Abstract
Introduction
Considering the prevalence of epilepsy and the failure of available treatments for many epileptic patients, finding more effective drugs in the treatment of epilepsy seems necessary. Oxidative stress has a special role in the pathogenesis of epileptic syndrome. Therefore, in the present study, we have examined the anti-epileptic and anti-oxidant properties of the Ferula Assa Foetida gum extract, using the pentylentetrazole (PTZ) kindling method.
In this experimental study, sixty male Albino mice weighing 25-30 g were selected and were randomly divided into 6 groups. 1- the control group, 2- PTZ-kindled mice, 3- positive control group which received valproate (100 mg/kg) as anti-convulsant drug, 4-5 & 6- the groups of kindled mice that pretreated with 25, 50 and 100 mg/kg doses of Ferula Assa Foetida gum extract.
Methods
Kindling has been induced in all groups, except for the control group via 11 PTZ injections (35 mg /kg; ip) every other day for 22 days. In the 24th day, the PTZ challenge dose was injected (75 mg / kg) to all groups except the control group. The intensity of seizures were observed and noted until 30 minutes after PTZ injection. At list, the mice were decapitated and the brains of all the mice were removed.. and their biochemical factors levels including malondialdehyde (MDA), superoxide dismutase (SOD) and nitric oxide (NO) were determined.
Results
Results of this study show that Ferula Assa Foetida gum extract is able to reduce seizure duration and its intensity. In addition, this extract has reduced MDA and NO levels and increased the level of SOD in the brain tissue compared to the PTZ- kindled mice.
Discussion
It can be concluded that Ferula Assa Foetida gum extract, in specific doses, is able to show an anti-epileptic effect because of its antioxidant properties, probably acting through an enzyme activity mechanism.
Keywords: Ferula Assa Foetida, Epilepsy, PTZ, Nitric Oxide, Superoxide Dismutase, Malondialdehyde
1. Introduction
Epilepsy is one of the most serious neurological disorders, which is induced by a sudden increment of stimulatory factors in the cortical neurons. About 0.5-3% of people experience it during their lifetime (Theodore & Fisher, 2007; Levav, Stephenson & Theodore, 1999). Epilepsy refers to a functional disorder of the brain with the occurrence of recurrent and unpredictable seizures (McNamara, 1994). Among the reasons of seizure attack are brain injury, stroke, serious events before birth, brain tumor, infection (encephalitis, bacterial meningitis) and genetic factors (Khaleghi-Ghadiri & Gorji, 2004).
It has been known that during epileptic attack, oxidative stress occurs, free radicals are produced and membrane lipid peroxidation happens, all of which cause tissue damage (Ilhan et al., 2005; Tuszkiewicz-Misztal, Opoka-Winiarska &, Postêpski, 2000).
MDA is the end product of unsaturated membrane fatty acid peroxidation and therefore, has been considered as the sign of lipid peroxidation (Chauhan, & Chauhan, 2006). NO is also one of the most abundant free radicals in the body (Evereklioglu et al., 2004) which its rapid reaction with other free radicals such as superoxide anion induces oxidizing product, neuronal toxicity and epileptic attack (Sogut et al., 2003; Buisson et al., 1993). In the presence of superoxide free radicals, the activity of SOD increases (Rukmini, Souza & Souza, 2004). Superoxide radical is also one of the free radicals which indirectly trigger lipid peroxidation (Dehpour et al., 2009). SOD can scavenger the superoxide anion by catalyzing it to H2O2 and O2 by which prevent the oxidative stress-induced cellular damage (Sogut et al., 2003). Despite the development of various anti-epileptic drugs, about one third of epileptic patients are resistant to current drug therapies. The resulted side effects from common toxic anti-epileptic drugs also lead to limited use of these drugs and failure of their optimal therapeutic effect in the treatment. Thus, considering the mentioned reports, focusing on new anti-epileptic drugs is necessary (Zargari, 1991).
In the recent years, there has been a revival in the use of medicinal plants. The milder side effects of these plants, the variety of effective compounds in plants, the recommendations of the World Health Organization for using medicinal plants have all contributed to the growth of this new trend (Zamani, 1999). In the Iranian and Indian traditional medicine, Ferula Assa Foetida has been suggested to eliminate seizures and also in Pakistan and Afghanistan it is used in food components. Its plant is distributed in East of Afghanistan and West of Iran (Taghi & Tehran, 1990; Moatar & Shams-Ardakani, 1999; Tabatabyi 1989). Ferula Assa Foetida has antispasmodic effects. It is used to eliminate the respiratory and gastrointestinal diseases with neural origins (Newall, Anderson & Phillipson, 1996) and is also prescribed for the treatment of inflammation, tremors and epilepsy in children (Gorgi & Madeja 2001). The most important substances in Ferula Assa Foetida, are sulfide compounds, Sesquiterpene compounds (Coumarin derivatives) and Monoterpenes including α-Pinene, β-,Pinene (With anti- epileptic properties) (Stefan et al., 2006), Phellandrene, Granyl acetate, α-terpineol, asaresinotannols and flavonoid compounds (with anti oxidant properties) and Luteolin (Dehpour et al., 2009).
In the researches performed on this plant, anticonvulsant, antioxidant and NO scavengering effects of this plant are mentioned (Sayyah et al., 2011). In this study we have investigated the anti-epileptic effects of Ferula Assa Foetida by evaluation of the antioxidant properties of this plant. In addition, by measuring the levels of NO as a free radical, MDA level as the most important indicator of lipid peroxidation, and SOD activity as an antioxidant enzyme in the PTZ-induced chemical kindling models, we examined the antioxidant properties of Ferula Assa Foetida.
2. Methods
2.1. Animals
In this experimental study, a total of 60 male Albino mice weighing 25-30 g (Razi Institue, Iran) were randomly divided into six experimental groups including; 1- the control group, 2- PTZ-kindled mice, 3- the posi- tive control group which received valproate (100 mg/kg) as anti-convulsant drug, 4-5 & 6- the treatment groups which received 25, 50 and 100 mg/kg; i.p. of Ferula Assa Foetida gum extract.
Ten mice per cage were kept in animal house of Shahed Medical University at temperature 21±2°C and under light cycle of 12 h darkness and 12 h lights. The mice had free access to standard food and tap water ad libitum. The experimental protocol was approved by the Ethic Committee of Shahed University.
2.2. Kindling Method
All groups of mice except the control group were kindled by a total of 11 injections of PTZ (35 mg/kg; i.p.) every second day and in a period of 22 days. PTZ (Sigma) was dissolved in isotonic saline solution (NaCl 9/0%). The animals were considered for 30 minutes after the last drug injection; after an additional 30 minutes the mice were observed for lethality. On day 24, the challenge dose of PTZ (75 mg / kg) was injected to all of the kindled mice, to induce tonic-clonic seizures and perhaps death.
In the positive control group and treatment groups (groups 3, 4, 5 and 6) PTZ was administrated 30 minutes after the first treatment with valproate and different doses of Ferula Assa Foetida gum extract. The intensity of seizure (0-6 phases) in the kindling model for 30 minutes after PTZ injection was evaluated using the following scale (Eracovic et al., 2001). The scale introduces six phases as follows:
No response
Ear and facial twitching
Axial convulsive waves through the body
Myoclonic body jerks
Generalized clonic convulsions turning over into side position
Generalized convulsions with tonic extension episode and status epilepticus
Mortality
2.3. Preparation of Plant Hydro-Alcoholic Extract
Ferula Assa Foetida gum was provided from the local stores and was scientifically approved by the department of Botany of Shahed University. To prepare the hydroalcoholic extract, using percolation method; 60 g of gum was grinded, and then the extract was obtained according to the Khalili method (Khalili et al., 2011; Atilla et al., 2006). The final concentration was 25%. However, the 25, 50 and 100 mg/kg extract doses were prepared from concentrated extract which were dissolved in saline.
2.4. Sample Preparation and Biochemical Assays
To evaluate the biochemical factors, after the 12th injection and behavioral observation, the mice were decapitated and their brains were quickly removed. The brains were washed two times in cold saline. They were placed in freezer (-30° C), in a glass bottle (for less than 10 hours). Then the brains were cut by scissors and were homogenized using four times ice-cold Tris-Hcl (50 mM, PH 7.4) buffer for 2 minutes at 5000 rpm. MDA and NO levels were measured at this phase. The homogenized solution was then centrifuged for 60 minutes at 5000×g to remove debris. The supernatant solution was then extracted with a mixture of ethanol/chloroform (a volume with ratio of 5:3). After centrifugation at 5000×g for 30 min, the clear upper layer (the ethanol phase) was taken and used for evaluation of the SOD activity. All experiments were carried out at +4° C (Oliver et al., 1990).
2.5. NO Measurement
Since nitric oxide is a highly unstable material that rapidly converts to nitrate (NO3-) and finally to nitrite (NO2-), the total nitrite amount using Griess method was used as an indicator of nitric oxide. Briefly, in this study nitrate in the samples was converted to nitrite by cadmium and followed by color development with Griess reagent (sulfanilamide and N-naphthyl ethylenediamine) in acidic medium. The absorbance was determined at 540 nm with a spectrophotometer (Ilhan, et al 2005).
2.6. SOD Enzyme Activity Evaluation
Total SOD activity was measured based on the Sun method. Supernatants of brain tissue samples were incubated with xantine and xanthine oxidase in potassium phosphate buffer (pH 7.8, 37°C) for 40 min and NBT was added. Blue formazan was then monitored spectro- photometrically at 550 nm. The inhibition of NBT reduction to 50% maximum by SOD enzyme was obtained and wasdefined as 1 nitrite unit (NU) of SOD activity (Sun & Oberley, 1988).
2.7. MDA Evaluation
Measurement of malondialdehyde levels (thiobarbituric acid reactive substances, TBARS) is in accordance with a method in which MDA at 100° C and pH = 2-3 reacts with thiobarbituric acid such that trichloroacetic acid and TBARS reagent have mixed with supernatant. After cooling on ice and centrifuging at 3000 rpm for 20 minutes, the supernatant light absorption was read at 532 nm (Oliver et al., 1990).
3. Statistical Analysis
The obtained results in this study were expressed as means ± S.E.M. Statistical analyses between experimental groups were carried out using repeated measurement of one way analysis of variance (ANOVA). The comparison between individual experimental groups was continued with complementary post-hoc Tukey test and p values less than 0.05 were considered as significant differences.
4. Results
4.1. Effect of Different Doses of Ferula Assa Foetida Gum Extract on the PTZ-Induced Seizure Intensity
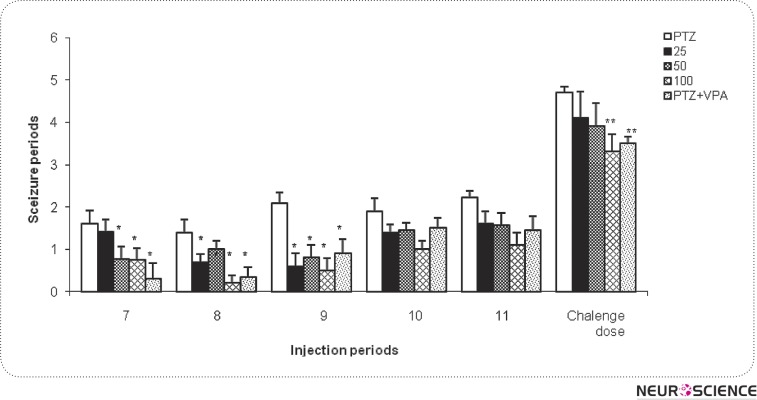
Statistical analysis of results (as shown in figure 1) indicates that Ferula Assa Foetida gum extract 25mg/kg could reduce seizure intensity significantly only at the 6th and 7th injections as compared to the PTZ group ) P< 0.05(. In addition, plant extract 50 mg/kg at the 6th, 7th and 9th injections was able to reduce seizure intensity significantly in comparison with PTZ group) P< 0.05(. Also, Ferula Assa Foetida gum extract 100 mg/kg in the 7th, 8th, and 9th injections, significantly reduced PTZ-induced seizure intensity as compared to the PTZ group)P< 0.05(. Finally, the last injection in the challenge dose of Ferula Assa Foetida gum extract (100 mg/kg) was able to reduce seizure rate significantly along with valproate) P< 0.01(. It is noteworthy that, in the positive control group, valproate (100 mg/kg) has reduced seizure intensity in most of the periods significantly (P < 0.05) relative to the PTZ group and had a more significant reducing effect in the challenge dose than in the other periods (P < 0.001).
Figure 1.
Effect of Ferula Assa Foetida pretreatment on the PTZ-induced kindling intensity. Ff shows Ferula Assa Foetida. *P < 0.05 and **P < 0.01 indicate significant differences as compared to PTZ-kindled group.
4.2. Effect of Different Doses of Ferula Assa Foetida Gum Extract on the Starting Time of Stage 5 of PTZ-Induced Seizure
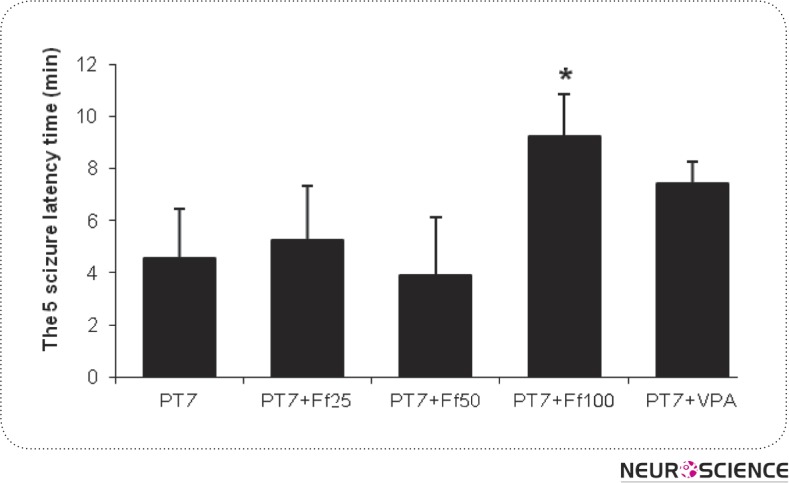
As it is indicated in figure 2, Ferula Assa Foetida gum extract 100 mg/kg has shown a significant increase in seizure starting time as compared to PTZ group)P< 0.05(. In the rest of the treatment groups, significant differences were not observed.
Figure 2.
Effect of valproate (100 mg/kg) and three doses of Ferula Assa Foetida (25, 50 and 100 mg/kg) on the latency to the onset of stage 4 seizure. n = 10 in each group. VPA and Ff indicate valproate and Ferula Assa Foetida respectively. *P < 0.05 indicate significant differences as compared to PTZ-kindled group
4.3. Effect of Different Doses of Ferula Assa Foetida Gum Extract on State 5 Duration Time
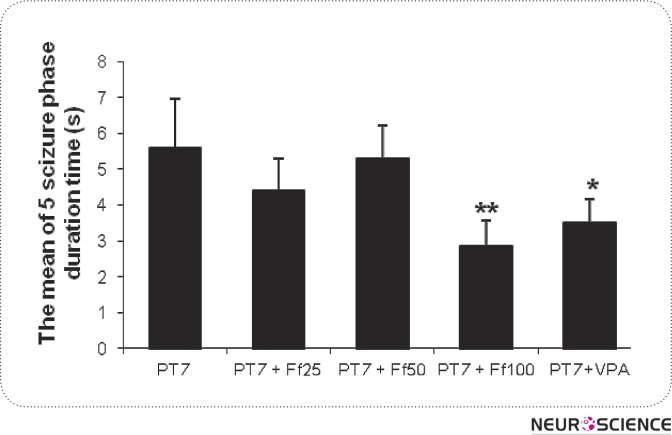
As it is shown in figure 3, only pretreatmented of mice with Ferula Assa Foetida gum extract 100 mg/kg and valproate 100 mg/kg are able to reduce the period that the mice remain in phase 5 of seizure significantly (P < 0.01 and P < 0.05 respectively).
Figure 3.
Effect of valproate (100 mg/kg) and three doses of Ferula Assa Foetida (25, 50 and 100 mg/kg) on the remaining time in the phase 5. n = 10 in each group. VPA and Ff indicate valproate and Ferula Assa Foetida respectively. *P < 0.05 shows significant difference as compared to PTZ-kindled group.
4.4. Effect of Different Doses of Ferula Assa Foetida Gum Extract on the NO and MDA Values, and SOD Activity
A comparison among the effects of different doses of Ferula Assa Foetida gum extract, valproate and PTZ on the biochemical factors of brain tissue that are the common oxidative stress markers, is shown in table 1. As it is shown in the table, in the PTZ-kindled group, MDA level of the brain tissue has increased significantly relative to the control group)P< 0.05( and SOD level has shown a significant reduction in this group compared to the control group)P< 0.05(. In this group, however, the NO level did not show any significant change compared to the control group.
Table 1.
The effect of valproate and three doses of Ferula Assa Foetida on the NO, MDA and SOD levels of brain tissue on the PTZ-kindled mice.
| Groups | SOD (U) | MDA ( nmol) | NO (mol) |
|---|---|---|---|
| Control | 0.127±0.004 | 17.69±1.89 | 0.527±0.027 |
| PTZ | 0.098±0.008 * | 25.63±2.11 * | 0.598±0.032 |
| PTZ + Valproate | 0.121±0.118 | 20.46±1.98 # | 0.398±0.042 * |
| PTZ + Ff 25 | 0.123±0.014 | 23.51±1.19 | 0.497±0.056 |
| PTZ + Ff 50 | 0.144±0.012 | 18.21±1.48 # | 0.401±0.019 # |
| PTZ + Ff 100 | 0.146±0.014 | 15.29±1.33 # # | 0.521±0.052 |
In the positive group which received valproate, the NO level showed a significant decrease compared to the control and PTZ groups (P< 0.05(. MDA levels in this group had significant reduction in comparison with the PTZ receiving group (P< 0.05(. However, valproate and different doses of plant extract were not able to induce any significant differences in SOD levels in the brain tissue in comparison with the PTZ and control groups.
In the experimental groups receiving different doses of plant extract, only Ferula Assa Foetida gum extract 50mg/kg induced significant reduction of the NO level compared with PTZ group)P< 0.05(. The mice which were pretreated with Ferula Assa Foetida gum extract 50 mg/kg and 100 mg/kg showed significant decrease of MDA levels compared to the PTZ group) P < 0.05 and P< 0.01 respectively (. Ferula Assa Foetida gum extract 25 mg/kg did not make any significant differences of enzymes amounts compared with PTZ and control groups.
Brain levels of NO, MDA and SOD are compared in six groups. In each group n = 10 and Ff indicates Ferula Assa Foetida * and # show significant differences as compared to control and PTZ-kindled groups respectively (P < 0.05).
5. Discussion
For centuries in the Iranian and Indian traditional medicine, Ferula Assa Foetida has been administered as an anti-seizure drug (Zargari, 1991). Additionally, in the western tradition of herbal medicine, it was used for the treatment of asthma, epilepsy, convulsions, and muscle cramps (Arky, 1996). In general, results of this research indicated that Ferula Assa Foetida gum extract has a lowering effect on seizure intensity and is able to enhance the seizure threshold. Our result is in consistence with previous studies that pointed to the anti-epileptic properties of this plant family in the PTZ model of epilepsy (Yajima, et al., 2000). All administrated doses of Ferula Assa Foetida gum extract could partly prevent the development of epilepsy. Ferula Assa Foetida gum extract at higher doses could significantly reduce the remaining period in the stage 5 of seizures. In addition, Ferula Assa Foetida gum extract 100 mg/kg postponed the starting time of phase 5 of the seizure. However, in a recent study it is reported that some species of Ferula did not suppress the PTZ-induced seizure (Bagheri, 2010). The discrepancy could rise from different parameters. The use of Ferula 300mg/kg in their experiment (which might be toxic), difference in the species of plant, the single dose of PTZ that they have used instead of chronic administration and the use of kindling in our experiment are some possible reasons for different results.
Rogawski and colleagues have pointed to this issue that PTZ causes epilepsy through the activation of glutamate receptors (NMDA) and stimulation of the calcium ions for entering into the nerve cells (Rogawski & Porter, 1995). Additionally, according to previous reports, PTZ induced epilepsy (absence epilepsy) could be inhibited by T-type calcium current lowering drugs such as ethosuximide (Coulter, Hugenard & Prince, 1989). Researchers have also shown that family members of this plant could exert their analgesic effects through stimulation of opioid receptors in the central nervous system (Fazly Bazaz et al, 1997). It has also been proven recently that opioid receptor stimulation has an anti-seizure effect (Yajima et al., 2000). Since the opioid receptor agonists mainly affects calcium channels and inhibits calcium entry into nerve cells (Werz & Macdonald, 1984), probably the ferula family, through the stimulation of opioid receptors with inhibition of calcium entry into post-synaptic neurons, could reduce nervous stimulation in the central nervous system and exert inhibitory effects on seizure.
In the present study, the maximum anti-epileptic response of Ferula Assa Foetida gum extract in terms of suppressing seizure attacks, the latency of epileptic response, and the duration of the epileptic statues was related to the dose of 100 mg/kg. This dose is the most effective dose of the plant in this field and the more the doses reduce, the more the effects are lowered. Therefore, it seems that these effects are dose dependent. Of course this issue can be investigated more thoroughly by increasing the number of cases.
The generated oxidative stress in the brain is a common mechanism of cellular damage in many acute neurological attacks, such as seizure activity and diseases like Alzheimer's Disease (Oliver et al., 1990). Also membrane lipids are full of unsaturated fatty acids such as arachidonic acid and most of them are susceptible to lipid peroxidation process which leads to the destruction of the membrane and hindering its functions (Kim et al, 2000; Mandegary, Sayyah & Heidari, 2004). Of course, in the normal system body, the harmful effects of oxidative stress and free radical are controlled to some extent by antioxidant systems such as SOD enzyme (Freitas R, 2009). In epileptic patients serum levels of antioxidants are reduced and due to elevation of free radical levels, lipid peroxidation will be increased (Sudha, Rao & Rao, 2001). Ilhan and colleagues also reported that PTZ will lead to seizure through the induction of oxidative stress, and that anti-oxidant administration significantly reduces both oxidative stress and PTZ-induced seizure (Ilhan, 2005). It is also expressed in other resources that probably PTZ is the initiator of membrane structure lysis process and it causes the release of lipid peroxidases and free radicals (Obay, 2008).
In the present study in the PTZ receiving group, SOD enzyme levels as an antioxidant enzyme reduced and MDA levels as an indicator of lipid peroxidation showed a significant increase indicating the oxidative stress effects of PTZ administration. Thus, in accordance with other studies, our research indicates that the increment in free radicals and oxidative stress which is induced by PTZ is probably one of the causes of epilepsy (Khalili et al., 2011).
In previous studies by Sayyah and his colleagues, it was indicated that approximately seventy percent of the plant extracts contain α and β- Pinene (Sayyah et al., 2001). Existence of monoterpenes including α-Pinene and β-,Pinene (with anti-epileptic properties) (Stefan et al, 2006) is reported in Ferula Assa Foetida (Dehpour et al, 2009). It is also shown that Pinene analogs prevent idiopathic epilepsy in prone mice (Guzmán-Gutiérrez, 2012). Therefore, from this information one can conclude that the suppressing effect of the extract on the PTZ-induced seizure may be mediated by Pinene compounds. Previous studies have identified that sesquiterpenes are able to exert inhibitory effects on arachidonic acid-metabolizing enzymes and NO synthesis enzyme (as a free radical). Regarding the existence of sesquiterpenes in the Ferula species (Dehpour et al, 2009) by which prevent the formation of metabolites than that caused by arachidonic acid metabolism in membranes, probably these plants act as antioxidants. In addition, flavonoid compounds with anti-oxidant properties are among the fractions of ferula plants and could be another candidate by which the anticonvulsant effect of ferula is occured.
NO is a molecule that is associated with regulation of neuronal stimulation ability and epileptic activity. Involvement of NO molecule in epilepsy and seizure making has been proven by numerous experiments via systemic injection of the NO synthetas inhibitor (Buisson et al., 1993). Furthermore, it has been shown that the NOS inhibitors act against acute seizures and progression of PTZ-induced kindling (Tsuda, Suzuki & Misawa, 1997). PTZ administration increases the NOS gene expression via activation of the calcium - calmodoline route in nerve cells and thus increases NO production in the brain (Swamy et al., 2010). The results of our study are also in consistence with this theory that NO levels are increased in PTZ receiving mice. NO increases cGMP in the cell, and the amino acid glutamate, as stimulatory neurotransmitter in the brain through increased cGMP levels causes increment of neuronal excitability (Oliveira et al., 1997). Taking together these evidences, one can conclude that additional NO in the brain increases cGMP that finally causes increment of neuronal activity and irritability, for providing an epileptic condition.
Our findings are also in consistence with previous studies that showed Ferula assa Foetida methanolic gum extract has NO scavengering properties (Dehpour et al., 2009) and probably the anticonvulsant effect of this extract is induced via NO reduction in mice tissue. Thus it seems that this plant is an effective plant in NO scavengering, and giving an absolute theory in this field needs more investigations.
In the body's normal physiological conditions, antioxidants defense system controls free radicals-induced tissue damage (Ilhan et al., 2006). Superoxide free radical is converted to hydrogen peroxide by the SOD enzyme. Thus, SOD enzyme protects cells against harmful superoxide radicals and the resulting oxidative stress. In this study, SOD enzyme activity in mouse brain tissue was significantly decreased in the PTZ group compared to the control group. Reduction of this antioxidant enzyme during seizure is probably due to excess consumption of this enzyme resulted from free radical production during PTZ-induced seizure. However, our plant could not scavenge the superoxide radical from the surface of the animal's brain via increasing the superoxide dismutase enzyme and had little effect in this regard.
MDA is also a lipid peroxidation marker that is caused by free radicals and therefore is an oxidation product (Ilhan et al., 2006). Significant increase of this index in PTZ group compared to the control group showed that during PTZ-induced seizures, free radicals are produced and neuronal membrane lipids suffer from oxidative stress induced peroxidation.
Considerable reduction of MDA levels in the plant extract treatment groups compared to the PTZ group implies that probably Ferula assa Foetida gum extract causes a decrease in oxidative damage and lipid peroxidation due to its antioxidant properties. In summary, according to the findings of present research, probably the lowering effects of hydro-alcoholic Ferula assa Foetida gum extracts on the PTZ-induced seizures is probably due to its antioxidant properties and decrease of oxidative stress.
References
- Arky, R. (1996) Physicians’ desk reference. Medical Economics Montvale. 50nd ed. New Jersey. 846 [Google Scholar]
- Atilla, I., Mustafa, I., Suat, K.Y. (2006) Pentylenetetrazol-induced kindling seizure attenuated by Ginkgo biloba extract (EGb 761) in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry, 30(8), 1504–1510 [DOI] [PubMed] [Google Scholar]
- Bagheri, S. M., Sahebkar, A., Gohari, A. R., Saeidnia, S., Malmir, M., Iranshahi, M. (2010) Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm. Biol, 48(3), 242–6 [DOI] [PubMed] [Google Scholar]
- Buisson, A., Lakhmeche, N., Verrecchia, C., Plotkine, M., Boulu, R. G. (1993) Nitric oxide: an endogenous anticonvulsant substance. NeuroReport., 4, 444–446 [PubMed] [Google Scholar]
- Chauhan, A., Chauhan, V. (2006) Review of Oxidative stress in autism. Pathophysiology, 13, 171–181 [DOI] [PubMed] [Google Scholar]
- Coulter, D. A., Hugenard, J. R., Prince, D. A. (1989) Characterization of the ethosuximide reduction of low-threshold calcium current in thalamic neurons. Annals Neurology., 25, 582–593 [DOI] [PubMed] [Google Scholar]
- Dehpour, A. A., Ebrahimzade, M. A., Nabavi, S. F., Nabavi, S. M. (2009) Antioxidant activity of the methanol extract of Ferula assa foetida and its essential oil composition. Grasas Y Aceites, 60(4), 405–412 [Google Scholar]
- Erakovic, V., Zupan, G., Varljen, J., Laginja, J., Simonic, A. (2001). Altered activities of rat brain metabolic enzymes caused bypentylenetetrazol kindling and pentylenetetrazolinduced seizures. Epilepsy Research 43, 165–173 [DOI] [PubMed] [Google Scholar]
- Evereklioglu, C., Turkoz, Y., Calis, M., Duygulu, F., Karabulut, A. B. (2004) Tumour necrosis factor a, lipid peroxidation and NO are increased and associated with decreased free-radical scavenging enzymes in patients with Weill Marchesani syndrome. Mediators of Inflammation, 13(3), 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazly Bazaz, B. S., Parsaei, H., Haririzadeh, G. (1997) Evaluation of antinociceptive and antimicrobial activities of galbanum plant (Ferula gummosa). Daru, 7, 1–22 [Google Scholar]
- Freitas, R. M. (2009) Investigation of oxidative stress involvement in hippocampus in epilepsy model induced by pilocarpine. Neurosci Lett., 462(3), 225–9 [DOI] [PubMed] [Google Scholar]
- Gorgi, A., Madeja, M. (2001) Lowering of the potassium concentration induces epileptiform activity in guinea pig hippocampal slices. Brain Res., 908, 130–139 [DOI] [PubMed] [Google Scholar]
- Guzmán-Gutiérrez, S. L., Gómez-Cansino, R., García-Zebadúa, J. C., Jiménez-Pérez, N. C., Reyes-Chilpa, R. (2012) Antidepressant activity of Litsea glaucescens essential oil: Identification of β-pinene and linalool as active principles. J Ethnopharmacol., 143(2), 673–9 [DOI] [PubMed] [Google Scholar]
- Ilhan, A., Iraz, M., Gurel, A., Armutcu, F., Kamilsi, S. (2005) Antiepileptogenic and antioxidant effects of Nigella sativa oil against pentylentetrazole-induced kindling in mice. Neuropharm., 49, 456–64 [DOI] [PubMed] [Google Scholar]
- Ilhan, A., Iraz, M., Kamisli, S., Yigitoglu, R. (2006) Pentylenetetrazol-induced Kindling seizure attenuated by Ginkgo biloba extract (Egb 761) in mice. Neuro-Psychopharmacology & Biological Psychiatry., 30, 1504–1510 [DOI] [PubMed] [Google Scholar]
- Khaleghi Ghadiri, M., Gorji, A. (2004) Natural remedies for impotence in medieval Persia. International Journal of Impotence Research, 16, 80–83 [DOI] [PubMed] [Google Scholar]
- Khalili M, Kiasalari Z, Roghani M, Azizi Y. (2011) Anticonvulsant and antioxidant effect of hydroalcoholic extract of Cyperus rotundus rhizome on pentylentetrazole-induced kindling model in male mice. Journal of Medicinal Plants Research, 5(7): 1140–1146 [Google Scholar]
- Kim, H. C., Jhoo, W. K., Bing, G., Shin, E. J., Wie, M. B., Kim, W. K., Ko, K. H. (2000) Phenidone prevents kainate-induced neurotoxicity via antioxidant mechanisms. Brain Res, 874(1): 15–23 [DOI] [PubMed] [Google Scholar]
- Levav, I., Stephenson, C., Theodore, W. H. (1999) Epilepsy in Latin America and the Caribbean: a survey on needs and resources. Pan Am J Public Health., 6(5), 342–345 [DOI] [PubMed] [Google Scholar]
- Mandegary, A., Sayyah, M., Heidari, M. R. (2004) Anti-nociceptive and Anti-inflammatory activity of the seed and root extracts of Ferula Gummosa Boiss in mice and rats. Daru., 12(2), 58–62 [Google Scholar]
- McNamara, J. O. (1999) Cellular and molecular basis of epilepsy. J.Neurosci., 14, 3413–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatar, F., Shams, Ardakani, M. (1999) Instructions for herbal therapy. Tehran. The Academy of Med Sci, 2, 73–75 [Google Scholar]
- Newall, C.A., Anderson, L. A., Phillipson, J. D. (1996) Herbal medicines. London: The pharmaceutical press., 96, 38–9 [Google Scholar]
- Obay, B. D., Tademir, E., Tümer, C., Bilgin, H. M., Atmaca, M. (2008) Dose dependent effects of ghrelin on pentylenetetrazole-induced oxidative stress in a rat seizure model. Peptides., 29(3), 448–455 [DOI] [PubMed] [Google Scholar]
- Oliver, C. N., Starke-Reed, P. E., Stadtman, E. R., Lin, G. J., Correy, J. M., Floyd, R,A. (1990) Oxidative damage to brain proteins, loss of glutamine synthetase activity and production of free radicals during ischemia/reperfusion induced injury to gerbil brain. Proc Natl Acad Sci USA., 87, 5144–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, P. R., Del-Bel, E. A., Oliveira, J. A. C., Mishra, P. K., Jobe, P. C., Carcia- Cairasco N. (1997) Anticonvulsant and proconvulsant roles of nitric oxide in experimental epilepsy models. Braz J Med Bio Res., 30(8), 971–979 [DOI] [PubMed] [Google Scholar]
- Rogawski, M. A., Porter, R. J. (1995) Antiepileptic drugs and pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacological Review., 42, 223–286 [PubMed] [Google Scholar]
- Rukmini, M. S., D'Souza, B., D'Souza, V. (2004) Superoxide dismutase and catalase activities and their correlation with malondialdehyde in schizophrenic patients. Indian Journal of Clinical Biochemistry., 19(2), 114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyah, M., Kamalinejad, M., Bahrami Hidage, R., Rustaiyan, A. (2001) Antiepileptic Potentional and Composition of the Fruit Essential Oil of Ferula Gummosa boiss. Iranian Biomed J., 2, 69–72 [Google Scholar]
- Sogut, S., Zoroglub, S. S., Zyurtc, H., Yılmazd, H. R., Zugurluc, F., Sivaslıe, E. (2003) Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clinica Chimica Acta., 331, 111–117 [DOI] [PubMed] [Google Scholar]
- Stefan, H., Lopes da silva, F. H., Loscher, W., Schmidt, D., Perucca, E., Brudie, M. (2006) J.Epilptogenesis and rational therapeutic strategies. Aeta Neural Scand., 113, 139–155 [DOI] [PubMed] [Google Scholar]
- Sudha, K., Rao, A. V., Rao, A. (2001) Oxidative stress and antioxidants in epilepsy. Clin. Chem., 303, 19–24 [DOI] [PubMed] [Google Scholar]
- Sun, Y., Oberley, L. W., Li, Y. (1988) A simple method for clinical assay of superoxide dismutase. Clinical Chemistry., 34, 497–500 [PubMed] [Google Scholar]
- Swamy, M., Salleh, M. J., Sirajudeen, K. N., Yusof, W. R., Chandran, G. (2010) Nitric oxide (NO), citrulline - no cycle enzymes, glutamine synthetase and oxidative stress in anoxia (hypobaric hypoxia) and reperfusion in rat brain. Int. J. Med. Sci., 7(3), 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabyi, S. M. (1989) In translate of Alhavi of Zakaria Razi. Tehran, Mashhad Med Sci University publication., 1, 127–153 [Google Scholar]
- Taghi, M. M. (1990) Tehran. In translate of Genuine authority of Ansari Shirazi. Razi med Distribution Company, 2, 136. [Google Scholar]
- Theodore, W. H., Fisher, R. (2007) Brain stimulation for epilepsy. Acta Neurochir Supp., 97(2), 261–272 [DOI] [PubMed] [Google Scholar]
- Tsuda, M., Suzuki, T., Misawa, M. (1997) Aggrivation of DM- CM-induced seizure by nitric oxide synthase inhibitors in mice. Life Sci., 60, 339–343 [DOI] [PubMed] [Google Scholar]
- Tuszkiewicz - Misztal, E., Opoka - Winiarska, V., Postêpski, J. (2000) Significance of dietary antioxidants for child proper development and health. Pediatr Pol., 75, 359–366 [Google Scholar]
- Werz, R. L., Macdonald, M. A. (1984) Dynorphin reduces voltage – dependent calcium conductance of mouse dorsal root ganglion neurons. Neuropept., 5, 253–6 [DOI] [PubMed] [Google Scholar]
- Yajima, Y., Naritaa, M., Takahashi-Nakano, Y., Misawa, M., Nagase, H., Mizoguchi, H. (2000) Effects of differential modulation of μ-, δ- and κ-opioid systems on bicuculline-induced convulsions in the mouse. Brain Res., 862, 120–26 [DOI] [PubMed] [Google Scholar]
- Zamani, S. (1999) Medicinal plants. Tehran Med. Sci. University, 2, 5–8 [Google Scholar]
- Zargari, A. (1991) Medicinal plants. Tehran Med. Sci. University, 5, 231–226 [Google Scholar]