Abstract
During the past 20 years, non-invasive brain stimulation has become an emerging field in clinical neuroscience due to its capability to transiently modulate corticospinal excitability, motor and cognitive functions. Whereas transcranial magnetic stimulation has been used extensively since more than two decades ago as a potential “neuromodulator”, transcranial current stimulation (tCS) has more recently gathered increased scientific interests. The primary aim of this narrative review is to describe characteristics of different tCS paradigms. tCS is an umbrella term for a number of brain modulating paradigms such as transcranial direct current stimulation (tDCS), transcranial alternative current stimulation (tACS), and transcranial random noise stimulation (tRNS). Their efficacy is dependent on two current parameters: intensity and length of application. Unlike tACS and tRNS, tDCS is polarity dependent. These techniques could be used as stand-alone techniques or can be used to prime the effects of other movement trainings.
The review also summarises safety issues, the mechanisms of tDCS-induced neuroplasticity, limitations of current state of knowledge in the literature, tool that could be used to understand brain plasticity effects in motor regions and tool that could be used to understand motor learning effects.
Keywords: Non-Invasive Brain Stimulation, Transcranial Direct Current Stimulation, Transcranial Alternating Current Stimulation, Transcranial Pulsed Current Stimulation, Transcranial Random Noise Stimulation, Neuroplasticity
1. Lifelong Brain Plasticity
The old concept that the brain structures become unalterable after childhood has been deserted based on the evidence that all areas of the brain remain plastic in adulthood and during physiological ageing, with even some evidence for neurogenesis (Bütefisch 2004). This capacity of a neural system to acquire or improve skills, and to adapt to new environments through a learning process has been labelled “neuroplasticity” (Rakic 2002; Overman Carmichael 2013; Zagrebelsky Korte 2013). Neuroplasticity refers to the ability of the nervous system to change its structure and function, as part of the processes that underlie learning and memory, to adapt to environmental changes, and to recover function after brain lesions. In recent years, new techniques have been developed for the understanding and induction of human neuroplasticity. An important contribution has come from the introduction of non-invasive brain stimulation (NIBS) (Wassermann et al. 2008; Kuo et al. 2013; Marcos 2013). The development of NIBS techniques to induce neuroplasticity constitutes a major breakthrough in our ability to study how changes in brain states account for behavioural changes such as motor performance.
2. Non-Invasive Brain Stimulation
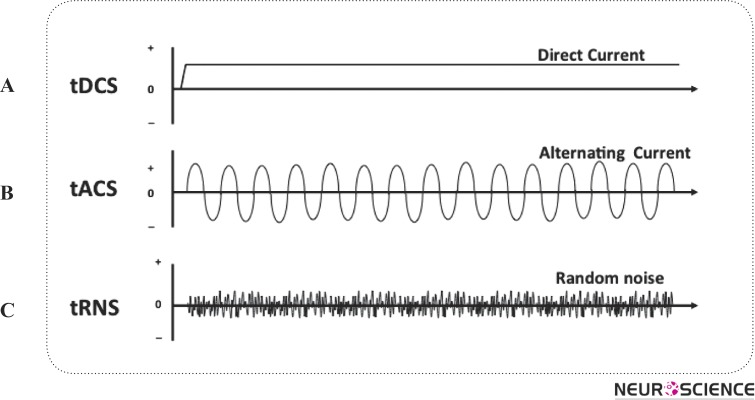
Several NIBS strategies aimed at modifying corticomotor excitability have emerged in recent years. These include transcranial magnetic stimulation (TMS), repetitive TMS (rTMS) (Pascual-Leone et al. 1994) which activates axons via short-pulsed stimulation and leads to new action potentials; and transcranial current stimulation (tCS) (Paulus 2011), which uses ultra-low intensity current, to manipulate the membrane potential of neurons and modulate spontaneous firing rates, but is insufficient on its own to discharge resting neurons or axons. tCS is an umbrella term for a number of brain modulating paradigms such as transcranial direct current stimulation (tDCS) (Nitsche et al. 2007; Kuo et al. 2013) transcranial alternative current stimulation (tACS) (Antal et al. 2008) and transcranial random noise stimulation (tRNS) (Terney et al. 2008) (Figure 1).
Figure 1.
tCS paradigms. tDCS: transcranial direct current stimulation; tACS: transcranial alternative current stimulation; tRNS: transcranial random noise stimulation.
Compared to TMS, tCS (Figure 1A) has a number of advantages. tCS has no or minimal side effects such as itching and burning sensations and it can be applied by an inexpensive battery-operated device which is very simple to operate (Jeffery et al. 2007; Bolognini et al. 2009), even by patients. tCS has a very long history in the literature with tDCS the most studied paradigm (Paulus 2011).
Transcranial Direct Current Stimulation
tDCS has been receiving increased interest in recent years as a tool for modulating cortical excitability and motor performance in a range of clinical settings and experimental conditions. tDCS involves application of weak, direct current (1-2 mA) to the scalp via spongebased rectangular pads (nominally 25-35 cm2) (Webster et al. 2006). This produces a sub-sensory level of electrical stimulation, which remains imperceptible by most people during its application. In a small percentage of participants it may cause minimal discomfort with a mild tingling sensation, which usually disappears after a few seconds (Nitsche et al. 2003). Skin burn is another side effect of tDCS, which should be avoided. A minor flaw in application of the technique such as small electrode size can easily result in skin burns.
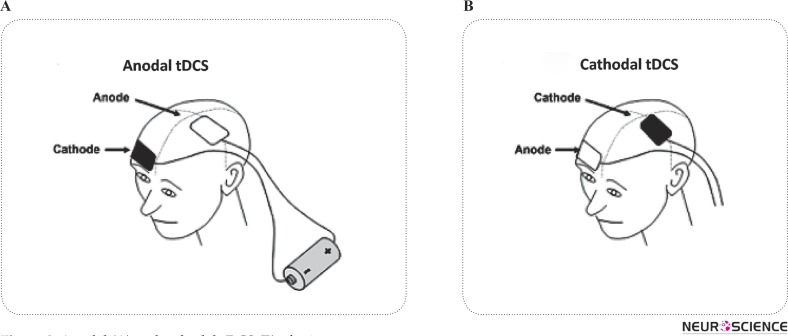
The applied current modifies the transmembrane neuronal potential and thus influences the level of excitability (Nitsche et al. 2008). The nature of these modulations depends on tDCS polarity, which may increase or decrease corticospinal excitability (CSE) (Nitsche et al. 2003). Anodal tDCS (a-tDCS), application of anode over cortical target area (i.e. primary motor cortex), increases CSE and Cathodal tDCS (c-tDCS), application of cathode over cortical target area, decreases CSE. In both cases the reference electrode could be placed on the opposite supraorbital area (Figure 2). This is just one of the most applicable type of montages.
Figure 2.
Anodal (A) and cathodal-tDCS (B) of primary motor cortex.
The respective changes evolve during tDCS, remain for up to 1 hour after it ceases (Nitsche Paulus 2001; Nitsche et al. 2003). These effects are probably intracortical. This was evidenced by increase in the size of TMSinduced MEPs and no changes in transcranial electrical stimulation (TES)-induced MEPs which are indicators of spinal changes (Nitsche Paulus 2000; Nitsche Paulus 2001; Nitsche et al. 2003). tDCS can be used as a standalone paradigm or as an add-on paradigm to prime the effects of motor training (Hummel et al. 2005; Hesse et al. 2007).
Effects of Motor Cortex Stimulation on Motor Skill Learning
Precise motor performance is essential to almost everything we do, from typing, to driving, to playing sports. Having a motor skill implies a level of performance in a given task that is only achievable through practice and motor learning. Motor learning is always associated with enhancement of M1 corticospinal excitability (Pascual-Leone et al. 1999; Muellbacher et al. 2002), which refers to both practice-related expansions in cortical representation area(s) of the involved muscles and increases in its strength of activations (Poldrack 2000). This increase is caused by recruitment of additional cortical units, which is evidenced by increase in size of TMS induced MEPS (Poldrack 2000). NIBS techniques facilitate motor skill learning by increasing the corticospinal excitability. Evidence from recent studies suggests links between a-tDCS induced corticospinal excitability, skill learning (Boggio et al. 2006; Galea Celnik 2009; Hunter et al. 2009; Reis et al. 2009) Bastani Jaberzadeh 2012 and motor performance (Nitsche et al. 2003; Hummel et al. 2010). Therefore, understanding of the interaction between modulations of corticospinal excitability and motor learning is critical for clinical approaches. A growing number of studies have shown added effects of a-tDCS for improvement of motor learning in healthy adults (Nitsche et al. 2003; Fregni et al. 2005; Hummel et al. 2005; Fregni et al. 2006; Hummel Cohen 2006; Fregni Pascual-Leone 2007; Matsuo et al. 2011). Within-session performance improvements (online effects) occur in the minutes of a single training session and continue over days and weeks of repeated training sessions (offline effects) (Reis et al. 2009). This improvement can be retained to varying degrees over weeks to months after the completion of training (long-term retention) (Savion- Lemieux Penhune 2005).
Priming the Effects of Motor Training Paradigms
Literature indicates that, there has been an effort to prime training strategies after brain lesions such as constraint-induced movement therapy, bilateral arm training, mirror and randomised training schedules or robotic-based approaches (Cauraugh Kim 2003; Wittenberg et al. 2003; Luft et al. 2004; Summers et al. 2007; Cramer 2008; Lo et al. 2009; Tanaka et al. 2010). Additionally, new technical approaches have been proposed to facilitate the beneficial effects of training on motor skill learning in the setting of rehabilitation interventions like somatosensory stimulation (Conforto et al. 2007) and non-invasive brain stimulation techniques, such as transcranial tDCS. Within the past two decades these techniques have been used to explore possible causal relations between activity in specific brain areas and particular behaviours (Hallett 2000; Nitsche et al. 2008). Improved understanding of the involvement of a brain region in a type of behaviour was followed by attempts to modulate activity in specific cortical areas with the goal to enhance motor performance (Hummel et al. 2005; Hummel Cohen 2006; Webster et al. 2006; Fregni Pascual-Leone 2007; Reis et al. 2008; Tanaka et al. 2011). Research studies in patients suffering from chronic stroke showed that a-tDCS on M1 of the affected hemisphere can beneficially influence motor performance of the paretic hand (Fregni et al. 2005; Hummel et al. 2005) (Hummel et al. 2006; O'Shea et al. 2013). Similar effects are also reported in the subacute stage of the post stroke patients (Kim et al. 2009). Refer to review by Gomez Palacio Schjetnan (2013) and Bastani and Jaberzadeh (2012) for further details (Bastani Jaberzadeh 2012; Gomez Palacio Schjetnan et al. 2013).
The Effects of Electrode Size and Electrode Montage
One important parameter in tDCS is electrode montage. In fact one of the reasons for the lack of significant effects for early tDCS studies (before the 90s) is electrode montage that result in lack of significant current being applied over the targeted cortical areas (Murphy et al. 2009). Nitsche and Paulus showed that tDCS-induced cortical excitability depends on the location of the electrodes (Nitsche Paulus 2000). During tDCS, electrodes are placed and secured to the scalp over the desired areas and current are delivered to the underlying cortical tissue. The direction of current flow determines the effects on the underlying tissue. Anodal tDCS, using the anodal electrode over M1 and the cathodal electrode over the contralateral supra orbital area, enhances cortical excitability, which increases the amplitude of motor evoked potentials (MEPs). On the other hand, cathodal tDCS, with the cathodal electrode over M1, shows the opposite effect (Nitsche Paulus 2000). Similar results were obtained in a modelling study (Wagner et al. 2007).
The spatial focality (targeting) of tDCS is considered pivotal for efficacy and safety in many biomedical applications. Focality is limited, in part, by the electrode size used. Traditional tDCS designs include two sponge-based electrodes, saturated with saline and connected to the stimulator via conductive rubber electrodes. The electrode on the target area is called active electrode and the one, which is usually placed on the contralateral supraorbital area, is called indifferent electrode. Decreasing active electrode size can improve spatial focality which may enhance cortico-plasticity (Bastani Jaberzadeh 2013). Indeed, by using smaller active electrodes we may avoid some inhibitory effects from stimulation of nearby cortical areas that might be functionally connected to M1 (Bastani Jaberzadeh 2013). Literature also indicates that, there are other methods that have been utilised to improve stimulation focality. Array electrodes and tripolar-electrodes configuration are among such examples (Datta et al. 2008). On the other hand, any decrease in electrode size, increases current density (Nitsche et al. 2007; Datta et al. 2008), which increases concerns related to safety issues such as skin irritation.
Inter hemispheric competition (rivalry model) and intra-hemispheric cortico-cortical connections (functional connectivity model) provide a number of tDCS strategies which could be used to promote M1 excitability and enhance motor performance (Nitsche et al. 2003; Boggio et al. 2006; Vines et al. 2006; Vines et al. 2008; Lindenberg et al. 2013). Interhemispheric rivalry assumes that any increase in motor performance may arise from excitation of contralateral cortex and inhibition from the ipsilateral cortex. Hence, motor performance might be facilitated by upregulating the excitability of the contralateral motor cortex through anodal tDCS or by downregulating the excitability of the ipsilateral motor cortex through cathodal tDCS (Nitsche et al. 2003; Boggio et al. 2006; Vines et al. 2006; Vines et al. 2008; Lindenberg et al. 2013). This is the basis for dual stimulation technique. The concept of functional connectivity is viewed as central for understanding the organized behaviour of anatomic regions in the brain during their activity (Kirimoto et al. 2011). This organization is thought to be based on the interaction between different and differently specialized cortical sites. For example, motor association cortex has inhibitory effects on M1 (Kirimoto et al. 2011) while premotor cortex facilitates M1 by reducing short-interval intracortical inhibition (Boros et al. 2008). Although these previous neurophysiological and modelling studies provided important insights regarding the optimal location for electrode placement it is critical to systematically test for different montages with different electrode sizes.
tDCS Safety
Safety of brain stimulation depends on the strength of current, the size of the electrodes and the duration of the stimulation (Nitsche et al. 2003; Iyer et al. 2005). In an MRI study, it was found that tDCS protocols, which are known to result in cortical excitability changes persisting for an hour post-stimulation, did not induce brain edema or alterations of the blood–brain barrier or cerebral tissue (Nitsche et al. 2004). The only main published safety study of DC stimulation, evaluated 103 subjects, (Iyer et al. 2005) found no adverse effects on cognitive and psychomotor measures, nor EEG changes during or after 20 min of treatment. In a double-blind, sham-controlled study (Gandiga et al. 2006) it has been shown that comparing tDCS and sham stimulation of the motor cortex elicited minimal discomfort and difference in the duration of tingling sensations. This study concluded that there have been no differences in self-rated attention or fatigue, or investigators could not distinguish real tDCS from sham.
Mechanisms of tDCS-Induced Neuroplasticity
Weak tDCS with a homogenous DC field at intensities of around 1 mA induces long-lasting changes in the brain. tDCS can be used to manipulate brain excitability via membrane polarisation: cathodal stimulation hyperpolarises, while anodal stimulation depolarises the resting membrane (Bindman et al. 1964; Nitsche et al. 2003). The induced after-effects of tDCS depend on Nmethyl-d-aspartate (NMDA) receptor efficacy changes (Liebetanz et al. 2002). There is also evidence for both GABAergic (Nitsche et al. 2004) and dopaminergic modulation of tDCS-induced effects (Nitsche et al. 2006). Relevant mechanisms underlying these after-effects include synaptic long-term potentiation (LTP) and long term depression (LTD) (Cooke Bliss 2006).
Intracortical inhibition and facilitation were prominently modulated by tDCS (Nitsche et al. 2005). For the short-lasting after-effects (7 min tDCS), inhibition was diminished and facilitation increased by anodal tDCS, whereas the effect of cathodal tDCS was the reverse. This result fits well with the fact that the after-effects of tDCS as well as intracortical inhibition and facilitation are at least partly controlled by NMDA receptor activity (Ziemann et al. 1998; Nitsche et al. 2003). Essentially, the results are identical for the long-lasting after-effects (9 or 13 min tDCS) (Nitsche et al. 2005).
A variety of other parameters influence tDCS effects. Co-application of neuropharmacologically active drugs may most impressively prolong or even reverse stimulation effects (Nitsche et al. 2003; Kuo et al. 2008). For example, administration of the NMDA antagonists decreased while GABA antagonists increased the tDCS effects (Nitsche et al. 2004). These findings provide evidence for involvement of these receptors in induced changes.
Other tCS Paradigms
Transcranial Alternating Current Stimulation (tACS)
tACS (Figure 1B) is another brain stimulation approach which involves application of alternating current through the skull over the target cortex of the brain (Antal et al. 2008). In this paradigm sinusoidally applied transcranial alternating current allows manipulation of intrinsic cortical oscillations with externally applied electrical frequencies. Of course, any combination of any frequency is possible. Motor learning under an implicit motor learning paradigm (Nitsche et al. 2003) was however better with 250 than with 140 Hz.
Transcranial Random Noise Stimulation (tRNS)
This paradigm (Figure 1C) is a form of tACS applied at random frequencies between 0.1 and 640 Hz, which can lead to an increase in performance of implicit motor or perceptual learning tasks (Terney et al. 2008; Ambrus et al. 2011; Fertonani et al. 2011; Saiote et al. 2013). Its effects on cortical excitability have also been shown to depend on the frequency range used for stimulation: high-frequency tRNS (101–640 Hz) increases cortical excitability whereas low-frequency tRNS (0.1–100 Hz) does not induce significant alterations (Terney et al. 2008).
A consistent CSE increase lasting at least 60 minutes, was induced by 10 minutes of tRNS (Terney et al. 2008). This effect may either be attributed to the repeated opening of NA channels or to a higher sensitivity of neuronal networks to field modulation than the average single neuron threshold (Francis et al. 2003).
Advantages of this technique compared to tDCS include it's insensitivity to electrode polarity and further reduction of skin sensations under the electrodes during stimulation. It is also easier to blind than tDCS (Ambrus et al. 2010).
Limitations of Current State of Knowledge
Although a considerable body of research has demonstrated the effects of tDCS paradigms in humans on cortical excitability and motor performance, there are considerable limitations with the studies that have been done to date.
While the neural substrates of motor skill learning involve functional changes in a distributed network that includes the primary motor cortex (M1), premotor cortex (PMC), supplementary motor area (SMA), somotosensory cortex (S1), dorsolateral prefrontal cortex (DLPFC), posterior parietal cortex (PPC), cerebellum, thalamic nuclei, and the striatum (Bo et al. 2008; Shadmehr Krakauer 2008; Doyon et al. 2009; Seidler 2010), most tDCS studies carried out so far have focused on efforts to only modulate activity within M1. The impact of cortical functional connectivity on motor learning and motor performance has not been fully understood yet.
Minimal research has investigated whether the effect of tDCS depends on what motor training paradigm is associated with.
Different paradigms of tCS (tDCS, tACS and tRNS) have developed in isolation from each other and no comparative studies have looked at whether or one or more of these plasticity paradigms have greater or lesser effects than the others.
Of particular relevance to neurorehabilitation is the finding of increased tDCS after effects with repetitive stimulation over days (Reis et al. 2009). Thus, the most efficient training protocols may turn out to be daily repetitions, further optimised with repetitive tDCS applications. Thus far little attention has been directed to the importance of daily repetition of tDCS sessions, number and interval between sessions.
Only a few studies have actually attempted to understand the mechanisms through which these paradigms change cortical activity (Nitsche et al. 2003; Stagg et al. 2011; Stagg Nitsche 2011). However, scientific rigor of double-blinded, randomised controlled trials was not carefully followed.
These limitations substantially inhibit the translation of the findings of this basic research into clinical applications. Where clinical applications have been developed, the choice of tCS paradigm and parameters has been rather idiosyncratic as opposed to being driven by knowledge of the effects of these stimulation parameters on brain function. Clearly, improving our knowledge of optimal tCS parameters and mechanisms would markedly enhance our capacity to develop effective clinical interventions.
References
- Ambrus, G. G. , Paulus, W. and Antal, A. (2010). Cutaneous perception thresholds of electrical stimulation methods: comparison of tDCS and tRNS. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 121(11), 1908–1914 [DOI] [PubMed] [Google Scholar]
- Ambrus, G. G. , Zimmer, M. , Kincses, Z. T. , Harza, I. , Kovacs, G. , and Paulus, W. , et al. (2011). The enhancement of cortical excitability over the DLPFC before and during training impairs categorization in the prototype distortion task. Neuropsychologia, 49(7), 1974–1980 [DOI] [PubMed] [Google Scholar]
- Antal, A. , Boros, K. , Poreisz, C. , Chaieb, L. , Terney, D. and Paulus, W. (2008). Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain stimulation, 1(2), 97–105 [DOI] [PubMed] [Google Scholar]
- Bastani, A. & Jaberzadeh, S. (2012). Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta-analysis. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 123(4), 644–657 [DOI] [PubMed] [Google Scholar]
- Bastani, A. & Jaberzadeh, S. (2013). a-tDCS Differential Modulation of Corticospinal Excitability: The Effects of Electrode Size. Brain stimulation, April 28, (Epub ahead of print) [DOI] [PubMed]
- Bindman, L. J. , Lippold, O. C. and Redfearn, J. W. (1964). The Action of Brief Polarizing Currents on the Cerebral Cortex of the Rat (1) during Current Flow and (2) in the Production of Long-Lasting after-Effects. The Journal of physiology, 172(369–382). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo, J. , Langan, J. and Seidler, R. D. (2008). Cognitive Neuroscience of Skill Acquisition Advances in Psychology, 139(11). [Google Scholar]
- Boggio, P. S. , Castro, L. O. , Savagim, E. A. , Braite, R. , Cruz, V. C. , and Rocha, R. R. , et al. (2006). Enhancement of nondominant hand motor function by anodal transcranial direct current stimulation. Neuroscience letters, 404(1-2), 232–236 [DOI] [PubMed] [Google Scholar]
- Bolognini, N. , Pascual-Leone, A. and Fregni, F. (2009). Using non-invasive brain stimulation to augment motor traininginduced plasticity. Journal of NeuroEngineering and Rehabilitation, 6(8):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros, K. , Poreisz, C. , Munchau, A. , Paulus, W. and Nitsche, M. A. (2008). Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. The European journal of neuroscience, 27(5), 1292–1300 [DOI] [PubMed] [Google Scholar]
- Bütefisch, C. (2004). Plasticity in the human cerebral cortex: lessons from the normal brain and from stroke. Neuroscientist, 10(2), 10–6 [DOI] [PubMed] [Google Scholar]
- Cauraugh, J. H. & Kim, S. B. (2003). Stroke motor recovery: active neuromuscular stimulation and repetitive practice schedules. Journal of neurology, neurosurgery, and psychiatry, 74(11), 1562–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforto, A. B. , Cohen, L. G. , dos Santos, R. L. , Scaff, M. and Marie, S. K. (2007). Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. Journal of neurology, 254(3), 333–339 [DOI] [PubMed] [Google Scholar]
- Cooke, S. F. & Bliss, T. V. (2006). Plasticity in the human central nervous system. Brain: a journal of neurology, 129(Pt 7), 1659–1673 [DOI] [PubMed] [Google Scholar]
- Cramer, S. C. (2008). Repairing the human brain after stroke. II. Restorative therapies. Annals of neurology, 63(5), 549–560 [DOI] [PubMed] [Google Scholar]
- Datta, A. , Elwassif, M. , Battaglia, F. and Bikson, M. (2008). Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. Journal of neural engineering, 5(2), 163–174 [DOI] [PubMed] [Google Scholar]
- Doyon, J. , Bellec, P. , Amsel, R. , Penhune, V. , Monchi, O. , Carrier, J. , et al. (2009). Contributions of the basal ganglia and functionally related brain structures to motor learning. Behavioural brain research, 199(1), 61–75 [DOI] [PubMed] [Google Scholar]
- Fertonani, A. , Pirulli, C. and Miniussi, C. (2011). Random noise stimulation improves neuroplasticity in perceptual learning. The Journal of neuroscience: the official journal of the Society for Neuroscience, 31(43), 15416–15423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, J. T. , Gluckman, B. J. and Schiff, S. J. (2003). Sensitivity of neurons to weak electric fields. The Journal of neuroscience: the official journal of the Society for Neuroscience, 23(19), 7255–7261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni, F. , Boggio, P. S. , Nitsche, M. , Bermpohl, F. , Antal, A. , and Feredoes, E. , et al. (2005). Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale, 166(1), 23–30 [DOI] [PubMed] [Google Scholar]
- Fregni, F. , Boggio, P. S. , Santos, M. C. , Lima, M. , Vieira, A. L. , and Rigonatti, S. P. , et al. (2006). Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson's disease. Mov Disord, 21(10), 1693–1702 [DOI] [PubMed] [Google Scholar]
- Fregni, F. & Pascual-Leone, A. (2007). Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nature clinical practice. Neurology, 3(7), 383–393 [DOI] [PubMed] [Google Scholar]
- Galea, J. M. & Celnik, P. (2009). Brain polarization enhances the formation and retention of motor memories. Journal of neurophysiology, 102(1), 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga, P. C. , Hummel, F. C. and Cohen, L. G. (2006). Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 117(4), 845–850 [DOI] [PubMed] [Google Scholar]
- Gomez Palacio Schjetnan, A. , Faraji, J. , Metz, G. A. , Tatsuno, M. and Luczak, A. (2013). Transcranial direct current stimulation in stroke rehabilitation: a review of recent advancements. Stroke research and treatment, 2013 (170256) Epub Feb 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett, M. (2000). Transcranial magnetic stimulation and the human brain. Nature, 406(6792), 147–150 [DOI] [PubMed] [Google Scholar]
- Hesse, S. , Werner, C. , Schonhardt, E. M. , Bardeleben, A. , Jenrich, W. and Kirker, S. G. (2007). Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restorative neurology and neuroscience, 25(1), 9–15 [PubMed] [Google Scholar]
- Hummel, F. , Celnik, P. , Giraux, P. , Floel, A. , Wu, W. H. , and Gerloff, C. , et al. (2005). Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain, 128(Pt 3), 490–499 [DOI] [PubMed] [Google Scholar]
- Hummel, F. C. & Cohen, L. G. (2006). Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet neurology, 5(8), 708–712 [DOI] [PubMed] [Google Scholar]
- Hummel, F. C. , Heise, K. , Celnik, P. , Floel, A. , Gerloff, C. and Cohen, L. G. (2010). Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiol Aging, 31(12), 2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel, F. C. , Voller, B. , Celnik, P. , Floel, A. , Giraux, P. , and Gerloff, C. , et al. (2006). Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC neuroscience, 7(73). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, T. , Sacco, P. , Nitsche, M. A. and Turner, D. L. (2009). Modulation of internal model formation during force fieldinduced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol, 587(Pt 12), 2949–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, M. B. , Mattu, U. , Grafman, J. , Lomarev, M. , Sato, S. and Wassermann, E. M. (2005). Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology, 64(5), 872–875 [DOI] [PubMed] [Google Scholar]
- Jeffery, D. T. , Norton, J. A. , Roy, F. D. and Gorassini, M. A. (2007). Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale, 182(2), 281–287 [DOI] [PubMed] [Google Scholar]
- Kim, D. Y. , Ohn, S. H. , Yang, E. J. , Park, C. I. and Jung, K. J. (2009). Enhancing motor performance by anodal transcranial direct current stimulation in subacute stroke patients. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists, 88(10), 829–836 [DOI] [PubMed] [Google Scholar]
- Kirimoto, H. , Ogata, K. , Onishi, H. , Oyama, M. , Goto, Y. and Tobimatsu, S. (2011). Transcranial direct current stimulation over the motor association cortex induces plastic changes in ipsilateral primary motor and somatosensory cortices. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 122(4), 777–783 [DOI] [PubMed] [Google Scholar]
- Kuo, M. F. , Paulus, W. and Nitsche, M. A. (2008). Boosting focally-induced brain plasticity by dopamine. Cereb Cortex, 18(3), 648–651 [DOI] [PubMed] [Google Scholar]
- Kuo, M. F. , Paulus, W. and Nitsche, M. A. (2013). Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage, E Publication, Ahead of print [DOI] [PubMed] [Google Scholar]
- Liebetanz, D. , Nitsche, M. A. , Tergau, F. and Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125(Pt 10), 2238–2247 [DOI] [PubMed] [Google Scholar]
- Lindenberg, R. , Nachtigall, L. , Meinzer, M. , Sieg, M. M. and Floel, A. (2013). Differential effects of dual and unihemispheric motor cortex stimulation in older adults. The Journal of neuroscience: the official journal of the Society for Neuroscience, 33(21), 9176–9183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, A. C. , Guarino, P. , Krebs, H. I. , Volpe, B. T. , Bever, C. T. , and Duncan, P. W. , et al. (2009). Multicenter randomized trial of robot-assisted rehabilitation for chronic stroke: methods and entry characteristics for VA ROBOTICS. Neurorehabilitation and neural repair, 23(8), 775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft, A. R. , McCombe-Waller, S. , Whitall, J. , Forrester, L. W. , Macko, R. , and Sorkin, J. D. , et al. (2004). Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA: the journal of the American Medical Association, 292(15), 1853–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos, Z. (2013). Alvaro Pascual-Leone: a pioneer of noninvasive brain stimulation. Lancet neurology, E Publication, Ahead of print [DOI] [PubMed] [Google Scholar]
- Matsuo, A. , Maeoka, H. , Hiyamizu, M. , Shomoto, K. , Morioka, S. and Seki, K. (2011). Enhancement of precise hand movement by transcranial direct current stimulation. Neuroreport, 22(2), 78–82 [DOI] [PubMed] [Google Scholar]
- Muellbacher, W. , Ziemann, U. , Wissel, J. , Dang, N. , Kofler, M. , and Facchini, S. , et al. (2002). Early consolidation in human primary motor cortex. Nature, 415(6872), 640–644 [DOI] [PubMed] [Google Scholar]
- Murphy, D. N. , Boggio, P. and Fregni, F. (2009). Transcranial direct current stimulation as a therapeutic tool for the treatment of major depression: insights from past and recent clinical studies. Current opinion in psychiatry, 22(3), 306–311 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Cohen, L. G. , Wassermann, E. M. , Priori, A. , Lang, N. , and Antal, A. , et al. (2008). Transcranial direct current stimulation: State of the art 2008. Brain stimulation, 1(3), 206–223 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Doemkes, S. , Karakose, T. , Antal, A. , Liebetanz, D. , and Lang, N. , et al. (2007). Shaping the effects of transcranial direct current stimulation of the human motor cortex. Journal of neurophysiology, 97(4), 3109–3117 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Fricke, K. , Henschke, U. , Schlitterlau, A. , Liebetanz, D. , and Lang, N. , et al. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. The Journal of Physiology, 553(Pt 1), 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche, M. A. , Lampe, C. , Antal, A. , Liebetanz, D. , Lang, N. , and Tergau, F. , et al. (2006). Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. The European journal of neuroscience, 23(6), 1651–1657 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Liebetanz, D. , Antal, A. , Lang, N. , Tergau, F. and Paulus, W. (2003). Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Supplements to Clinical neurophysiology, 56(255–276). [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Liebetanz, D. , Lang, N. , Antal, A. , Tergau, F. and Paulus, W. (2003). Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 114(11), 2220–2222; author reply 2222-2223 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Liebetanz, D. , Schlitterlau, A. , Henschke, U. , Fricke, K. , and Frommann, K. , et al. (2004). GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. The European journal of neuroscience, 19(10), 2720–2726 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Niehaus, L. , Hoffmann, K. T. , Hengst, S. , Liebetanz, D. , and Paulus, W. , et al. (2004). MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 115(10), 2419–2423 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 15(527), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche, M. A. & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899–1901 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Roth, A. , Kuo, M. F. , Fischer, A. K. , Liebetanz, D. , and Lang, N. , et al. (2007). Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci, 27(14), 3807–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche, M. A. , Schauenburg, A. , Lang, N. , Liebetanz, D. , Exner, C. , and Paulus, W. , et al. (2003). Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. Journal of cognitive neuroscience, 15(4), 619–626 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Seeber, A. , Frommann, K. , Klein, C. C. , Rochford, C. , and Nitsche, M. S. , et al. (2005). Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol, 568(Pt 1), 291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea, J. , Boudrias, M. H. , Stagg, C. J. , Bachtiar, V. , Kischka, U. , and Blicher, J. U. , et al. (2013). Predicting behavioural response to TDCS in chronic motor stroke. NeuroImage, E Publication, Ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman, J. J. & Carmichael, S. T. (2013). Plasticity in the Injured Brain: More than Molecules Matter. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry, [DOI] [PubMed] [Google Scholar]
- Pascual-Leone, A. , Tarazona, F. and Catala, M. D. (1999). Applications of transcranial magnetic stimulation in studies on motor learning. Electroencephalography and clinical neurophysiology. Supplement, 51(157–161). [PubMed] [Google Scholar]
- Pascual-Leone, A. , Valls-Solé, J. , Wassermann, E. M. and Hallett, M. (1994). Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain, 117(4), 11. [DOI] [PubMed] [Google Scholar]
- Paulus, W. (2011). Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychological rehabilitation, 21(5), 602–617 [DOI] [PubMed] [Google Scholar]
- Poldrack, R. A. (2000). Imaging brain plasticity: conceptual and methodological issues--a theoretical review. NeuroImage, 12(1), 1–13 [DOI] [PubMed] [Google Scholar]
- Rakic, P. (2002). Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nature reviews. Neuroscience, 3(1), 65–71 [DOI] [PubMed] [Google Scholar]
- Reis, J. , Robertson, E. , Krakauer, J. W. , Rothwell, J. , Marshall, L. , and Gerloff, C. , et al. (2008). Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain stimulation, 1(4), 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, J. , Schambra, H. M. , Cohen, L. G. , Buch, E. R. , Fritsch, B. , and Zarahn, E. , et al. (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America, 106(5), 1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, J. , Schambra, H. M. , Cohen, L. G. , Buch, E. R. , Fritsch, B. , and Zarahn, E. , et al. (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A, 106(5), 1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiote, C. , Polania, R. , Rosenberger, K. , Paulus, W. and Antal, A. (2013). High-frequency TRNS reduces BOLD activity during visuomotor learning. PloS one, 8(3), e59669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savion-Lemieux, T. and Penhune, V. B. (2005). The effects of practice and delay on motor skill learning and retention. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale, 161(4), 423–431 [DOI] [PubMed] [Google Scholar]
- Seidler, R. D. (2010). Neural correlates of motor learning, transfer of learning, and learning to learn. Exercise and sport sciences reviews, 38(1), 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr, R. & Krakauer, J. W. (2008). A computational neuroanatomy for motor control. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale, 185(3), 359–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg, C. J. , Jayaram, G. , Pastor, D. , Kincses, Z. T. , Matthews, P. M. and Johansen-Berg, H. (2011). Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia, 49(5), 800–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg, C. J. & Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry, 17(1), 37–53 [DOI] [PubMed] [Google Scholar]
- Summers, J. J. , Kagerer, F. A. , Garry, M. I. , Hiraga, C. Y. , Loftus, A. and Cauraugh, J. H. (2007). Bilateral and unilateral movement training on upper limb function in chronic stroke patients: A TMS study. Journal of the neurological sciences, 252(1), 76–82 [DOI] [PubMed] [Google Scholar]
- Tanaka, S. , Honda, M. , Hanakawa, T. and Cohen, L. G. (2010). Differential contribution of the supplementary motor area to stabilization of a procedural motor skill acquired through different practice schedules. Cerebral cortex, 20(9), 2114–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, S. , Takeda, K. , Otaka, Y. , Kita, K. , Osu, R. , and Honda, M. , et al. (2011). Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabilitation and neural repair, 25(6), 565–569 [DOI] [PubMed] [Google Scholar]
- Terney, D. , Chaieb, L. , Moliadze, V. , Antal, A. and Paulus, W. (2008). Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci, 28(52), 14147–14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines, B. W. , Cerruti, C. and Schlaug, G. (2008). Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC neuroscience, 9(103). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines, B. W. , Nair, D. G. and Schlaug, G. (2006). Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport, 17(6), 671–674 [DOI] [PubMed] [Google Scholar]
- Wagner, T. , Fregni, F. , Fecteau, S. , Grodzinsky, A. , Zahn, M. and Pascual-Leone, A. (2007). Transcranial direct current stimulation: a computer-based human model study. NeuroImage, 35(3), 1113–1124 [DOI] [PubMed] [Google Scholar]
- Wassermann, E. M. , Epstei, C. M. , Ziemann, U. , Walsh, V. , Paul, T. and Lisanby, S. H. 2008. The Oxford handbook of transcranial stimulation. New York. Oxford University Press, NY [Google Scholar]
- Webster, B. R. , Celnik, P. A. and Cohen, L. G. (2006). Noninvasive brain stimulation in stroke rehabilitation. NeuroRx, 3(4), 474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg, G. F. , Chen, R. , Ishii, K. , Bushara, K. O. , Eckloff, S. , and Croarkin, E. , et al. (2003). Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabilitation and neural repair, 17(1), 48–57 [DOI] [PubMed] [Google Scholar]
- Zagrebelsky, M. & Korte, M. (2013). Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology, E Publication, Ahead of print [DOI] [PubMed] [Google Scholar]
- Ziemann, U. , Chen, R. , Cohen, L. G. and Hallett, M. (1998). Dextromethorphan decreases the excitability of the human motor cortex. Neurology, 51(5), 1320–1324 [DOI] [PubMed] [Google Scholar]