Abstract
Introduction
Exposure to 3-4, methylenedioxymethamphetamine (MDMA) leads to cell death. Herein, we studied the protective effects of ginger on MDMA- induced apoptosis.
Methods
15 Sprague dawley male rats were administrated with 0, 10 mg/kg MDMA, or MDMA along with 100mg/kg ginger, IP for 7 days. Brains were removed to study the caspase 3, 8, and 9 expressions in the hippocampus by RT-PCR. Data was analyzed by SPSS 16 software using the one-way ANOVA test.
Results
MDMA treatment resulted in a significant increase in caspase 3, 8, and 9 as compared to the sham group (p < 0.001). Ginger administration however, appeared to significantly decrease the same (p < 0.001).
Discussion
Our findings suggest that ginger consumption may lead to the improvement of MDMA-induced neurotoxicity.
Keywords: Apoptosis, Ginger, MDMA, Caspase
1. Introduction
Ecstasy or 3, 4-methylenedioxymethamphetamine (MDMA), is a synthetic amphetamine derivative and an illicit drug of abuse which is primarily consumed by young people in dance and music environments. Many studies have demonstrated that MDMA is neurotoxic to the serotonin neurons (O'Leary et al., 2001). These effects seem to be dose- related, leading to memory impairment (Vorhees et al., 2009) and apoptosis in the hippocampus (Soleimani et al., 2012). It has been shown that MDMA induces cell death trough an apoptotic pathway through the release od cytochrome C and activation of the caspase cascade (Jiménez et al., 2004). The neurotoxicity associated with MDMA exposure may be the result of oxidative stress leading to the formation of hydroxyl radicals (Shankaran et al., 1999), lipid peroxidation (Alves et al., 2009) and an increase in the number of tunnel positive cells in the hippocampus. The imbalance between reactive oxygen species (ROS) and the internal antioxidants result in the oxidative stress. Glutathione is an important intracellular antioxidant that protects cells against oxidative stress (Franco and Cidlowski, 2012).
It has been repeatedly reported that MDMA treatment may decrease the glutathione concentration (Miranda et al., 2007).
As glutathione is unable to cross the BBB, possible treatment options would include the glutathione analog as a suitable candidate for therapeutic applications (Slivka et al., 1987).
There is evidence that dietary components could improve brain damage and cognitive function (Bisson et al., 2008 and Head, 2009). Ginger, member of the family of zingiberaceae, is widely used as a spicy seasoning. Moreover, it is used in Asian traditional medicine for treatment of stomach ache (Mascolo et al., 1989), nausea, diarrhea, and joint and muscle pain (Ojewole et al., 2006).
Ginger's antioxidant activity ( Nanjundaiah et al., 2009) and neuroprotective effects (Waggas et al., 2009) have been identified well. Kyung et al. suggest that ginger can reduce cell death and restore the motor function in rats with spinal cord injury (2006). Our aim was to investigate the effects of ginger on the caspase expression following MDMA treatment. Our hypothesis was if ginger has any anti apoptotic effect, then MDMA plus ginger treated rats should exhibit diminished caspase expression in their hippocampui.
2. Methods
2.1. MDMA and Ginger Preparation
3, 4-methylenedioxymethamphetamine was obtained from the Drug Control Headquarters. Zingiber officinale rhizomes with herbarium code no. 1483 were obtained from the Iranian institute of medicinal plant. About 500 g of dried rhizomes powder of Zingiber officinale were extracted with 3 liter 70% aqueous ethanol using percolation method at room temperature. The extracts were filtered through Whatman filter paper and evaporated to dryness under reduced pressure at a maximum of 40°C using a rotary evaporator. Zingiber officinale yielded 33.28% dried extract. For treatment ginger was solved in normal saline.
2.2. Animals
We included 15 male Sprague Dawley rats, weighing 200-250 g (Razi Institute, Iran). Animals were allowed to acclimatize to the colony room for one week prior to administration of MDMA. Rats were kept in the colony room at a temperature of 21 ± 1°C, relative humidity of 55± 5%, on a 12-h light/12-h dark cycle with access to water and food ad libitum. All experimental procedures were performed in accordance with the Guidelines of the Ethical Committee of Tehran University of Medical Sciences.
Animals were assigned to the following groups:
Sham (saline) group (n = 5) received normal saline,1 ml/kg, intraperitoneally (i.p.), daily for 1 week
MDMA groups (n = 5) received 10 mg/kg MDMA,i.p., daily for 1 week.
Treatment group (n = 7) received 100 mg/kg ginger, i.p. at 9:00 plus 10 mg/kg MDMA at 13:00, i.p., daily for 1 week (Mehdizadeh et al., 2012).
2.3. RT-PCR Experiment
The day after the last treatment, animals were euthanized by cervical dislocation. The brains were removed; hippocampi were immediately dissected out on ice, and then frozen in liquid nitrogen and kept at -80 °C until use.
Total mRNA was extracted from the frozen hippocampi by using phenol-chloroform method. Tissue samples were homogenized in 1000 µl RNATM (Cinnagen, Tehran, Iran) then 200 µl ice-cold chloroform was added. The homogenates were centrifuged (Eppendrof, Hamburg, Germany) at 12000 g for 20 min in 4°C. The RNA of the supernatant was precipitated with isopropanol, and washed with ethanol 75%. The air-dried RNA pellet was dissolved in RNase free water. cDNA first-strand synthesis was performed using a cDNA synthesis Kit (Quiagen, Hilden, Germany). First strand cDNA was used as template for subsequent PCR with a PCR master kit (Cinnagen, Tehran, Iran), and primers (Cinnagen, Tehran-Iran) as follow:
β-actin: forward 5TGGAGAAGAGCTATGAGCTGCCTG3
reverse 5GTGCCACCAGACAGCACTGTGTTG3
caspase3: forward 5TTTGGAACGAACGGACCTGT3
reverse 5CACGGGATCTGTTTCTTTGC3
caspase 8: forward GCAGGACATGTGGGACTCGCC 3
reverse 5 TCAGGCACAGGCACCGCTTTC 3
caspase 9 forward 5GAAGAACGACCTGACTGCTAAG 3
reverse 5 AGGAGACAAAACCTGGGAAG 3
The PCR reactions included initial denaturation at 95 °C for 3 min, followed by 35 cycles with 95 °C for 50 s, 58 °C for 45 s and 72 °C for 50 s for caspase3, and 9 and 35 cycles with 95 °C for 50 s, 59 °C for 45 and 72 °C for 50 s for caspase 8. The reactions were terminated by 72 °C for 7 min of elongation period. The same annealing temperature was used for β-actin. PCR products were separated by electrophoresis in 1% agarose gel at 100V. Our semi-quantitative analysis were made using a digital imaging system (UVIdoc,Houston,Texas,USA).
2.4. Statistical Analysis
Statistical analyses were performed using the SPSS 16 software. Data were presented as the mean ± S.E.M and the results were analyzed by one -way ANOVA with Tukey post-hoc comparison test. The p ≤ 0.05 was considered statistically significant.
3. Results
3.1. Protective Effect of ginger on Caspase-3 Expression
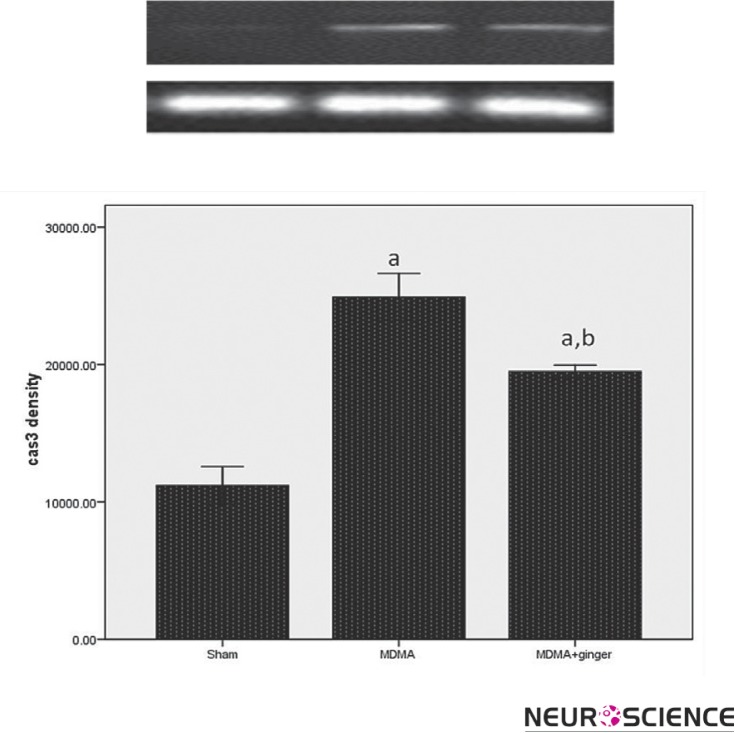
As shown in Figure 1, there was a significant increase in caspase 3 expression in the MDMA group as compared to the sham group (P < 0.001). Ginger pretreatment significantly decreased caspase 3 expression compared to the MDMA group (p < 0.001).
Figure 1.
RT-PCR analysis of the caspase-3 expression in sham and treatment groups. A significant difference in the caspase-3 expression between sham and other groups was noted ( ap < 0.001 vs. sham). Administration of ginger down-regulated the caspase-3 expression (b p < 0.001 vs. MDMA group).
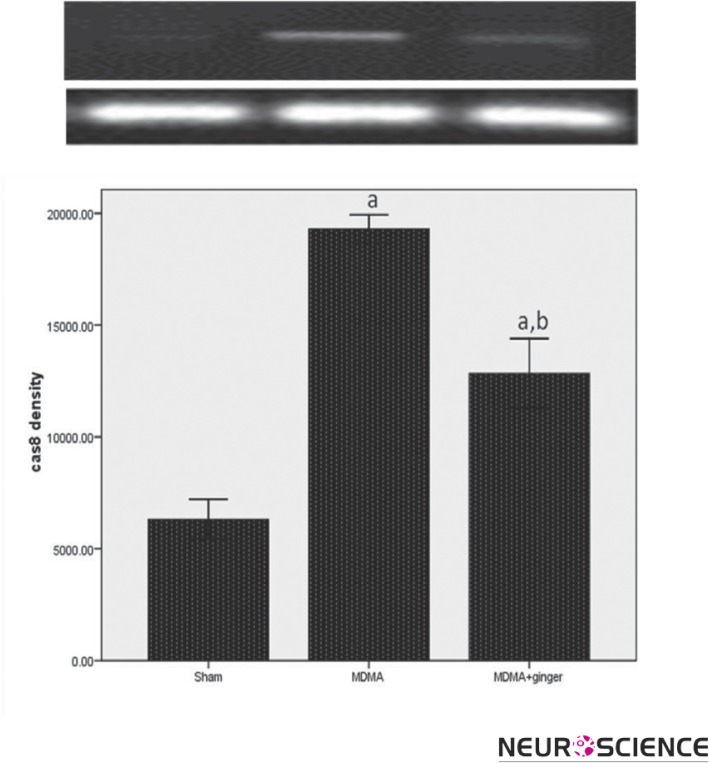
3.2. Protective Effect of Ginger On Caspase-8 Expression
With regards to the caspase 3 mRNA expression, there was significant difference between the MDMA and control group for caspase8 expression (p < 0.001, Figure 2). Moreover, subsequent analysis revealed that exposure to ginger resulted in significant reduction in caspase 8 expression compared to MDMA- treated rats (p < 0.001).
Figure 2.
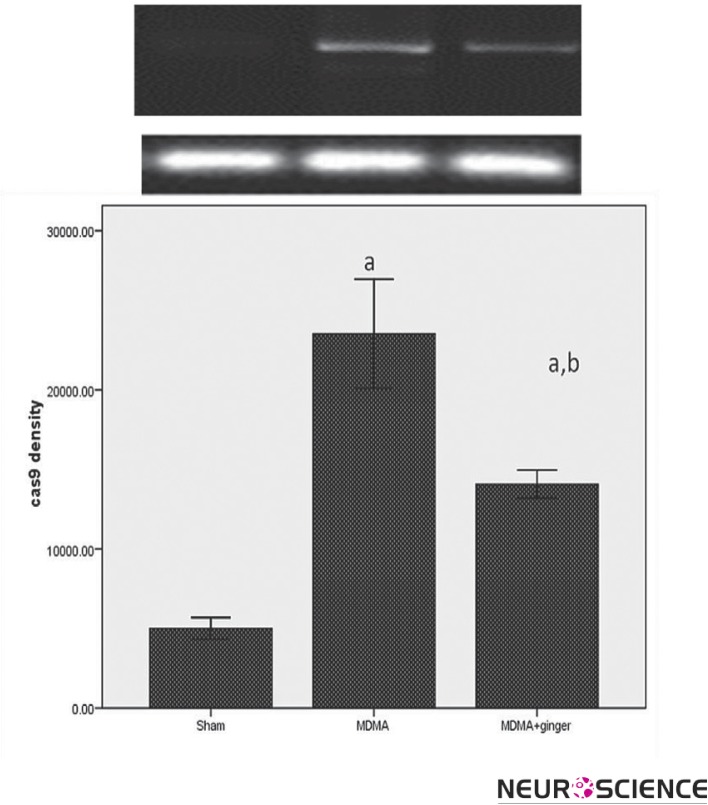
RT-PCR analysis of the caspase-8 expression in sham and treatment groups. MDMA treatment led to a significant increase in the caspae-9 expression ( ap < 0.001 vs. sham). Administration of ginger down-regulated the caspase-9 expression (b p < 0.001 vs. MDMA group).
3.3. Protective Effect of Ginger on Caspase-9 Expression
Densitometry from the electrophoresis gel showed significantly more expression of the caspase-9 gene in the MDMA group than the other groups (P < 0.001, Figure 3). Ginger pretreatment caused a significant reduction in caspase-9 expression as compared to the MDMA group (p < 0.001).
Figure 3.
RT-PCR analysis of the caspase-9 expression in sham and treatment groups. Exposure to MDMA caused an increase in the caspae-8 expression ( ap < 0.001 vs. sham). Administration of ginger down-regulated the caspase-8 expression (b p < 0.001 vs. MDMA group).
4. Discussion
The major finding of this study was the attenuation of caspase expression by ginger following MDMA treatment. Pretreatment with ginger was protective against MDMA- induced neurotoxicity in the hippocampus.
The brain is sensitive to oxidative stress due to low antioxidant and cell membrane lipids (Café et al., 1995). Therefore, the use of an external antioxidant is one of the most common therapeutic strategies for the treatment of neurotoxicity. Ginger is a spice that contains thiol group, which has antioxidant activity (Nanjundaiah et al., 2009) and neuroprotective effect (Waggas et al., 2009).
The hippocampus is an important brain structure substantially involved in learning and memory (Deng et al., 2012). Several studies have suggested that many neurotoxic factors can induce neuronal damage in the hippocampus (Che et al., 2010 and Singh et al., 2010), thus the use of external antioxidant may improve these insults (Alipanahzadeh et al., 2012, Soleimani et al., 2011).
Consistent to other study, our results demonstrated a well noted MDMA-induced neuronal death in the hippocampus (Wang et al., 2009 and Escubedo et al., 2011). MDMA causes rapid intracellular Ca2+ influx, mitochondrial membrane depolarization, ROS production and Caspase-9 activation (Montgomery et al., 2010). Injection of methamphetamine as another amphetamine derivates triggers the activation of the programmed cell death pathway and causes up-regulation of Bax and down regulation of Bcl-2 (Jayanthi et al., 2001).
Programmed cell death or apoptosis depends on activation of caspases such as caspases 3, 8, and 9 that cleave a number of substrates resulting in the biochemical and morphological changes typical for this form of death (For review see Favaloro et al., 2012).
In this study, we showed down- regulation of caspase 3, 8, and 9 genes in ginger plus MDMA treated group in comparison with MDMA group. Our results are consistent with the other studies. For instance, Mehdizadeh et al. reported that ginger could alter MDMA- induced apoptosis (Mehdizadeh et al., 2012). They showed that ginger pretreatment up- regulated anti apoptotic Bcl- 2 and down- regulated proapoptotic Bax proteins in MDMA- treated rats. Furthermore, 6-shagoal purified from ginger could lead to prominent decrease in PARP apoptosis protein and increase in the expression of Bcl-2 antiapoptotic protein in spinal cord injury (Kyung et al., 2006). It has been reported that ginger can decrease the oxidative stress by increasing the activity of SOD in cerebral cortex, hippocampus, and striatum and increases the activity of CAT and GSH in cerebral cortex and hippocampus resulting in the decrease of lipid peroxidation level in all areas mentioned earlier (Kyung et al., 2006). Therefore the neuroprotective effect of Z.afficinale extract might be related to its antioxidant effects.
In conclusion, our findings suggested that ginger could decrease MDMA-induced apoptosis in the hippocampus of male rats. Therefore, ginger appears to be a useful medicinal herb as a potential treatment for the MDMA- associated adverse effects.
Acknowledgments
This project was supported by the Research Institute for Islamic and Complementary Medicine (RICM) (p26/ m/t/548).
References
- Alipanahzadeh, H., Soleimani, M., Soleimani Asl, S., Mehdizadeh, M. (2012). The Effect of TGF-alpha on Neurogenesis in Subventricular Zone of Rat Brain after Ischemia-Reperfusion. Journal of Basic and Clinical Neurosciences, 3(2),12–15 [Google Scholar]
- Alves, E., Summavielle, T., Alves, C.J, Custódio, J.B., Fernandes, E., De Lourdes Bastos, M., et al. (2012). Ecstasy-induced oxidative stress to adolescent rat brain mitochondria in vivo: influence of monoamine oxidase type A. Addict Biol, 14,185–193 [DOI] [PubMed] [Google Scholar]
- Bisson, J.F., Nejdi, A., Rozan, P., Hidalgo, S., Lalonde, R., Messaoudi, M.(2008). Effects of long-term administration of a cocoa polyphenolic extract (Acticoa powder) on cognitive performances in aged rats. Br J Nutr, 100,94–101 [DOI] [PubMed] [Google Scholar]
- Café, C., Torri, C., Bertorelli, L., Tartara, F., Tancioni, F., Gaetani, P.(1995). Oxidative events in neuronal and glial cellenriched fractions of rat cerebral cortex. Free Radic Biol Med, 19, 853–857 [DOI] [PubMed] [Google Scholar]
- Che, Y., Wang, J.F., Shao, L., Young, T.(2010). Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. J Psychiatry Neurosci, 35,296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W., Aimone, J.B., Gage, F.H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory?. Nat Rev Neurosci, 11,339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escubedo, E., Abad, S., Torres, I., Camarasa, J., Pubill, D. (2011). Comparative neurochemical profile of 3,4-methylenedioxymethamphetamine and its metabolite alpha-methyldopamine on key targets of MDMA neurotoxicity. Neurochem Int, 58,92–101 [DOI] [PubMed] [Google Scholar]
- Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. (2012). Role of apoptosis in disease. Aging (Albany NY), 4(5),330–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, R., Cidlowski, J.A. (2012). Glutathione efflux and cell death. Antioxid. Redox. Signal.. 15,1694–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head, E. (2009). Oxidative damage and cognitive dysfunction: antioxidant treatments to promote healthy brain aging. Neurochem Res. 34,670–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi, S., Deng, X., Bordelon, M., McCoy, M.T., Cadet, J.L. (2001). Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J, 15,1745–1752 [DOI] [PubMed] [Google Scholar]
- Jiménez, A., Jordà, E.G., Verdaguer, E., Pubill, D., Sureda, F.X., Canudas, A.M., et al. (2004). Neurotoxicity of amphetamine derivatives is mediated by caspase pathway activation in rat cerebellar granule cells. Toxicol Appl Pharmacol, 15,223–234 [DOI] [PubMed] [Google Scholar]
- Kyung, K.S., Gon, J.H., Geun, K.Y., Sup, J.J., Suk, W.J., Ho, K.J.(2006). 6-Shogaol, a natural product, reduces cell death and restores motor function in rat spinal cord injury. Eur J Neurosci, 24,1042–1052 [DOI] [PubMed] [Google Scholar]
- Mascolo, N., Jain, R., Jain, S.C., Capasso, F. (1989). Ethnopharmacologic investigation of ginger (Zingiber officinale). J Ethnopharmacol, 27,129–140 [DOI] [PubMed] [Google Scholar]
- Mehdizadeh, M., Nejhadi, A., Fallah huseini, H., Choopani, S., Mohammadzadeh Kazorgah, F., Shekarriz, N.et al. (2012). Zingiber fficinale alters 3, 4-methylenedioxymetham-induced neurotoxicity in rat's brain. Cell Journal Cell Journal(Yakhteh), 14(3),177–184 [PMC free article] [PubMed] [Google Scholar]
- Miranda, M., Bosch-Morell, F., Johnsen-Soriano, S., Barcia, J., Almansa, I., Asensio, S.et al. (2007). Oxidative stress in rat retina and hippocampus after chronic MDMA (‘ecstasy’) administration. Neurochem Res, 32(7),1156–1162 [DOI] [PubMed] [Google Scholar]
- Montgomery, T., Sitte, H., McBean, G.J. (2010). 4-Methylthioamphetamine (4-MTA) induces mitochondrial-dependent apoptosis in SH-SY5Y cells independently of dopamine and noradrenaline transporters. BMC Pharmacology, 10(Suppl 1),A22 [Google Scholar]
- Nanjundaiah, S.M., Annaiah, H.N., Dharmesh, S. (2009) Gastroprotective Effect of Ginger Rhizome (Zingiber officinale) Extract: Role of Gallic Acid and Cinnamic Acid in H+, K + ATPase/H. pylori Inhibition and Anti-oxidative Mechanism. Jul 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojewole, J.A. (2006). Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res, 20,764–772 [DOI] [PubMed] [Google Scholar]
- O'Leary, G., Nargiso, J., Weiss, R.D. (2001). 3, 4-methylenedioxymethamphetamine (MDMA): a review. Curr Psychiatry Rep, 3(6),477–483 [DOI] [PubMed] [Google Scholar]
- Shankaran, M., Yamamoto, B.K., Gudelsky, G.A. (1999). Involvement of the serotonin transporter in the formation of hydroxyl radicals induced by 3,4-methylenedioxymethamphetamine. Eur J Pharmacol, 3,103–110 [DOI] [PubMed] [Google Scholar]
- Singh, M., Nam, D.T., Arseneault, M., Ramassamy, C. (2010). Role of by-products of lipid oxidation in Alzheimer's disease brain: a focus on acrolein. J Alzheimers Dis, 21,741–756 [DOI] [PubMed] [Google Scholar]
- Slivka, A., Mytilineou, C., Cohen, G. (1987). Histochemical evaluation of glutathione in brain. Brain Research. 21,275–284 [DOI] [PubMed] [Google Scholar]
- Soleimani Asl, S., Falahati, P., Shekarriz, N., Molavi, N., Esmaeili, F.et al. (2011). Protective effects of N-acetyl-L-cystein on 3,4-methylene dioxymethamphetamie-induced neurotoxicity in cerebellum of male rats. Journal of Basic and Clinical Neurosciences, 3(1),45–48 [Google Scholar]
- Soleimani Asl, S., Farhadi, M.H., Moosavizadeh, K., Samadi Kuchak Saraei, A., Soleimani, M. (2012). Evaluation of Bcl-2 family gene expression in hippocampus of 3-4 methylenedioxymethamphetamine treated rats. Cell Journal, 13,275–280 [PMC free article] [PubMed] [Google Scholar]
- Vorhees, C.V., Schaefer, T.L., Skelton, M.R., Grace, C.E., Herring, N.R., Williams, M.T. (2009). (+/-)3,4-Methylenedioxymethamphetamine (MDMA) dose-dependently impairs spatial learning in the morris water maze after exposure of rats to different five-day intervals from birth to postnatal day twenty. Dev Neurosciences, 31:107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggas, A.M. (2009). Neuroprotective evaluation of extract of ginger (Zingiber officinale) root in monosodium glutamateinduced toxicity in different brain areas male albino rats. Pak J Biol Sci, 12:201–212 [DOI] [PubMed] [Google Scholar]
- Wang, X., Zhu, S.P., Kuang, W.H., Li, J., Sun, X., Huang, M.S, Sun, X.L. (2009). Neuron apoptosis induced by 3,4-methylenedioxy methamphetamine and expression of apoptosisrelated factors in rat brain. Sichuan Da Xue Xue Bao Yi Xue Ban, 40,1000–1002, 1037 [PubMed] [Google Scholar]