Abstract
The most popular animal models of Alzheimer's disease (AD) are transgenic mice expressing human genes with known mutations which do not represent the most abundant sporadic form of the disease. An increasing number of genetic, vascular and psychosocial data strongly support that the Octodon degus, a moderate-sized and diurnal precocial rodent, provides a naturalistic model for the study of the early neurodegenerative process associated with sporadic AD. In this minireview we describe and analyze the risk factors that contribute to Alzheimer-like characteristics in the degus, following recent publications, and establish some guidelines for future studies in this model of natural aging associated with the disease. Given the heterogeneity of current data derived from the diverse transgenic animal models of AD, now may be the time for the degus to become a strong attractor for academic research labs and companies involved with AD. This may help to understand the mechanisms responsible for the early neurodegenerative process associated with this devastating disease.
Keywords: Animal Model, Aging, Octodon Degus, Alzheimer's Disease
1. Introduction
Advancing age is the major risk factor for Alzheimer's disease (AD), a progressive neurodegenerative disorder characterized by cerebrovascular and neuronal dysfunctions leading to a gradual decline in cognition, daily functioning and behavioural alterations (Zlokovic, 2008). The main neuropathological hallmarks of AD include extracellular senile plaques containing β-amyloid (Aβ) derived from β-amyloid precursor protein (APP) after sequential cleavage by β-secretase and γ-secretase, and intracellular neurofibrillary tangles caused by abnormally phosphorylated tau protein (Duyckaerts, Delatour, and Potier, 2009).
Research progress over the past two decades, including the elucidation of AD susceptibility and causative genes as well as other proteins involved in the pathogenic process, has profoundly facilitated the development of genetically altered mouse models (see http://www.alzforum.org/res/com/tra for a listing of currently available models).
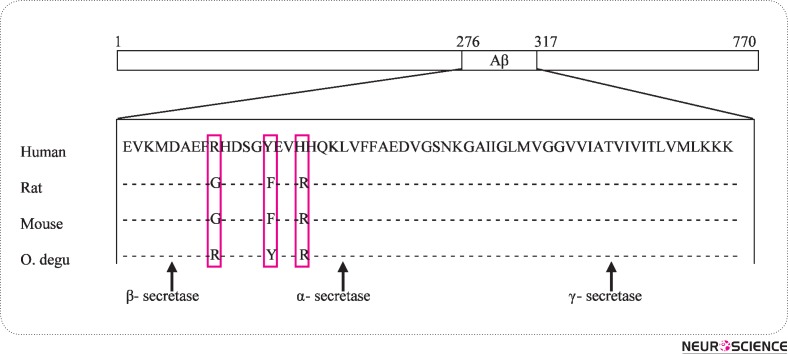
The most popular animal models of AD are transgenic mice expressing human genes with mutations known to lead to familial AD. Despite their undoubted value, these transgenic models do not represent the most abundant sporadic form of AD. Although several mammalian species (e.g., bears, cats, dogs, goats, monkeys, sheep) develop Alzheimer's-like pathology with aging, the aged wild-type central Chilean rodent, the Octodon degus (Od) a moderate-size and diurnal precocial rodent, also develops Alzheimer-like pathology (Inestrosa et al., 2005). If this is because of the remarkable close βA sequence homology (97, 5%) between human and Od remains to be shown. Different AD-like phenotypes between transgenic mice models and Octodon degus are compared in Table 1. Moreover, in old age, the laboratory bred Od, expresses cognitive deficits (Ortiz et al., 2005), anxiety (Popovic et al., 2009), unstable circadian rhythms of low amplitude (Vivanco et al., 2007), and spontaneous neuropathology in cerebral vessels and significant white matter alterations (van Groen et al., 2011), all of which are also signs seen in AD patients. It has recently been reported that postsynaptic dysfunction is associated with spatial and object recognition memory loss in the Od. This rodent exhibits an age-related accumulation of soluble Aβ oligomers and tau protein phosphorylation that correlates with cognitive decline in spatial memory (T-maze) and object recognition memory (ORM), as well as synaptic and neural plasticity dysfunction (Ardiles et al., 2012). Their results are consistent with the concept that soluble Aβ oligomers at prefibrillar stages, specially Aβ dodecamers (Aβ*56), can act as toxic ligands at postsynaptic compartments, and suggest that the Od provides a strong and naturalistic model for the study of the early neurodegenerative process associated with sporadic AD. It is also noted that neuronal loss is not present in significant numbers in the Od. Mice and rats do not develop Alzheimer pathology with aging, which is most likely related to the three amino-acid differences between the human and rodent sequence of Aβ (Guénette and Tanzi, 1999). Aged rats do not show these memory deficits (Rosenzweig and Barnes, 2003; Willig et al., 1987; Lukaszewska and Radulska, 1994) in clear contrast to the Od. This has obvious differences both in APP processing and in Aβ peptide fibrillization that is produced by the sequential cleavage of APP by the β- and γ-secretase activity (Guénette and Tanzi, 1999; Selkoe and Wolfe, 2007). However, molecular analysis of the Od APP has indicated that the Od Aβ presents only one amino-acid substitution with respect to the human sequence (Inestrosa et al., 2005) (Fig. 1).
Table 1.
Neuropathological features comparison between the main transgenic mouse models of Alzheimer disease and Octodon degus.
| Mouse model | Gene (mutation) fAD | Intraneuronal Aβ | Parenchymal Aβ plaques | Hyperphos-phorylated Tau | NFT | Neuronal - loss | Synaptic dysfunction | CAA | AD-like signs | Primary reference |
|---|---|---|---|---|---|---|---|---|---|---|
| PDAPP | APP (V717F) | - | Yes | Yes | No | No | Yes | - | < 2 years | Games et al. 1995 |
| Tg2576 | APP (K670N/M671L) | Yes | Yes | - | No | No | No | - | < 2 years | Hsiao et al. 1996 |
| TgCRND8 | APP (K670N/ M671L,V717F) | - | Yes | - | No | No | - | - | < 2 years | Chishti et al. 2001 |
| APP/PS1 | APP (K670N/M671L), PS1 (M146L) | - | Yes | - | - | - | - | - | < 2 years | Holcomb et al. 1998 |
| APP23 | APP (K670N/M671L) | - | Yes | Yes | No | Little | Yes | Yes | < 2 years | Sturchler-Pierrat et al. 1997 |
| Tg-SwDI | APP (E693Q, D694N) | - | Yes | - | - | - | - | Yes | < 2 years | Davis et al. 2004 |
| APPDutch | APP (E693Q) | - | Little | - | - | - | - | Yes | < 2 years | Herzig et al. 2004 |
| APPDutch/PS1 | APP (E693Q), PS1 (G384A) | - | Yes | - | - | - | - | Little | < 2 years | Herzig et al. 2004 |
| hAPP-Arc | APP (E693G, K670N/ M671L, V717F) | - | Yes | - | - | - | - | Little | < 2 years | Cheng et al. 2004 |
| Tg-ArcSwe | APP (E693G, K670N/ M671L) | Yes | Yes | - | - | - | - | Yes | < 2 years | Lord et al. 2006, Knobloch et al. 2007 |
| APPArc | APP (E693G) | - | Yes | - | - | - | - | Yes | < 2 years | Rönnbäck et al. 2011 |
| TAPP | APP (K670N/M671L), Tau (P301L) | - | Yes | - | Yes | - | - | - | < 2 years | Lewis et al. 2001 |
| 3xTg-AD | APP (K670N/M671L), Tau (P301L), PS1(M146V) | Yes | Yes | Yes | Yes | - | No | - | < 2 years | Oddo et al. 2003 |
| APPSL/PS1 | APP (K670N/ M671L,V717I), PS1 (M146L) | Yes | Yes | - | - | Yes | Yes | - | < 2 years | Wirths et al. 2002 |
| APP/PS1KI | PS1 (M233T/L235P) | Yes | Yes | - | - | Yes | Yes | - | < 2 years | Casas et al. 2004 |
| 5xFAD | APP (K670N/M671L, I716V, V717I), PS1 (M146L/L286V) | Yes | Yes | - | - | Yes | Yes | - | < 2 years | Oakley et al. 2006 |
| Octodon degus | SAD | Yes | Yes | Yes | Yes | Yes | Yes | ≥ 3 years | Inestrosa et al. 2005 |
fAD = familial AD; sAD = sporadic AD; NFT = Neurofibrillary tangles; CAA = cerebral amyloid angiopathy; Dash (-) = not reported. (modified from Schaeffer et al. 2011).
Figure 1.
Sequence alignment of Aβ from vertebrate species. Sequence alignment of Aβ and flanking sequences from the human, rat, mouse and octodon degus. Amino acid homology of deduced OdAβ sequence with human, rat and mouse Aβ sequences. The degu Aβ presents only one amino acid substitution with respect to the human sequence (Arg13 His, respectively; Inestrosa et al., 2005).
2. AD Risk-Factors Acting During Octodon Degus Aging
Based on epidemiological studies, genetic studies, neuroimaging methods and neuropathology research, three basic etiological hypotheses of the development of AD have been formulated: genetic, vascular and psychosocial (Povova J et al., 2012). It is also known that high blood pressure, serum cholesterol concentrations, and impaired glucose regulation are among the factors which are most associated with increased vascular risk of AD. Cerebral amyloid angiopathy precedes brain parenchyma Aβ pathology in the old Od (van Groen et al., 2011). The observed changes in the brain blood vessels may have been caused by hypertension, another risk factor for the development of AD (Helzner et al., 2009; van Groen et al., 2011). Several studies have shown that a cholesterol-fed rabbit model of AD displays as many as fourteen different pathological markers of AD including amyloid-β accumulation, thioflavin-S staining, blood brain barrier breach, microglia activation, cerebrovasculature changes, and alterations in learning and memory (Sparks, 2008; Deci et al., 2012). A significant increase in ventricular volume is one of the hallmarks of AD. Cholesterol-fed rabbits also show a significant increase in ventricular volume following 10 weeks on a diet of 2% cholesterol (Deci et al., 2012). It is still unknown if the Od naturally develops an increase in the ventricular size during aging. Previous data showed that the Od has a human-like lipoprotein metabolism and develops extensive atherosclerosis with cholesterol feeding in the presence of hyperglycemia (Homan et al., 2010). In fact, when fed a high cholesterol diet, the LDL fraction is enriched disproportionately, such that approximately 60% of cholesterol is carried in the LDL fraction, which more closely resembles the human lipoprotein profile (Tannock and King, 2010). Diabetes is also one of the risk factors for AD development (Sims-Robinson et al., 2010; Cheng et al., 2012). Interestingly, the wild Od is hyperglycemic and develops features suggestive of diabetic complications (Tannock and King, 2010), therefore breeding animals will need to receive an appropriate diet.
One challenge for social-affective neuroscience programs is to identify simple and yet valid animal models for studying the expression of basic social emotions and their role during different developmental windows, from infancy to adulthood (Colonnello et al., 2011). The Od is a very social animal with a broad array of communication methods (Edwards, 2009). Social animals are susceptible to high infection levels by contact-transmitted parasites due to increased nonspecific interaction. It has been shown that viral infections such as herpes play a role in the development of AD (Honjo, van Reekum, and Verhoeff, 2009; Itzhaki and Wozniak, 2008; Robinson, Dobson, and Lyons, 2004), and the wild Od is also often infected with Herpes-like viruses (Spear, Caple, and Sutherland, 1984) and with Trypanosoma (Galuppo et al., 2009).
Recent evidence suggests that circadian dysregulation may act to exacerbate AD pathology (Bedrosian and Nelson, 2012). The decline in melatonin production in aged individuals has been suggested as one of the primary contributing factors for the development of age-associated neurodegenerative diseases (Srinivasan et al., 2005). Melatonin, the principal hormonal output of the circadian system, is dysregulated in AD, and this may be important because melatonin is protective in cells exposed to toxic Aβ. In fact, because of its powerful antioxidant properties (Tan et al., 2000), melatonin has been proposed as a potential therapeutic agent in diseases in which oxidative stress is thought to be a major pathogenic factor. In this regard, oxidative damage is thought to be a significant contributing factor in the pathogenesis of AD. Most of the small laboratory animals are nocturnal and regulate melatonin synthesis by mechanisms that diverge from those of humans. However, the Od may provide an ideal model system for laboratory investigation of mechanisms of melatonin synthesis and secretion in diurnal mammals (Lee et al., 2009).
All these risk-factors acting during Od aging would likely lead to an increase in the chances of developing AD-like symptoms in these wild animals.
3. An Array of Research Possibilities in Octodon Degus
The amyloid theory suggests that deposits of Aβ42 are the key event of this disease and that they precede clinical signs by several years (Hardy and Selkoe, 2002; Jack et al., 2010; Selkoe, 2011). This peptide can be removed by capillaries, enzymatically degraded, or eliminated into cerebrospinal fluid (CSF) (Tanzi, Moir, and Wagner, 2004). The discovery of pathogenic mutations in the following three genes, encoding APP and the γ-secretase-complex components presenilin-1 and presenilin-2, in patients with autosomal dominant, early-onset familial AD, provided incontestable evidence that aberrant APP processing can be sufficient to trigger the pathological cascade leading to AD (Sleegers et al., 2010). However, the majority of late-onset AD patients (>99%) do not have a mutation that causes an increase in APP processing (Tanzi and Bertram, 2005) and, therefore, increases in Aβ production have not been found in late-onset AD. The pathological accumulation of Aβ in the far more common late-onset AD is more likely to be the result of defects in the clearance of Aβ (Mawuenyega et al., 2010). Clearance of Aβ from the brain occurs via active transport at the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB). In this way, it might be possible to open up, by using the Od, a spectrum of possibilities to investigate the mechanisms of the brain barriers involved in the clearance of Aβ during the early neurodegenerative process associated with sporadic AD. In their work, Ardiles et al. (2012), suggest that the decline in synaptic plasticity may be associated with an increase in Aβ peptides before the increase in tau phosphorylation and, because this process occurs naturally in the Od, it represents a unique opportunity to examine physiological mechanisms. Since the postsynaptic compartments are more susceptible to the effects of Aβ peptides, as shown by the reduction of PSD-95 and glutamate receptors in the aged Od (Ardiles et al., 2012), an array of research possibilities is open to new drugs and therapies for the initial phases of AD. The promise of effective therapy has created a great need for biomarkers able to detect AD in the predementia phase, because drugs will probably only be effective if neurodegeneration is not too advanced. In this regard, Od research also opens up new possibilities for CSF and plasma biomarkers, but it will be necessary to develop, from the methodological point of view, biological tools and reagents (antibodies, nucleotide sequences, etc) for these and other studies.
4. Discussion
An accumulation of recent evidence strongly support that the Od provides a naturalistic model for the study of the early neurodegenerative processes associated with sporadic AD. Given the heterogeneity of current data derived from the diverse transgenic animal models of AD, now may be the time for the Od to take its place as a strong attractor for academic research labs and companies involved with AD. This may help to understand the mechanisms responsible for the neurodegenerative process associated with this devastating disease.
Acknowledgments
This work was supported by the Canary Islands Government and by the FIS grant PI 10/00283.
References
- Ardiles, A.O., Tapia-Rojas, C.C., Mandal, M., Alexandre, F., Kirkwood, A., and Inestrosa, N.C., et al. (2012). Postsynaptic dysfunction is associated with spatial and object recognition memory loss in a natural model of Alzheimer's disease. Proc Natl Acad Sci U S A, 109(34), 13835–13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian, T.A., and Nelson, R.J. (2012). Pro: Alzheimer's disease and circadian dysfunction: chicken or egg? Alzheimers Res Ther, 4(4), 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas, C., Sergeant, N., Itier, J.M., Blanchard, V., Wirths, O., and van der Kolk, N., et al. (2004). Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol, 165(4), 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, G., Huang, C., Deng, H., and Wang, H. (2012). Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J, 42(5), 484–491 [DOI] [PubMed] [Google Scholar]
- Cheng, I.H., Palop, J.J., Esposito, L.A., Bien-Ly, N., Yan, F., and Mucke, L. (2004). Aggressive amyloidosis in mice expressing human amyloid peptides with the Arctic mutation. Nat Med, 10(11), 1190–1192 [DOI] [PubMed] [Google Scholar]
- Chishti, M.A., Yang, D.S., Janus, C., Phinney, A.L., Horne, P., and Pearson, J. (2001). Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem, 276(24), 21562–21570 [DOI] [PubMed] [Google Scholar]
- Colonnello, V., Iacobucci, P., Fuchs, T., Newberry, R.C., and Panksepp, J. (2011). Octodon degus. A useful animal model for social-affective neuroscience research: basic description of separation distress, social attachments and play. Neurosci Biobehav Rev, 35(9), 1854–1863 [DOI] [PubMed] [Google Scholar]
- Davis, J., Xu, F., Deane, R., Romanov, G., Previti, M.L., and Zeigler, K., et al. (2004). Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem, 279(19), 20296–20306 [DOI] [PubMed] [Google Scholar]
- Deci, S., Lemieux, S.K., Smith-Bell, C.A., Sparks, D.L., and Schreurs, B.G. (2012). Cholesterol increases ventricular volume in a rabbit model of Alzheimer's disease. J Alzheimers Dis, 29(2), 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts, C., Delatour, B., and Potier, M.C. (2009). Classification and basic pathology of Alzheimer disease. Acta Neuropathol, 118(1), 5–36 [DOI] [PubMed] [Google Scholar]
- Edwards, M.S. (2009). Nutrition and behavior of degus (Octodon degus). Vet Clin North Am Exot Anim Pract, 12(2), 237–253 [DOI] [PubMed] [Google Scholar]
- Galuppo, S., Bacigalupo, A., García, A., Ortiz, S., Coronado, X., and Cattan, P.E., et al. (2009). Predominance of Trypanosoma cruzi genotypes in two reservoirs infected by sylvatic Triatoma infestans of an endemic area of Chile. Acta Trop, 111(1), 90–93 [DOI] [PubMed] [Google Scholar]
- Games, D., Adams, D., Alessandrini, R., Barbour, R., Berthelette, P., and Blackwell, C., et al. (1995). Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature, 373(6514), 523–527 [DOI] [PubMed] [Google Scholar]
- Guénette, S.Y., and Tanzi, R.E. (1999). Progress toward valid transgenic mouse models for Alzheimer's disease. Neurobiol. Aging, 20(2), 201–211 [DOI] [PubMed] [Google Scholar]
- Hardy, J., and Selkoe, D.J. (2002). The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science, 297(5580), 353–356 [DOI] [PubMed] [Google Scholar]
- Helzner, E.P., Luchsinger, J.A., Scarmeas, N., Cosentino, S., Brickman, A.M., and Glymour, M.M., et al. (2009). Contribution of vascular risk factors to the progression in Alzheimer disease. Arch. Neurol, 66(3), 343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig, M.C., Winkler, D.T., Burgermeister, P., Pfeifer, M., Kohler, E., and Schmidt, S.D., et al. (2004). Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci, 7(9), 954–960 [DOI] [PubMed] [Google Scholar]
- Holcomb, L., Gordon, M.N., McGowan, E., Yu, X., Benkovic, S., and Jantzen, P., et al. (1998). Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med, 4(1), 97–100 [DOI] [PubMed] [Google Scholar]
- Homan, R., Hanselman, J.C., Bak-Mueller, S., Washburn, M., Lester, P., and Jensen, H.E., et al. (2010). Atherosclerosis in Octodon degus (degu) as a model for human disease. Atherosclerosis, 212(1), 48–54 [DOI] [PubMed] [Google Scholar]
- Honjo, K., van Reekum, R., and Verhoeff, N.P. (2009). Alzheimer's disease and infection: do infectious agents contribute to progression of Alzheimer's disease? Alzheimers Dement, 5(4), 348–360 [DOI] [PubMed] [Google Scholar]
- Hsiao, K., Chapman, P., Nilsen, S., Eckman, C., Harigaya, Y., and Younkin, S., et al. (1996). Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science, 274(5284), 99–102 [DOI] [PubMed] [Google Scholar]
- Inestrosa, N.C., Reyes, A.E., Chacón, M.A., Cerpa, W., Villalón, A., and Montiel, J., et al. (2005). Human-like rodent amyloidbeta-peptide determines Alzheimer pathology in aged wildtype Octodon degu. Neurobiol Aging, 26(7), 1023–1028 [DOI] [PubMed] [Google Scholar]
- Itzhaki, R.F., and Wozniak, M.A. (2008). Herpes simplex virus type 1 in Alzheimer's disease: the enemy within. J Alzheimers Dis, 13(4), 393–405 [DOI] [PubMed] [Google Scholar]
- Jack, C.R. Jr., Knopman, D.S., Jagust, W.J., Shaw, L.M., Aisen, P.S., and Weiner, M.W., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol, 9(1), 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch, M., Konietzko, U., Krebs, D.C., and Nitsch, R.M. (2007). Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol Aging, 28(9), 1297–1306 [DOI] [PubMed] [Google Scholar]
- Lee, S.J., Liu, T., Chattoraj, A., Zhang, S.L., Wang, L., and Lee, T.M., et al. (2009). Posttranscriptional regulation of pineal melatonin synthesis in Octodon degus. J Pineal Res, 47(1), 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J., Dickson, D.W., Lin, W.L., Chisholm, L., Corral, A., and Jones, G., et al. (2001). Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science, 293(5534), 1487–1491 [DOI] [PubMed] [Google Scholar]
- Lord, A., Kalimo, H., Eckman, C., Zhang, X.Q., Lannfelt, L., and Nilsson, L.N. (2006). The Arctic Alzheimer mutation facilitates early intraneuronal Abeta aggregation and senile plaque formation in transgenic mice. Neurobiol Aging, 27(1), 67–77 [DOI] [PubMed] [Google Scholar]
- Lukaszewska, I., and Radulska, A. (1994). Object recognition is not impaired in old rats. Acta Neurobiol Exp (Wars), 54(2), 143–150 [PubMed] [Google Scholar]
- Mawuenyega, K.G., Sigurdson, W., Ovod, V., Munsell, L., Kasten, T., and Morris, J.C., et al. (2010). Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science, 330(6012), 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley, H., Cole, S.L., Logan, S., Maus, E., Shao, P., and Craft, J., et al. (2006). Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci, 26(40), 10129–10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo, S., Caccamo, A., Shepherd, J.D., Murphy, M.P., Golde, T.E., and Kayed, R., et al. (2003). Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron, 39(3), 409–421 [DOI] [PubMed] [Google Scholar]
- Ortiz, V., Lozano, J.P., Rol de Lama, M.A., and Madrid, J.A. (2005). Rendimiento y deterioro cognitivo en Octodon degu, un nuevo modelo para ensayos conductuales. In: XLVII Congreso de la Sociedad Española de Geriatría y Gerontología, Málaga, Spain, June8–11 [Google Scholar]
- Popovic, N., Baño-Otálora, B., Rol, M.A., Caballero-Bleda, M., Madrid, J.A., and Popovic, M. (2009). Aging and time-of-day effects on anxiety in female Octodon degus. Behav. Brain Res, 200(1), 117–121 [DOI] [PubMed] [Google Scholar]
- Povova, J., Ambroz, P., Bar, M., Pavukova, V., Sery, O., and Tomaskova, H., et al. (2012). Epidemiological of and risk factors for Alzheimer's disease: A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 156(2), 108–114 [DOI] [PubMed] [Google Scholar]
- Robinson, S.R., Dobson, C., and Lyons, J. (2004). Challenges and directions for the pathogen hypothesis of Alzheimer's disease. Neurobiol Aging, 25(5), 629–637 [DOI] [PubMed] [Google Scholar]
- Rönnbäck, A., Zhu, S., Dillner, K., Aoki, M., Lilius, L., and Näslund, J., et al. (2011). Progressive neuropathology and cognitive decline in a single Arctic APP transgenic mouse model. Neurobiol Aging, 32(2), 280–292 [DOI] [PubMed] [Google Scholar]
- Rosenzweig, E.S., and Barnes, C.A. (2003). Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol, 69(3), 143–179 [DOI] [PubMed] [Google Scholar]
- Schaeffer, E.L., Figueiro, M., and Gattaz, W.F. (2011). Insights into Alzheimer disease pathogenesis from studies in transgenic animal models. Clinics (Sao Paulo), 66(Suppl 1), 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe, D.J. (2011). Resolving controversies on the path to Alzheimer's therapeutics. Nat Med, 17(9), 1060–1065 [DOI] [PubMed] [Google Scholar]
- Selkoe, D.J., and Wolfe, M.S. (2007). Presenilin: running with scissors in the membrane. Cell, 131(2), 215–221 [DOI] [PubMed] [Google Scholar]
- Sims-Robinson, C., Kim, B., Rosko, A., and Feldman, E.L. (2010). How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol, 6(10), 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleegers, K., Lambert, J.C., Bertram, L., Cruts, M., Amouyel, P., and Van Broeckhoven, C. (2010). The pursuit of susceptibility genes for Alzheimer's disease: progress and prospects. Trends Genet, 26(2), 84–93 [DOI] [PubMed] [Google Scholar]
- Sparks, D.L. (2008). The early and ongoing experience with the cholesterol-fed rabbit as a model of Alzheimer's disease: the old, the new and the pilot. J Alzheimers Dis, 15(4), 641–656 [DOI] [PubMed] [Google Scholar]
- Spear, G.S., Caple, M.V., and Sutherland, L.R. (1984). The pancreas in the degu. Exp Mol Pathol, 40(3), 295–310 [DOI] [PubMed] [Google Scholar]
- Srinivasan, V., Pandi-Perumal, S.R., Maestroni, G.J., Esquifino, A.I., Hardeland, R., and Cardinali, D.P. (2005). Role of melatonin in neurodegenerative diseases. Neurotox Res, 7(4), 293–318 [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat, C., Abramowski, D., Duke, M., Wiederhold, K.H., Mistl, C., and Rothacher, S., et al. (1997). Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA, 94(24), 13287–13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, D.X., Manchester, L.C., Reiter, R.J., Qi, W.B., Karbownik, M., and Calvo, J.R. (2000). Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept, 9(3-4), 137–159 [DOI] [PubMed] [Google Scholar]
- Tannock, L.R., and King, V.L. (2010). Animal models of atherosclerosis: more than mice. Atherosclerosis, 212(1), 32–33 [DOI] [PubMed] [Google Scholar]
- Tanzi, R.E., and Bertram, L. (2005). Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell, 120(4), 545–555 [DOI] [PubMed] [Google Scholar]
- Tanzi, R.E., Moir, R.D., and Wagner, S.L. (2004). Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron, 43(5), 605–608 [DOI] [PubMed] [Google Scholar]
- Van Groen, T., Kadish, I., Popović, N., Popović, M., Caballero-Bleda, M., Baño-Otálora, B., Vivanco P, Rol MÁ, and Madrid JAet al. (2011). Age-related brain pathology in Octodon degu: blood vessel, white matter and Alzheimer-like pathology. Neurobiol Aging, 32(9), 1651–1661 [DOI] [PubMed] [Google Scholar]
- Vivanco, P., Ortiz, V., Rol, M.A., and Madrid, J.A. (2007). Looking for the keys to diurnality downstream from the circadian clock: role of melatonin in a dual-phasing rodent, Octodon degus. J Pineal Res, 42(3), 280–290 [DOI] [PubMed] [Google Scholar]
- Willig, F., Palacios, A., Monmaur, P., M'Harzi, M., Laurent, J., and Delacour, J. (1987). Short-term memory, exploration and locomotor activity in aged rats. Neurobiol Aging, 8(5), 393–402 [DOI] [PubMed] [Google Scholar]
- Wirths, O., Multhaup, G., Czech, C., Feldmann, N., Blanchard, V., and Tremp, G., et al. (2002). Intraneuronal APP/A beta trafficking and plaque formation in beta-amyloid precursor protein and presenilin-1 transgenic mice. Brain Pathol, 12(3), 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic, B.V. (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron, 57(2), 178–201 [DOI] [PubMed] [Google Scholar]