Abstract
Introduction
Tanacetum sonbolii (Asteraceae) is an endemic species in Iran. In the present study, we examined the effects of Tanacetum sonbolii hydroalcoholic extract on the formalin test in mice.
Methods
126 Swiss albino mice weighing 230-280g were used as subjects. The formalin test was performed on two control groups (marked as intact and saline groups; n = 6 in each group) and an experimental group. In all groups, the formalin test was recorded for 60 min after administration of extract and drugs in mice.
Results
The results showed that Tanacetum sonbolii (150 and 300 mg/kg) produced significant antinociception in phase 2. In addition, different doses of Tanacetum sonbolii extract (600, 900 and 1200 mg/kg) also induced antinociceptive effects in phase1 and phase 2. On the other hand, morphine could induce antinociception in a dose-dependent manner. Diclofenac (10 mg/kg) failed to affect the pain scores compared to Tanacetum sonbolii (300 mg/kg) group.
Discussion
It seems that administration of hydroalcoholic extract of Tanacetum sonbolii has the potential to relieve pain through both central and peripheral mechanisms in persistent inflammatory nociception.
Keywords: Tanacetum Sonbolii (Asteraceae), Morphine, Formalin Test, Mice
1. Introduction
Since the existing antinociceptive drugs have a wide range of side effects, finding new antinociceptive compounds is a priority for researchers (Amin, Vyas, Attur, Leszczynska-Piziak, Patel, Weissmann& Abramson 1995). Today, researchers believe that plants could be a valuable resource of chemical compounds with powerful therapeutic effects. Therefore, the study of plant species traditionally used for reducing pains with the capacity to produce analgesics seems to be necessary (Mojtahedin, Tamaddonfard, & Zanbouri, 2009).
The genus Tanacetum is one of the largest and most widely distributed genera of the family Asteraceae, tribe Anthemideae. This genus is represented in Iran by 36 specie (Rajaei, Nejadsattari, Maassoumi, Mozaffarian, & Sonboli, 2011). Tanacetum sonbolii is an endemic species in west Azerbaijan, Iran. Tanacetum species are rich in essential oils and sesquiterpene lactones. Locally, a decoction prepared from aerial flowering part of Tanacetum sonbolii is used to treat intestinal disorder such as diarrhea. Some species of the genus Tanacetum L. i used in Turkish traditional medicine (Tabanca, Demirci, Demirci, Wedge, & Baser, 2007). Various remedies containing Tanacetum species are used in the treatment of arthritis, fever, migraine, menstrual disorders, stomachache, toothache, and insect bites (Norata , Passamonti, Pirillo, Violi& Catapano, 2007;Tabanca, Demirci, Demirci, Wedge& Baser, 2007;Zhou, Kou, & Stevenson, 1999). Additionally, Tanacetum microphyllum is useful for treating various inflammatory disorders(Abad, Bermejo& Villar, 2003). Bioactive compounds isolated from Tanacetum vulgare have also been reported to exhibit antibacterial and antihelminthic activities (Abad, Bermejo& Villar, 2003). This genus is found to contain acetylenes and sesquiterpene lactones and lactones are described amongst the major constituents in species belonging to the genus Tanacetum (Aljancic, Vajs, Bulatovic, Menkovic, & Milosavljevic, 2001; Mahmood, Kaul, & Singh, 2002). Moreover, other constituents such as aromatic compounds (e.g., camphor) and flavonoids (e.g., luteolin and apigenin) isolating from Tanacetum parthenium (Wu Chen, Wang, Kim, He, Haley-Zitlin& Huang, 2006). Tanacetum parthenium has a long history in the traditional medicine of Iran for the treatment of migraine (Mirjalili, Salehi, Sonboli& MohammadiVala, 2007), cancer (Koochek, 2002) and menstrual cramps (Avallone et al., 2000) (Mirjalili, 2007). Also, it is used as sedative (Besharati-Seidani, Jabbari, Yamini& Saharkhiz 2006;Parvini, Hosseini, & Bakhtiarian, 2007) , antimicrobial (Fidler, Loprinzi, O'Fallon, Leitch, Lee, Hayes, Novotny, Clemens-Schutjer, Bartel& Michalak, 1996) and anti-inflammatory agents (Besharati-Seidani, Jabbari, Yamini& Saharkhiz 2006;Koochek, Pipelzadeh& Mardan, 2002;Mirjalili, Salehi, Sonboli& MohammadiVala, 2007;Minaiyan, Ghassemi-Dehkordi& Mohammadzadeh, 2006), and other common stressrelated problems (Fidler Loprinzi, O'Fallon, Leitch, Lee, Hayes, Novotny, Clemens-Schutjer, Bartel& Michalak., 1996). In a recent study (Firozy, Talebpour, & Sonboli, 2012), several major components in the essential oil of Tanacetum sonbolii were characterized including α-cadinol (35.3%), globulol (20.1%), and 1,8-cineole (8.6%). Moreover, the ethyl acetate extract of this plant showed better β-carotene bleaching capacity with high level of total phenolics. In another study (Esmaeili, 2010), the antioxidant activity of ethanolic extract of Tanacetum sonbolii from Iran is reported to be moderate. Since Tanacetum sonbolii is known to be effective in treating inflammatory disorders, the present study was aimed to investigate the effect of hydro alcoholic extract of aerial flowering parts of Tanacetum sonbolii on tonic and acute inflammatory pain model associated with formalin in male mice.
2. Methods
2.1. Animals
A total of 126 Swiss albino mice (25-35 g) were supplied by Razi Research Center of Hesarak, Karaj, Iran. The animals were kept under standard laboratory conditions with tap water and regular mice chow ad libitum. The animals were housed in groups of 10-12 per cage (45cm×30cm×15cm) at temperature -controlled room (23±1 °C) and maintained on a 12-h light/dark cycle (light on at 07:00 h) with free access to standard rodent breeding diet and tap water. Each animal was used only once and killed immediately after the experiment. All experiments were executed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 80-23, revised 1996) and approved by the Research and Ethics Committee of Qazvin University of Medical Sciences, Qazvin, Iran.
2.2. Preparation of Extracts
The aerial parts of Tanacetum sonbolii were collected from Takab, Iran on June 2011, and a voucher herbarium specimen (998) was deposited in the herbarium of Medicinal Plants and Drugs Research Institute (MPH) of Shahid Beheshti University, Tehran, Iran. The aerial flowering parts were air-dried, protected from direct sunlight, and then powdered. The powdered plant material (50 g) was extracted three times with a mixture of absolute ethanol and distilled water (1:1) at room temperature overnight. The extracts were thoroughly mixed and concentrated under reduced pressure on a rotary evaporator, filtered and then lyophilized. The extract was stored in sealed vials at 4 °C until analysis.
2.3. Drug Administration
In the present study, different doses of extract (75, 150, 300, 600, and 900 mg/kg) were prepared. Diclofenac, a non-steroidal anti-inflammatory drug (10 mg/kg), and morphine, as an opioid receptor agonist receptor (1.5, 3, and 6 mg/kg), were dissolved in saline.
2.4. Formalin Test
Initially, animals were moved to the test room at least 1 hour before the commencement of the experiment. The mice were later acclimatized for 30 min in an acrylic observation chamber (15x30 cm in diameter and 30 cm in height) followed by subcutaneous injection of 50 μl of 2% formalin into the plantar surface of the right hind paw with a 30 gauge needle. Each mouse was then immediately returned to the observation box, and the behavioral recording commenced. A mirror, placed at a 45° angle beneath the box, permitted the observation of behaviors without moving the box. Pain behaviors was scored as follows: 0, the injected paw was not favored; 1, the injected paw had little or no weight placed on; 2, the injected paw was elevated and not in contact with any surface; and 3, the injected paw was licked or bit. The Scores were continuously observed for the duration of experiment (60 min). The mean nociceptive behaviors score for each 3-min interval was calculated as the weighted average of the number of seconds spent in each nociceptive behavioral response, from the start of the experiment. In each group, the behavioral responses of each mouse during the first phase (1-7 min), interphase (8-14 min), and the second phase (15-60 min) were separately evaluated.
2.5. Experimental Protocols
The formalin test was performed on two control groups (marked as intact and saline groups; n = 6 in each group) and an experimental group. In all groups, the formalin test was recorded for 60 min after administration of extract and drugs in mice.
2.5.1. Effect of Hydroalcoholic Extract of Tanacetum sonbolii on Formalin-induced Pain behaviors
To evaluate the effect of hydroalcoholic extract of Tanacetum sonboliiMozaff on antinociceptive responses, the study animals systemically received extract of Tanacetum sonbolii (75, 150, 300, 600, 900, and 1200 mg/kg; n = 6-8 in each group) and 30 min later, the painrelated behaviors were monitored for 60 min.
2.5.2. Effect of Morphine on Formalin-induced Pain Behaviors
To evaluate the effect of morphine on pain-related behaviors, the mice received morphine (1.6, 3, and 6 mg/kg; n = 7 in each group) 30 min before formalin injection. Recording of the nociceptive behaviors began immediately after formalin injection (time 0) and continued for 60 min.
2.5.3. Effect of Diclofenac on Formalin-induced Pain Behaviors
The effect of Diclofenac on antinociceptive responses was investigated through administration of this drug (10 mg/kg; n = 7 in each group), to the mice 30 min before formalin injection into the right hind paw. Recording of the nociceptive behaviors began immediately after formalin injection and continued for 60 min.
2.6. Statistical Analysis
The data are presented as mean ±SEM (standard error of mean). The mean nociceptive scores in each phase (I, interphase, and II) of the formalin test was analyzed using ANOVA followed by Dunnett's or Newman-Keuls post hoc test, as needed. Phase 1 (1–7 min), inter-phase (8–14 min), and the phase 2 (15–60 min) of the formalin test were analyzed separately. The defined level for statistical significance was P < 0.05.
3. Results
The results obtained for formalin pain score revealed that there were no significant differences in formalin pain scores at any time intervals between the intact (n = 7) and vehicle (Saline; n = 7) groups. Hence, in all experimental animals, the pain scores in formalin tests were compared to respective saline groups as control and the results considered as baseline in all time set intervals.
3.1. Effect of Hydroalcoholic Extract of Tanacetum sonbolii on Formalin-induced Pain Behaviors
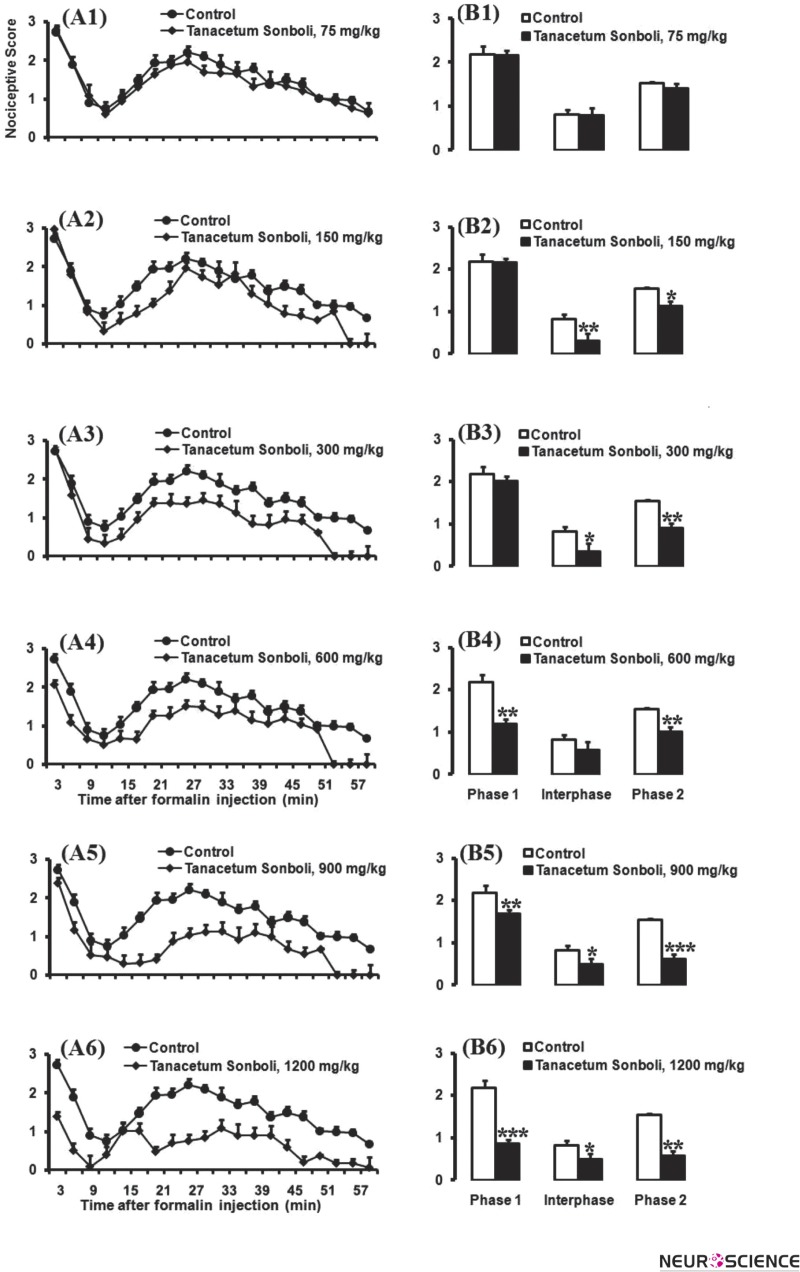
In this set of experiments, we evaluated different doses of extract of Tanacetum sonbolii (75, 150, 300, 600, 900, and 1200 mg/kg; n = 8-10 mice in each group) on antinociceptive response during a 60-min period, 30 min before the formalin test as persistent inflammatory model of pain. As shown in Fig. 1, the administration of different doses of extract of Tanacetum sonbolii (150 and 300 mg/kg) significantly induced antinociception in both inter phase and phase 2. Student t-test showed that there were significant differences in calculated areas under curve (AUC) values of pain scores. Moreover, higher doses of Tanacetum sonbolii extract (600, 900, and 1200 mg/kg; n = 8-10 mice in each group) also induced significant antinociceptive effect in phase1 and phase 2 at time set intervals and/or AUC calculated value in comparison with control group.
Figure 1.
Pain scores of formalin-induced nociceptive behaviors (mean ±S.E.M. of 8–10 mice per group) following injection of saline (10 ml/kg) or hydroalcoholic extract of Tanacetum sonbolii (A1-A6) measured every 3 min for a total 60-min period and bar chart for different doses of Tanacetum sonbolii 75, 150, 300, 600, 900, and 1200 mg/kg in the formalin test and control group (B1-B6). Columns represent the mean nociceptive score in each phase: phase 1 (minutes 1–7), inter phase (minutes 8–14), and phase 2 (minutes 15–60) (B1-B6). The mice received Tanacetum sonbolii Mozaff 30 min before formalin injection in the right hind paw. Recording of the nociceptive behaviors began immediately after formalin injection (time 0) and continued for 60 min. For clarity, each dose of Tanacetum sonbolii was plotted in separate graphs, with the same vehicle group in each.
*P < 0.05; ** P < 0.01 and ***P < 0.001 compared with saline
3.2. Effect of Morphine on Formalin-induced Pain Behaviors
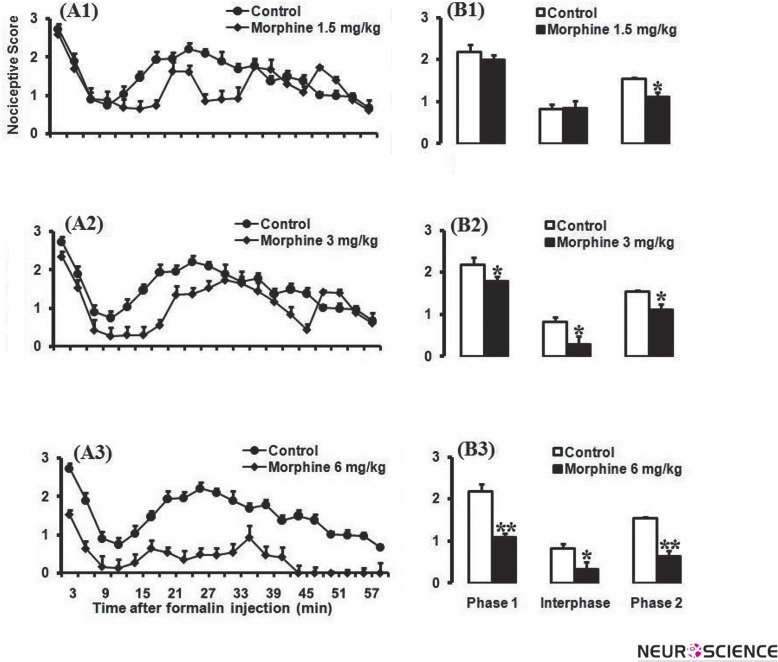
The antinociceptive effect of different doses of morphine (1.5, 3, and 6 mg/kg; n = 8–10 mice per group) was examined during a 60-min period in acute inflammatory model of pain. Administration of different doses of morphine (1.5, 3, and 6 mg/kg; n = 8–10 mice per group) caused a significant antinociceptive effect (Fig. A1–A3). Furthermore, as shown in Fig. B1–B3, the student t-test revealed that there were significant differences in calculated AUC values for pain scores in morphine group of experiments (Fig. 2).
Figure 2.
Pain scores of formalin-induced nociceptive behaviours (mean ±S.E.M. of 8–10 mice per group) following injection of saline (10 ml/kg) or morphine (A1-A3) measured every 3 minutes for a total 60 minutes (A) and bar chart for different doses of morphine 1.5, 3, and 6 mg/kg in the formalin test and control site (B1-B3). Columns represent the mean nociceptive score in each phase: phase 1 (minutes 1–7), inter phase (minutes 8–14), and phase 2 (minutes 15–60) (B1-B3). The mice received morphine 30 minutes before formalin injection in the right hind paw. Recording of the nociceptive behaviors began immediately after formalin injection (time 0) and continued for 60 minutes. For clarity, each dose of morphine was plotted in separate graphs, with the same control group in each.
*P < 0.05; ***P < 0.001 compared with saline
3.3. Effect of Diclofenac on Formalin-induced Pain Behaviors
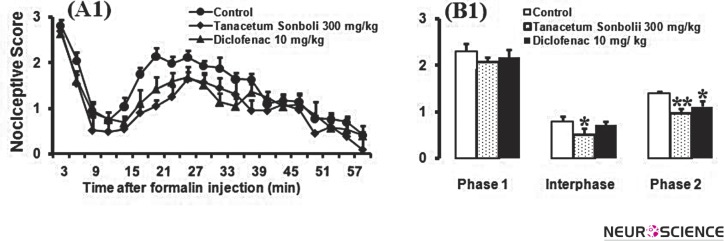
In this set of experiments, we examined the antinociception effects of Diclofenac (10 mg/kg), as a nonsteroidal anti-inflammatory drug, on formalin-induced pain behaviors in formalin test during a 60-min period. As shown in Fig. 3B-1, one-way ANOVA followed by Newman-Keuls multiple comparison test revealed that the administration of Diclofenac (10 mg/kg) significantly decreased the antinociceptive effect demonstrated by calculated AUC values for pain scores compared to those found in phase 2 [F(2,26) = 14.08, P < 0.0001] for control group . However, Diclofenac (10 mg/kg) in phase 1 [F(2,26) = 0.5547, P= 0.5547] and inter phase [F(2,26) = 3.029, P= 0.0672] failed to significantly decrease the antinociceptive effect. On the other hand, Diclofenac (10 mg/kg) failed to affect the pain scores at time set intervals and/or AUC calculated values compared with those in Tanacetum sonbolii (300, mg/kg; n = 6) group.
Figure 3.
Pain scores of formalin induced nociceptive behaviors (mean ±S.E.M. of 8–10 mice per group) following injection of saline (10 ml/kg) or Diclofenac measured every 3 minutes for a total 60 minutes (A) and bar chart for different doses of morphine 1.5, 3, and 6 mg/kg in the formalin test and control site (B1-B3). Columns represent the mean nociceptive score in each phase: phase 1 (minutes 1–7), inter phase (minutes 8–14), and phase 2 (minutes 15–60) (B). The mice received the drug 30 minutes before formalin injection in the right hind paw. Recording of the nociceptive behaviors began immediately after formalin injection (time 0) and continued for 60 minutes.
*P < 0.05; **P < 0.01; and ***P < 0.001 compared with saline
4. Discussion
The purpose of this study was to evaluate the involvement of the hydroalcoholic extract of Tanacetum sonbolii in antinociceptive responses during persistent inflammatory pain model in mice. The major findings were (a) administration of hydroalcoholic extract of Tanacetum sonbolii dose-dependently decreased the formalin-induced nociceptive behaviors (b) administration of morphine dose-dependently induced antinociception in formalin test (c) systemic administration of Diclofenac produced antinociception in phase 2.
We showed that administration of hydroalcoholic extract of Tanacetum sonbolii dose-dependently decreased the formalin-induced nociceptive behaviors. There is an increasing attention over herbal extracts due to their potentially positive action against certain diseases including pain, cancer, diabetes, atherosclerosis, and coronary heart diseases. Several species of the genus Tanacetum are traditionally used in a diversity of health conditions including pain, inflammation, respiratory and gastrointestinal diseases (Bukhari, Jabbari, Yamini& Saharkhiz, 2007). The results of the present study demonstrated significant antinociceptive effects of hydroalcoholic extract of Tanacetum sonbolii in the first phase of the formalin test at doses of 600, 900, and 1200 mg/kg. Also, significant antinociceptive effects were shown in the second phase at doses of 150, 300, 600, 900, and 1200 mg/kg.
In this study, we compared the antinociceptive properties of Tanacetum sonbolii with Morphine and Diclofenac as positive control for their abilities to decrease the formalininduced nociceptive behaviors. Peripherally acting drugs, such as Diclofenac only decreased formalin-induced nociceptive behaviors in the second phase (Castaneda Hernandez, Granados-Soto, & Ortiz, 2005). Therefore, it seems that the hydroalcoholic extract of Tanacetum sonbolii affect peripherally and centrally at low and high doses, respectively. Furthermore, the effect of hydroalcoholic extract of Tanacetum sonbolii on both phases of the formalin test at high doses suggests that the action of this extract may be resulted from its peripheral and centrally effects which needs to be examined by further investigations. In Esmaeili et al. (2010) study, the HPLC analysis revealed the presence of various compounds in Tanacetum sonbolii. These compounds were recorded at 290, 340 and 360 nm confirming the presence of caffeic acid, ferulic acid, luteolin, apigenin, and rutin as the main compounds in plant extracts. Furthermore, p-coumaric acid, quercetin, and kaempferol were not traceable in their samples (Esmaeili, Sonboli, & Ayyari, 2010). Caffeic acid is found in the plant leaves and it is reported that this compound has anti-inflammatory activity (da Cunha Duma, Assreuy, Buzzi, Niero, Campos& Calixto, 2004; Norata, Marchesi, Passamonti, Pirillo, Violi& Catapano, 2007) and might be considered for antinociceptive effect of hydroalcoholic extract of Tanacetum sonbolii. Several mechanisms of action were suggested for antinociceptive and anti-inflammatory effects of this herbal extract and some studies emphasized on participation of the opioidergic system and GABAergic inhibitory mechanisms.
In conclusion, our data suggest that the administration of hydroalcoholic extract of Tanacetum sonbolii can relieve pain through both central and peripheral mechanisms in persistent inflammatory nociception. However, further pharmacological investigations are needed to elucidate the hypothesis concerning the actual role of opioid and other receptors in antinociceptive effect of Tanacetum sonbolii extract in animal model of persistent inflammatory pain.
Acknowledgments
This research was supported by a grant from Qazvin University of Medical Sciences, Qazvin, Iran. The authors wish to express their gratitude to Dr. Ali Pahlevan for his meticulous work on revision of the final English version of the manuscript.
References
- Abad, M.J., Bermejo, P., Villar, A. M. (2003). The phytochemical and pharmacological investigations on Tanacetum microphyllum. Recent Research Developments in Life Sciences, 1, 371–95 [Google Scholar]
- Aljancic, I., Vajs, V., Bulatovic, V., Menkovic, N., & Milosavljevic, S. (2001). Parthenolide from the aerial parts of Tanacetum larvatum. Biochem Syst Ecol, 29(6), 655–57 [DOI] [PubMed] [Google Scholar]
- Amin, A. R., Vyas, P., Attur, M., Leszczynska-Piziak, J., Patel, I. R., Weissmann, G., & Abramson, S.B. (1995). The mode of action of aspirin-like drugs: effect on inducible nitric oxide synthase. Proc Natl Acad Sci U S A, 92(17), 7926–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallone, R., Zanoli, P., Puia, G., Kleinschnitz, M., Schreier, P., & Baraldi, M. (2000). Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol, 59(11), 1387–94 [DOI] [PubMed] [Google Scholar]
- Besharati-Seidani, A., Jabbari, A., Yamini, Y., Saharkhiz, M.J. (2006). Rapid extraction and analysis of volatile organic compounds of Iranian feverfew (Tanacetum parthenium) using headspace solvent microextraction (HSME), and gas chromatography/mass spectrometry. Flavour and Fragrance Journal, 21, 502–9 [Google Scholar]
- Bukhari, I. A., Khan, R. A., Gilani, A. U., Shah, A. J., Hussain, J., & Ahmad, V. U. (2007). The analgesic, anti-inflammatory and calcium antagonist potential of Tanacetum artemisioides. Archive of Pharmacilogy Reserch, 30(3), 303–12 [DOI] [PubMed] [Google Scholar]
- Castaneda-Hernandez, G., Granados-Soto, V., & Ortiz, M. I. (2005). Pinacidil increases diclofenac antinociception in the formalin test. Proceedings of the Western Pharmacology Society's, 48, 55–8 [PubMed] [Google Scholar]
- Da Cunha, F. M., Duma, D., Assreuy, J., Buzzi, F. C., Niero, R., & Campos, M. M. (2004). Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties. Free Radical Research, 38(11), 1241–53 [DOI] [PubMed] [Google Scholar]
- Esmaeili, M. A., Sonboli, A., Ayyari, M. (2010). Antioxidant and protective properties of six Tanacetum species against hydrogen peroxide-induced oxidative stress in K562 cell line: A comparative study. Food Chemistry, 121, 148–55 [Google Scholar]
- Fidler, P., Loprinzi, C. L., O'Fallon, J. R., Leitch, J. M., Lee, J. K, & Hayes, D. L. (1996). Prospective evaluation of a chamomile mouthwash for prevention of 5-FU-induced oral mucositis. Cancer, 77(3), 522–25 [DOI] [PubMed] [Google Scholar]
- Firozy, M., Talebpour, Z., & Sonboli, A. (2012). Essential oil composition and antioxidant activities of the various extracts of Tanacetum sonbolii Mozaff. (Asteraceae) from Iran. Natural Product Research, 26(23), 2204–7 [DOI] [PubMed] [Google Scholar]
- Koochek, M. H., Pipelzadeh, M. H., & Mardan, H. (2002). The effectiveness of Viola odorata in the prevention and treatment of formalin-induced lung damage in the rat. Spices & Medicinal Plants, 10, 95–103 [Google Scholar]
- Mahmood, U., Kaul, V. K., & Singh, B. (2002). Sesquiterpene and long chain ester from Tanacetum longifolium. Phytochemistry, 61(8), 913–17 [DOI] [PubMed] [Google Scholar]
- Minaiyan, M., Ghassemi-Dehkordi, N., & Mohammadzadeh, B. (2006). Anti-ulcer effect of Tripleurospermumdisciforme (C.A. Mey) Shultz Bip on pylorus ligated (Shay) rats. Reasearch in Pharmaceutical Sciences, 1, 15–21 [Google Scholar]
- Mirjalili, M. H., Salehi, P., Sonboli, A., & Mohammadi-Vala, M. (2007). Essential oil composition of feverfew ( Tanacetum parthenium ) in wild and cultivated populations from Iran. Chemistry of Natural Compounds, 43, 181–82 [Google Scholar]
- Mojtahedin, A., Tamaddonfard, E., & Zanbouri, A. (2009). Role of central muscarinic cholinergic receptors in the formalininduced pain in rats. Indian Journal of Pharmacology, 41(3), 144–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norata, G. D., Marchesi, P., Passamonti, S., Pirillo, A., Violi, F., & Catapano, A. L. (2007). Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis, 191(2), 265–271 [DOI] [PubMed] [Google Scholar]
- Parvini, S., Hosseini, M. J., & Bakhtiarian, A. (2007). The Study of Analgesic Effects and Acute Toxicity of Tripleurospermum disciforme in Rats by Formalin Test. Toxicology Mechanisms and Methods, 17(9), 575–80 [DOI] [PubMed] [Google Scholar]
- Rajaei, P., Nejadsattari, T., Masoumi, A. A., Mozaffarian, V., & Sonboli, A. (2011). Micromorphology of glandular hairs, biological activity and composition of the essential oil of Tanacetum fisherae (Asteraceae-Anthemideae) from Iran. Natural product communications, 6(2), 259–62 [PubMed] [Google Scholar]
- Tabanca, N., Demirci, F., Demirci, B., Wedge, D. E., & Baser, K. H. (2007). Composition, enantiomeric distribution, and antimicrobial activity of Tanacetum argenteum subsp. flabellifolium essential oil. Journal of Pharmaceutical and Biomedical Analysis, 45(5), 714–19 [DOI] [PubMed] [Google Scholar]
- Wu, C., Chen, F., Wang, X., Kim, H.J., & He, G. (2006). Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chemistery, 96, 220–27 [Google Scholar]
- Zhou, J. Z., Kou, X., & Stevenson, D. (1999). Rapid extraction and high-performance liquid chromatographic determination of parthenolide in feverfew (Tanacetum parthenium). Journal of agricultural and food chemistry, 47(3), 1018–22 [DOI] [PubMed] [Google Scholar]