetnanský R., & Jezová D. (2001). Altered function of peripheral organ systems in rats exposed to chronic mild stress model of depression. Cellular and Molecular Neurobiology, 21(4), 403–11. [DOI] [PubMed] [Google Scholar]
- Edenfield T. M., & Saeed S. A. (2012). An update on mindfulness meditation as a self-help treatment for anxiety and depression. Psychology Research and Behavior Management, 5, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. M., Davis R. B., Ettner S. L., Appel S., Wilkey S., Van R. M., Kessler R. C. (1998). Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. Journal of the American Medical Association, 280, 1569–1575. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J., Hernández M., & Ramos J. A. (2010). Cannabinoid–Dopamine Interaction in the Pathophysiology and Treatment of CNS Disorders. CNS Neuroscience & Therapeutics, 16(3), e72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E., Bregman T., & Kirkham T. C. (2005). Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood. The Journal of Experimental Biology and Medicine, 230, 225–234. [DOI] [PubMed] [Google Scholar]
- Gobbi G., Bambico F. R., Mangieri R., Bortolato M., Campolongo P., Solinas M., Cassano T., Morgese M. G., Debonnel G., Duranti A., Tontini A., Tarzia G., Mor M., Trezza V., Goldberg S. R., Cuomo V., Piomelli D. (2005). Anti-depressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proceedings of the National Academy of Sciences, 102(51), 18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P. B., Feitosa M. L., Silva M. I., Noronha E. C., Moura B.A., Venâncio E. T., Rios E. R., de Sousa D. P., de Vasconcelos S. M., Fonteles M.M., de Sousa F.C. (2010). Anxiolytic-like effect of the monoterpene 1, 4-cineole in mice. Pharmacology Biochemistry & Behavior, 96, 287–93. [DOI] [PubMed] [Google Scholar]
- Haller J., Varga B., Ledent C., & Freund T. F. (2004). CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behavioural Pharmacology, 15, 299–304. [DOI] [PubMed] [Google Scholar]
- Handley S. L., & Mithani S. (1984). Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn-Schmiedeberg's Archives of Pharmacology, 327, 1–5. [DOI] [PubMed] [Google Scholar]
- Hill M. N., & Gorzalka B. B. (2009). The endocannabinoid system and the treatment of mood and anxiety disorders, CNS & neurological disorders. Drug Targets, 8, 451–458. [DOI] [PubMed] [Google Scholar]
- Hoffman B. B., Michel T., Kilpatrick D. M., Lefkowitz R. J., Tolbert M. E., Gilman H., Fain J. N. (1980). Agonist versus antagonist binding to a-adrenergic receptors. Proceedings of the National Academy of Sciences, 77(8), 4569–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B. D., Hébert Terence E., & Kelly M. E. M. (2010). Physical and functional interaction between CB1 cannabinoid receptors and b adrenoceptors. British Journal of Pharmacology, 160, 627–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R. G. (1987). The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology, 92, 180–185. [DOI] [PubMed] [Google Scholar]
- López-Moreno J. A., González-Cuevas G., Moreno G., & Navarro M. (2008). The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addiction Biology, 13, 160–187. [DOI] [PubMed] [Google Scholar]
- Martin M., Ledent C., Parmentier M., Maldonado R., & Valverde O. (2002). Involvement of CB1 cannabinoid receptors in emotional behavior. Psychopharmacology (Berl), 159(4), 379–87. [DOI] [PubMed] [Google Scholar]
- Maul B., Becker M., Gembardt F., Becker A., Schultheiss H. P., Siems W. E., Walther T. (2012). Genetic deficiency in neprilysin or its pharmacological inhibition initiate excessive stress-induced alcohol consumption in mice. Plos One, 7(11), 50187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiguren A., & Pineda J. (2006). Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. European Journal of Pharmacology, 534(1–3), 83–8. [DOI] [PubMed] [Google Scholar]
- Millan M. J. (2003). The neurobiology and control of anxious states. Progress in Neurobiology, 70, 83–244. [DOI] [PubMed] [Google Scholar]
- Miller A. S., & Walker J. M. (1996). Electrophysiological effects of a cannabinoid on neural activity in the globuspallidus. European Journal of Pharmacology, 304(1-3), 29–35. [DOI] [PubMed] [Google Scholar]
- Moreira F. A., & Lutz B. (2008). The endocannabinoid system: Emotion, learning and addiction. Addiction Biology, 13, 196–212. [DOI] [PubMed] [Google Scholar]
- Morrish A. C., Hill M. N., Riebe C. J., & Gorzalka B. B. (2009). Protracted cannabinoid administration elicits antidepressant behavioral responses in rats: role of gender and noradrenergic transmission. Physiology & Behavior, 98, 118–24. [DOI] [PubMed] [Google Scholar]
- Muntoni A. L., Pillolla G., Melis M., Perra S., Gessa G. L., & Pistis M. (2006). Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. European Journal of Neuroscience, 23, 2385–94. [DOI] [PubMed] [Google Scholar]
- Musty RE. Cannabinoids as Therapeutics: Cannabinoids and anxiety, Department of Psychology, University of Vermont, Burlington, VT 05405,USA, 2005; p 141–147.
- Oropeza V. C., Mackie K., & Bockstaele E. J. V. (2007). Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Research, 5, 1127(1), 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P., Bátkai S., & Kunos G. (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological Reviews, 58, 389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. E., Oropeza V. C., & Bockstaele E. J. V. (2008). Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neuroscience Letters, 431(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S., & File S. E. (1986). Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacology Biochemistry and Behavior, 24, 525–9. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File S. E, & Briley M. (1985). Validation of open and closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods, 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Pitkänen A., Mathiesen C., Rønn L. C., Møller A., & Nissinen J. (2007). Effect of novel AMPA antagonist, NS1209, on status epilepticus. An experimental study in rat. Epilepsy Research, 74(1), 45–54. [DOI] [PubMed] [Google Scholar]
- Ragu Varman D., Marimuthu G., & Rajan K. E. (2012). Environmental enrichment upregulates micro-RNA-183 and alters acetylcholinesterase splice variants to reduce anxiety-like behavior in the little Indian field mouse (Musbooduga). Journal of Neuroscience Research, 14, 20–21. [DOI] [PubMed] [Google Scholar]
- Ramos B. P., & Arnsten A. F. T. (2007). Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology & Therapeutics, 113, 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B. A. S, Szot P., Sikkema C., Cathel A. M., Kirby L. G., & Bockstaele E. J. V. (2012). Stress-induced sensitization of cortical adrenergic receptors following a history of cannabinoid exposure. Experimental Neurology, 236, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B. A. S., Rosario J. C., Piana P. M. T., & Bockstaele E. J. V. (2009). Cannabinoid modulation of cortical adrenergic receptors and transporters. Journal of Neuroscience Research, 87, 3671–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T., Realini N., Castiglioni C., Guidali C., Vigano D., Marras E., Petrosino S., Perletti G., Maccarrone M., DiMarzo V., Parolaro D. (2008). Role of anxiety behavior of the endocannabinoid system in the prefrontal cortex. cerebral cortex, 18, 1292–1301. [DOI] [PubMed] [Google Scholar]
- Ruehle S., Rey A. A., Remmers F., & Lutz B. (2012). The endocannabinoid system in anxiety, fear memory and habituation. The Journal of Psychopharmacology, 26(1), 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone J. L., Mackie K., & Bockstaele E. J. V. (2010). Characterization of cannabinoid-1 receptors in the locus coeruleus: Relationship with mu-opioid receptors. Brain Research, 1312, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto-Maior F. N., Carvalho F. L., Morais L. C., Netto S. M., de Sousa D. P., & Almeida R. N. (2011). Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacology Biochemistry & Behavior, 100, 259–63. [DOI] [PubMed] [Google Scholar]
- Takahashi R. N., Berton O., Mormède P., & Chaouloff F. (2001). Strain-dependent effects of diazepam and the 5-HT2B/2C receptor antagonist SB 206553 in spontaneously hypertensive and Lewis rats tested in the elevated plus-maze. Brazilian Journal of medical Biological Research, 34, 675–682. [DOI] [PubMed] [Google Scholar]
- Wing V. C., & Shoaib M. (2010). Second-order schedules of nicotine reinforcement in rats: effect of AM251. Addiction Biology, 15(4), 393–402. [DOI] [PubMed] [Google Scholar]
- You J. S., Peng M., Shi J. L., Zheng H. Z., Liu Y., Zhao B. S., & Guo J. Y. (2012). Evaluation of anxiolytic activity of compound Valerianajatamansi Jones in mice. BMC Complementary and Alternative Medicine, 2(1), 12, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast M. R., Eslahi N., Rezayof A., Rostami P., & Zahmatkesh M. (2012). Modulation of ventral tegmental area dopamine receptors inhibit nicotine-induced anxiogenic-like behavior in the central amygdale. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 21, 11–17. [DOI] [PubMed] [Google Scholar]