Abstract
Introduction
Amnesia or loss of memory is the cardinal hallmark of Alzheimer's disease (AD), a progressive neurodegenerative disorder associated with ageing process. Although, AD had been discovered over a century ago, drugs which could cure or halt the progression of the disease are yet to see the light of the day. However, there has been a growing interest in the use of phytomedicines with multipronged mechanisms of action that could target various aspects of the pathologies of AD. Jobelyn (JB) is a potent antioxidant African polyherbal formulation with active components that have been acclaimed to show neuroprotection. This investigation was carried out to evaluate whether JB has anti-amnesic and antioxidant activities.
Methods
The alteration of alternation behavior in the Y-maze paradigm was utilized as the test for memory function in mice. The effect of JB on acetylcholinesterase (AChE) activity, malondialdehyde (MDA) level and the concentrations of glutathione (GSH) in the frontal cortex and hippocampus were assessed in rats as means of providing insight into the mechanism underlying its anti-amnesic activity. The animals were given JB (1, 2.5 or 5mg/kg, i.p.) daily for 7 days before the biochemical assays or test for memory functions were carried out.
Results
JB was found to produce a significant increase in the level of alternation behavior compared with the control, suggesting anti-amnesic activity. Also, JB reversed the memory impairment induced by scopolamine, which further indicates anti-amnesic property. Furthermore, JB demonstrated a significant inhibition of MDA formation in the frontal cortex and hippocampus of rats, indicating antioxidant property. In addition, it increased the defense armory of the brain tissues, as it significantly increased the concentrations of GSH in the frontal cortex and hippocampus of rats. However, JB did not demonstrate any inhibitory effect against AChE activity in the frontal cortex and hippocampus of rats in comparison with the control group.
Discussion
This investigation provides evidence that suggests that JB has anti-amnesic and antioxidant properties. Although the present data suggest that the anti-amnesic property of JB might be related to its antioxidant activity, more studies are necessary to clarify this observation.
Keywords: Jobelyn, Anti-Amnesic, Antioxidant, Malondialdehyde Levels, Glutathione Concentrations
1. Introduction
Amnesia is characterized by loss of memory and/or the inability to form memories and it represents one of the cardinal hallmarks of Alzheimer's disease (AD), a progressive neurodegenerative disorder associated with the ageing process (Hsieh et al., 2010; Baron, Wright, & Wenger, 1998). In recent years, considerable data have accrued indicating that increased oxidative stress is the primary event involved in the pathogenesis of AD (Markesbery, 1997; Holttum & Gershon, 1992; Moreira et al., 2008). Oxidative stress occurs when reactive oxygen species (ROS) accumulate in cells, either from excessive production or insufficient degradation, resulting in injury to DNA, lipids, and proteins (Natalie, Kelsey, Heather, Wilkins, Linseman, 2010). Brain tissue is particularly susceptible to free radical-mediated injury because of its high content of readily oxidized fatty acids, high oxygen demand and low levels of antioxidant defense systems (Moreira et al., 2008). Thus, it has been proposed that the search for new drugs that could be used to cure or halt the progression of AD should be directed at the scavenging of ROS or inhibition of their formation (Natalie, Kelsey, Heather, Wilkins, Linseman, 2010).
Recently, there has been a renewed effort to search for compounds from natural products with antioxidant property that could be efficacious for the treatment of AD (Hsieh et al., 2010; Ming et al., 2010). Jobelyn (JB) is a potent African polyherbal antioxidant formulation with various phytochemicals obtained mainly from the leaves of Sorghum bicolor (Gramineae), a plant that has been used for over a century for the treatment of several diseases (Erah et al., 2003; Okochi et al., 2003). These phytochemicals, especially apigenin, luteolin and naringenin have been shown to exhibit neuroprotection and to reduce neuroinflammation, suggesting a beneficial role in neurodegenerative diseases like AD (Awika and Rooney, 2004; Heo et al., 2004; Liu et al., 2009). Although previous investigations have shown that JB has anti-anaemic effect (Erah et al. 2003; Okochi et al., 2003), there are no experimental data that suggest its usefulness in neurodegenerative disorders like AD. Thus, this study was carried out to evaluate whether JB has anti-amnesic and antioxidant activities in rodents.
2. Methods
2.1. Experimental Animals
Male albino Swiss mice (20–22 g) and male Sprague-Dawley rats (180-200 g) used in the study were obtained from the Central Animal House, University of Ibadan and housed in plastic cages at room temperature with 12:12 h light–dark cycle. They were fed with rodent pellets and water ad libitum. The animals were acclimatized for 7 days before use in all experiments. The study was carried out in accordance with the ethical guidelines of the University of Ibadan for the care and use of laboratory animals for experimental investigations.
2.2. Drugs and Chemicals
Jobelyln (Health Forever Products Ltd, Lagos, Nigeria), physostigmine-PHY (Burroughs Wellcome Co. London) and scopolamine-SC (BDH) were used in the study. The drugs were dissolved in distilled water immediately before use and were given intraperitoneally (i.p.). The doses of 1, 2.5 and 5 mg/kg of JB used in the study were selected based on the results obtained from preliminary investigations. All the experimental procedures were carried out on day 7, 30 min after last treatment.
Experimental Procedures
Behavioral Studies
Effect of JB on Memory Performance
The effect of JB on memory was assessed using the Y-maze paradigm as previously described (Casadesus et al., 2006). Male mice were randomly distributed into treatment groups (n = 6) and were given i.p. injection of JB (1, 2.5, 5 mg/kg), PHY (0.10 mg/kg), SC (5 mg/kg) or distilled water (10 ml/kg) daily for 7 days. On the day of the experiment, 30 min after treatment, the animals were placed individually in the maze specifically at the end of arm A, and allowed to explore all the three arms (A, B, C) freely for 5 min. The number of alternations was recorded for the 5 min duration and the percentage alternations, which indicates memory performance was calculated. The percentage alternation was calculated by dividing the total number of alternations by the total number of arm entries minus two, multiplied by 100 (Yan et al., 2001). The ability of JB to reverse memory impairment induced by SC was also assessed in the study, utilizing the Y-maze paradigm as earlier described. The animals (6/group) were pretreated alone with SC (5 mg/kg) or in combination with JB (1, 2.5 or 5 mg/kg) or PHY (0.10 mg/kg) daily for 7 days before testing for memory performance. The number of arms entries, which indicates the level of spontaneous motor activity, was assessed in the Y-maze paradigm.
Biochemical Assays
The animals (6 rats per group) received intraperitoneal injection of JB (1, 2.5 and 5 mg/kg), PHY (0.10 mg/ kg), SC (5 mg/kg) or distilled water (10 ml/kg) daily for 7 days. Thirty minutes after the last treatment, the animals were scarified through cervical dislocation. The brain was rapidly removed and kept in the refrigerator with ice block for 30 min. Thereafter, the frontal cortex and hippocampus were dissected from the solidified brain tissues, which were extracted, weighed and homogenized separately in phosphate buffer (0.1M, pH 7.4) at a concentration of 10% w/v. Each brain tissue homogenates was separated into 3 portions for the different biochemical assays.
Estimation of acetylcholinesterase activity
Acetylcholinesterase activity in the brain tissues was measured according to the method of Ellman, Courtney, Andre, & Featherstone (1961). Briefly, the acetylcholinesterase activity in the homogenate was measured by adding 2.6 ml of phosphate buffer (0.1M, pH 7.4), 0.1ml of 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB) and 0.4ml of the homogenate. Then, 0.1ml of acetylcholine iodide solution was added to the reaction mixture. The absorbance was read at 412nm using spectrophotometer and the change in absorbance was measured at two min interval for a period of ten min. Acetylcholinesterase activity was expressed as micromoles per minute per milligram tissue (µmol/min/mg tissue).
Determination of Reduced Glutathione Concentrations
The concentrations of reduced glutathione in the brain tissues were determined by the method of Moron, Depierre, Mannervik (1979). Equal volumes of each tissue homogenate (0.4ml) and 20% trichloroacetic acid (0.4ml) were mixed and then centrifuged at 10,000 rpm for 20 min at 4°C. 0.25ml of the supernatant was added to 2ml of 0.6mM DTNB. The final volume of the solution was made up to 3ml with phosphate buffer (0.2M, pH 8.0). The absorbance was then read at 412nm against blank reagent using a spectrophotometer. The concentrations of reduced GSH in the brain tissues were expressed as micromoles per gram tissue (µmol/g tissue).
Determination of Lipid Peroxidation
The levels of lipid peroxidation in the brain tissues were determined by estimating MDA formation using the thiobarbituric acid test (Ohkawa, Ohishi, & Yagi, 1979). Briefly, 0.5 ml of distilled water and 1.0 ml 10% TCA were to 0.5 ml of each homogenate of the brain tissues. The mixture was then centrifuged at 3000 rpm for 10 min and 0.1 ml of thiobarbituric acid (0.375%) was added to ml of the supernatant. The mixture was incubated in a water bath at 80°C for 40 min. Upon cooling, the absorbance of the supernatant was measured at 532 nm using a spectrophotometer. The concentrations of MDA in the brain tissues were expressed as micromoles per gram tissue (µmol/g tissue).
Statistical Analysis
The data were analyzed using Graph pad prism software version 4.00 and data are expressed as mean ± S.E.M. Statistical analysis of data was done using Oneway ANOVA, followed by Newman-Keuls post-hoc test. P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Effect of JB on Memory Performance
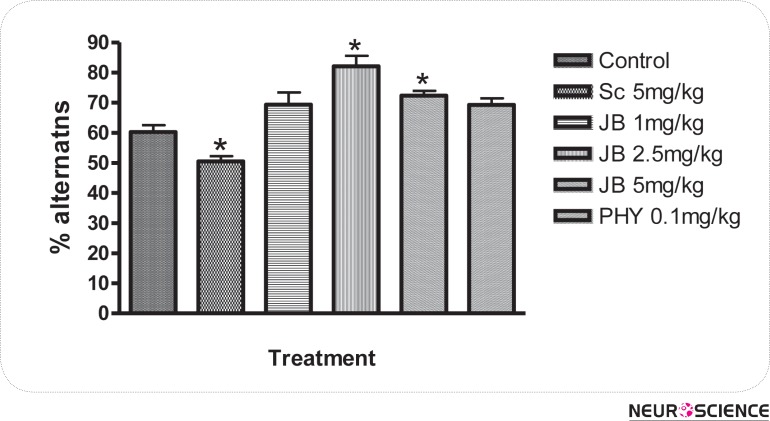
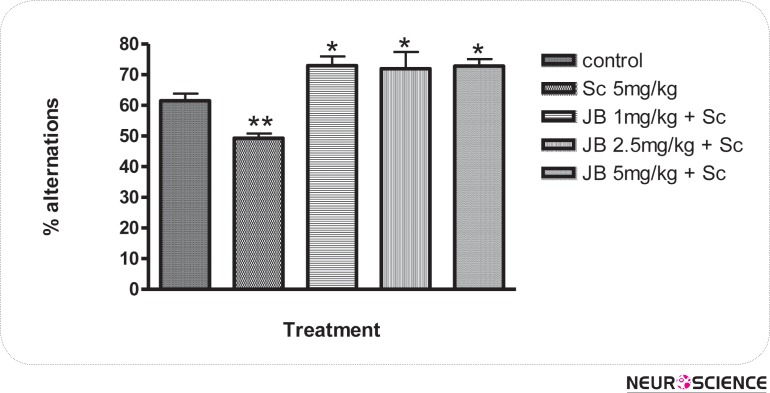
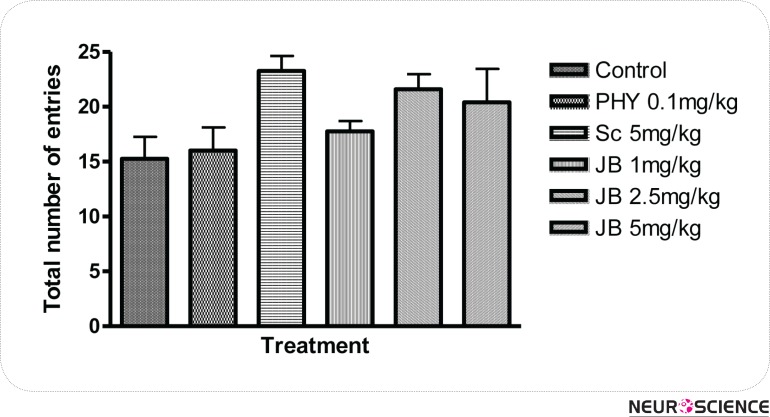
The effect of JB given daily for 7 days on memory as measured by the changes in alternation behaviors are shown in Figure 1. JB (2.5mg/kg) significantly (p < 0.05) increased the level of alternation behaviors in comparison with the control group, which suggest memory enhancing activity (Fig. 1). SC (5 mg/kg) given i.p daily for 7 days produced a significant (p < 0.05) reduction in alternation behaviors of mice when compared with the control, indicating memory impairment. However, the impairment in spatial memory induced by scopolamine was attenuated in a significant (p < 0.05) manner by JB (1, 2.5, 5mg/kg) in comparison with the group treated with SC alone (Fig. 2). The effect of JB administered daily for 7 days on SMA, as measured by the number of entries in the Y-maze is shown in Figure 3. It is evident from Figure 3 that JB (2.5 or 5mg/kg) did not significantly (p > 0.05) alter SMA when compared with the control group.
Figure 1.
Effects of Jobelyn on memory performance in the Y-maze paradigm in mice. Values represent the mean ± S.E.M for 6 animals per group. *p < 0.05 compared to control group (ANOVA followed by Newman Keuls test).
Figure 2.
Effect of Jobelyn on scopolamine-induced amnesia in mice. Values represent the mean ± S.E.M for 6 animals per group. *p < 0.05 treatment groups compared to scopolamine group and **p < 0.05 scopolamine group compared to control (ANOVA followed by Newman Keuls test).
Figure 3.
Effect of Jobelyn on the total number of entries in the Y-maze paradigm in mice. Values represent mean ± S.E.M for 6 animals per group. There are no significant (p > 0.05) differences in the total number of arm entries in treatment groups in comparism with the control group (ANOVA followed by Newman Keuls test).
3.2. Effect of JB on Acetylcholinesterase Activity
Table 1 shows the effect of JB (1, 2.5, or 5 mg/kg) given daily for 7 days on the level of AChE activity in the frontal cortex and hippocampus of rats. JB (1, 2.5 or 5mg/kg) did not inhibit the activity of AChE in the frontal cortex and hippocampus of rats compared with the control (Table 1). However, it is evident from Table 1 that JB significantly produced increase in the level of AChE activity in the brain regions in comparison with the control. Similar effects were found in the group pretreated with PHY (0.1 mg/kg) daily for 7 consecutive days (Table 1).
Table 1.
Effect of jobelyn on acetylcholinesterase activity in the cortex and hippocampus of rats
| Treatment | Dose (mg/kg) | AChE activity (µmol/min/mg tissue) | |
|---|---|---|---|
| Cortex | Hippocampus | ||
| Control | - | 364.3 ± 13.15 | 319.5 ± 6.51 |
| Scopolamine | 5 | 408.1 ± 8.79* | 443.2 ± 14.22* |
| Jobelyn | 1 | 521.1 ± 17.81* | 440.3 ± 16.41* |
| Jobelyn | 2.5 | 466.1 ± 12.61* | 480.6 ± 8.47* |
| Jobelyn | 5 | 349.3 ± 10.61 | 365.7 ± 16.97* |
| Physostigmine | 0.1 | 521.8 ± 12.74* | 510.3 ± 16.05* |
Values represent the mean ± S.E.M for 6 animals per group.
p < 0.05 compared to control group (ANOVA followed by Newman Keuls post hoc test).
3.3. Effect of JB on Reduced Glutathione (GSH) Concentrations in the Cortex and Hippocampus of Rats
The effects of JB (1, 2.5, or 5 mg/kg) given daily for 7 days on the concentrations of GSH in the cortex and hippocampus of rats are presented in Table 2. JB (1, 2.5 or 5mg/kg) was found to produce a significant (p < 0.05) increase in the concentrations of GSH in both the frontal cortex and hippocampus of rats in comparison with the control (Table 2). As evidenced from Table 2, PHY (0.1 mg/kg) but not scopolamine given i.p. for 7 consecutive days also significantly elevated the concentrations of GSH in both the cortex and hippocampus of rats.
Table 2.
Effect of JB on reduced glutathione concentration in the cortex and hippocampus of rats
| Treatment | Dose (mg/kg) | GSH concentration (µmol/g tissue) | |
|---|---|---|---|
| Cortex | Hippocampus | ||
| Control | - | 34.00± 4.59 | 28.00 ± 3.27 |
| Scopolamine | 5 | 27.00 ± 1.73 | 25.33 ±0.33 |
| Jobelyn | 1 | 65.60 ± 2.54* | 73.20 ± 2.76* |
| Jobelyn | 2.5 | 75.50 ± 1.44* | 99.75 ± 5.34* |
| Jobelyn | 5 | 38.25 ± 3.56 | 44.50 ± 3.38* |
| Physostigmine | 0.1 | 80.00 ± 2.39* | 83.80 ± 3.11* |
Values represent the mean ± S.E.M for 6 animals per group.
p < 0.05 compared to control group (ANOVA followed by Newman Keuls post hoc test).
3.4. Effect of Jobelyn on malondialdehyde levels in the cortex and hippocampus of rats
The effects of JB (1, 2.5, or 5 mg/kg) given daily for 7 days on the concentrations of MDA in the cortex and hippocampus of rats are shown in Table 3. JB (1, 2.5 or 5mg/kg) exhibited a significant inhibitory activity against MDA formation in both the frontal cortex and hippocampus of rats in comparism with the control group (Table 3). However, SC (5 mg/kg, i.p) but not PHY (0.1 mg/kg) given for 7 consecutive days significantly suppressed the concentrations of MDA in both the cortex and hippocampus of rats (Table 3).
Table 3.
Effect of Jobelyn on malondialdehyde (MDA) level in cortex and hippocampus of rats
| Treatment | Dose (mg/kg) | MDA level (µmol/g tissue) | |
|---|---|---|---|
| Cortex | Hippocampus | ||
| Control | - | 3.76 ± 0.09 | 4.56 ± 0.14 |
| Scopolamine | 5 | 5.38 ± 0.09* | 6.15 ±0.11* |
| Jobelyn | 1 | 0.08 ± 0.02* | 0.23 ± 0.04* |
| Jobelyn | 2.5 | 0.15 ± 0.03* | 0.19 ± 0.03* |
| Jobelyn | 5 | 3.33 ± 0.09* | 4.12 ± 0.08* |
| Physostigmine | 0.1 | 0.24 ± 0.03* | 0.25 ± 0.08* |
Values represent the mean ± S.E.M for 6 animals per group.
p < 0.05 compared to control group (ANOVA followed by Newman Keuls post hoc test).
4. Discussion
The results of the study showed that JB increased the levels of alternation behaviors in the Y-maze paradigm in mice, which suggest anti-amnesic activity. In addition, the ability of JB to reverse memory impairment induced by scopolamine further suggests anti-amnesic property. However, it did not significantly alter the number of arm entry in the Y-maze test, indicating absence of CNS stimulation. The primary behavioral parameter indicative of memory in the Y-maze test is the ability of rodents to remember the sequence of arms entry commonly known as spontaneous alternation (Blokland, 2005). It has been proposed that the list of arms visited is held in working memory and this aspect of memory is required to avoid making revisits to the former arm (Hooper, Fraser, Stone, 1996; Lee et al, 2010). Typically, a rodent always remember the least recently visited arm in order to alternate the arm choice and thus serves as a measure of short-term memory (Hooper, Fraser, Stone, 1996; Lee et al, 2010; Heo et al, 2009). Traditionally, the test is used for providing short-term spatial recognition memory and based on the ability of rodent to learn new information and recall past events critical to its existence in the natural environment (Dunning & During, 2003). The findings that JB increased alternation performance and reversed the amnesic effect of scopolamine suggest that it has memory enhancing activity.
Functional deficits in central cholinergic neurotransmission have been postulated to be involved in the pathogenesis of amnesia (Tabet et al., 2000; Blokland, 2005; Myhrer, 2003). Drugs such as AChE inhibitors that are capable of enhancing central cholinergic neuro-transmission have been shown to improve memory loss or intellectual impairment (Blokland, 2005; Myhrer, 2003; Brito, Davis, Stopp, & Stanton, 1983; Zhang and O'Donnell, 2000; Blokland, 2005; Bejar, Wang, & Weinstock, 1999). However, in this study, JB did not inhibit but rather like PHY increased the activity of AChE in the frontal cortex and hippocampus, the major brain regions involved in learning and memory. Although the reason for the increase in AChE activity in PHY-treated animals is not apparent in this study, it may be related to its duration of action and the dose used in the study. It is worthy to note that since PHY has short duration of action, it is possible that the enzyme might have regained its activity duration the course of the assay (Taylor, 2001). This may perhaps explain why the long acting AChE inhibitors like tacrine or donepezil are used clinically for the symptomatic relief of AD (Taylor, 2001; Kamal, Greig & Reale, 2009).
Current data that have accrued over the years revealed that increased oxidative stress is the primary event involved in the pathological abnormalities of AD, including β-amyloid deposition and cholinergic dysfunction (Markesbery, 1997; Tabet et al., 2000). Studies have shown that increase in lipid peroxidation and decreased polyunsaturated fatty acids occur in AD, which further support oxidative stress in the pathology of the disease (Lovell, Ehmann, Butler, Markesbery, 1995; Markesbery, 1997; Tabet et al., 2000). Oxidative stress occurs when reactive oxygen species (ROS) accumulate to toxic levels in cells, either from excessive production or insufficient degradation, resulting in injury to DNA, lipids, and proteins (Natalie, Kelsey, Heather, Wilkins, Linseman, 2010). Brain tissue is known to be more susceptible to the deleterious effects of ROS because unlike many other tissues, it contained small amounts of protective antioxidant defense systems (Markesbery, 1997). The memory impairment in scopolamine-induced animal model of AD was also shown to be associated with increased oxidative stress, which results in the degeneration of cholinergic neurons and of other neurotransmitter systems (Blokland, 2005; Myhrer, 2003; El-Sherbiny, Khalifa, Attia, Eldenshary, 2003; Jimenez-Jimenez, Alonso-Navarro, Avuso-Peralta, Jabbour-Wadih, 2006).
Oxidative stress in brain generates oxygen radicals, which initiate and propagate lipid peroxidation producing neuronal changes characterized in patients with AD (Coyle & Puttfarcven, 1993). In this study, JB reduced the brain levels of malondialdehyde, which is a measure of lipid peroxidation and free radical generation. At the same time there was a significant reduction in levels of glutathione, an endogenous antioxidant defense system that protect cells against the deleterious effects of ROS (Schulz, Linderau & Dichgans, 2000). These findings suggest that JB has antioxidant property and by virtue of this effect, it might be protecting neurons against the damaging effects of ROS, thereby retarding the progression of AD. Although more studies are necessary particularly on the effect of JB on scopolamine-induced oxidative stress, this investigation suggests that the antioxidant effect of JB might be playing a significant role in its anti-amnesic activity. Previous investigations have shown that JB possessed several phytochemicals with antioxidant and anti-amnesic properties (Awika & Rooney, 2004; Liu et al., 2009). In particular, naringenin and luteolin have been found to increase memory performance and to reverse scopolamine-induced amnesia in rodents (Liu et al., 2009; Heo et al., 2004). The anti-amnesic effect was associated with the antioxidant activity of these phytochemicals (Liu et al., 2009; Heo et al., 2004). Thus, the presence of these phytochemicals might be contributing a significant role in the antiamnesic and antioxidant activities of JB.
5. Conclusion
This investigation provides evidence which suggests that JB has anti-amnesic activity and might offer some promising effects for the treatment of memory deficits. Although more studies are necessary, the present data suggest that the anti-amnesic property of JB appears to be mediated through the scavenging of reactive oxygen species and/or inhibition of their formation.
Acknowledgements
The authors would like to thank the technical staff of department of Pharmacology and Therapeutics for their assistance. We also thank Dr O. Owoeye of the department of Anatomy and Dr. J. O. Olapade of the department of Veterinary Anatomy for their kind assistance and advice.
References
- Awika, J. M., & Rooney, L. W. (2004). Sorghum phytochemicals and their potential impact on human health. Phytochemistry, 65, 1199–1221 [DOI] [PubMed] [Google Scholar]
- Bejar, C., Wang, R. H., & Weinstock, M. (1999). Effect of rivastigmine on scopolamine induced memory impairment in rats. European Journal of Pharmacology, 383, 231–240 [DOI] [PubMed] [Google Scholar]
- Blokland, A. (2005). Scopolamine-induced deficits in cognitive performance: A review of animal studies. Scopolamine Review, 1–76
- Brito, G. N. O., Davis, B. J., Stopp, L. C., & Stanton, M. E. (1983). Memory and the septohippocampal cholinergic system in the rat. Psychopharmacology, 81, 315–320 [DOI] [PubMed] [Google Scholar]
- Casadesus, G., Webber, K. M., Atwood, C. S., Pappolla, M. A., Perry, G., Bowen, R. L., Smith, M. A. (2006). Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochimica Biophysica Acta, 1762, 447–452 [DOI] [PubMed] [Google Scholar]
- Coyle, T, & Puttfarcven, P. (1993). “Oxidative Stress, Glutamate and Neurodegenerative Disorder. Science,” 262, 89–695 [DOI] [PubMed] [Google Scholar]
- Dunning, J., & During, M. J. (2003). Molecular mechanisms of learning and memory. Expert Reviews in Molecular Medicine, 5(25), 1–11 [DOI] [PubMed] [Google Scholar]
- Ellman, G. L., Courtney, K. D., Andre, Jr. V., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacology, 7, 88–95 [DOI] [PubMed] [Google Scholar]
- El-Sherbiny, D. A., Khalifa, A. E., Attia, A. S., & Eldenshary, E. E. S. (2003). Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacology Biochemistry & Behaviour, 76, 523–533 [DOI] [PubMed] [Google Scholar]
- Erah, P. O., Asonye, C. C., & Okhamafe, A. O. (2003). Response of Trypanosoma brucei–induced anaemia to a commercial herbal preparation. African Journal of Biotechnology, 2, 307–311 [Google Scholar]
- Heo, H. J., Kim, M., Lee, J., Choi, S., Cho, H., Hong, B., Kim, H., Kim, E., & Shin, D. (2004). Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dementia and Geratric Cognitive Disorders, 17, 151–157 [DOI] [PubMed] [Google Scholar]
- Holttum, J. R., & Gershon, S. (1992). The cholinergic model of dementia, Alzheimer type: progression from the unitary transmitter concept. Dementia, 3, 174–185 [Google Scholar]
- Hooper, N., Fraser, C., & Stone, T. (1996). Effects of purine analogues on spontaneous alternation in mice. Psychopharmacology, 123, 250–257 [DOI] [PubMed] [Google Scholar]
- Hsieh, M.T., Peng, W.H., Wu, C.R., Ng, K.Y., Cheng, C.L., & Xu, H.X. (2010). Review on Experimental Research of Herbal Medicines with Anti-Amnesic Activity. Planta Med., 76, 203–217 [DOI] [PubMed] [Google Scholar]
- Jimenez-Jimenez, F. J., Alonso-Navarro, H., Avuso-Peralta, L., & Jabbour-Wadih, T. (2006). Oxidative stress and Alzheimer's disease. Review Neurol., 42, 419–427 [PubMed] [Google Scholar]
- Kamal, M.N., Greig, H.N, & Reale, M. (2009). Anti-Inflammatory Properties of Acetylcholinesterase Inhibitors Administred in Alzheimer's Disease. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 8, 85–100 [Google Scholar]
- Lee, M., Yun, B., Zhang, D., Liu, L., Wang, Z., Wang, C., Gu, L., Wang, C., Mo, E., Ly, S., & Sung, C. (2010). Effect of aqueous antler extract on scopolamine induced memory impairment in mice and antioxidant activities. Food Sci. Biotechnol., 19, 655–661 [Google Scholar]
- Liu, R., Gao, M., Qiang, G-F., Zhang, T-T., Lan, X., Ying, J., & Du, G-H. (2009). The anti-amnesic effects of luteolin against amyloid β25–35 peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience, 162, 1232–1243 [DOI] [PubMed] [Google Scholar]
- Lovell, M. A., Ehmann, W. D., Butler, S. M., Markesbery, W. R. (1995). Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurology, 45, 1594–1601 [DOI] [PubMed] [Google Scholar]
- Markesbery, W. R. (1997). Oxidative stress hypothesis in Alzheimer's disease. Free Radical Biology and Medicine, 23(1), 134–147 [DOI] [PubMed] [Google Scholar]
- Ming, T. H., Wen, H. P., Chi, R. W., Kit, Y. N., Chuen, L. C., & Hong, X. X. (2010). Review on Experimental Research of Herbal Medicines with Anti-Amnesic Activity. Planta Med, 76, 203–217 [DOI] [PubMed] [Google Scholar]
- Moreira, P. I., Santos, M. S., Oliveira, C. R., Shenk, J. C., Nunomura, A., Smith, M. A., Zhu, X., & Perry, G. (2008). Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurological Disorders and Drug Target, 7(1), 3–10 [DOI] [PubMed] [Google Scholar]
- Moron, M. S., Depierre, J. W., & Mannervik, B. (1979). Levels of glutathione, glutathione reductase and glutathione Stransferase activities in rat lung and liver. Biochimica et Biophysica ACTA, 582, 67–78 [DOI] [PubMed] [Google Scholar]
- Myhrer, T. (2003). Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Research Review, 41, 268–287 [DOI] [PubMed] [Google Scholar]
- Natalie, A., Kelsey, I., Heather, M., Wilkins, I., & Linseman, D. A. (2010). Nutraceutical Antioxidants as novel neuroprotective agents. Molecules, 15, 7792–7814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa, H., Ohishi, N., & Yagi, K. (1979). “Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction,” Analytical Biochemistry, 95(2), 351–358 [DOI] [PubMed] [Google Scholar]
- Okochi, V. I., Okpuzor, J., Okubena, M. O., & Awoyemi, A. K. (2003). The influence of African Herbal Formula on the haematological parameters of trypanosome infected rats. African Journal of Biotechnology, 2, 312–316 [Google Scholar]
- Schulz, J.B., Linderau, J., & Dichgans, J. L. (2000). Glutathione, Oxidative Stress and Neurodegeneration. European Journal of Biochemistry, Vol. 267, No.16, pp. 4904–4911 [DOI] [PubMed] [Google Scholar]
- Tabet, N., Mantle, D., & Orrell, M. (2000). Free radicals as mediators of toxicity in Alzheimer's disease: a review and hypothesis. Adverse Drug Reaction and Toxicol Review, 19(2), 127–152 [PubMed] [Google Scholar]
- Taylor, P. (2001). Anticholinerasterase agents. In Hardman J.B, Limbird L.E, & Gilman A. (Ed.), The Goodman and Gilman's Pharmacological Basis of Therapeutics, (pp. 175–191). McGraw-Hill: New York [Google Scholar]
- Yan, J., Cho, J., Kim, H., Jung, J., Huh, S., Suh, H., & Song, D. (2001). Protection against β-amyloid peptide toxicity in vivo with long term administration of ferulic acid. British Journal of pharmacology, 133, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. T., & O'Donnell, J. M. (2000). Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology, 150, 311–316 [DOI] [PubMed] [Google Scholar]