Abstract
Introduction
Excessive olivo-cerebellar burst-firing occurs during harmaline-induced tremor. We hypothesized that antiglutamatergic agents would suppress harmaline tremor. From this point of view, the aim of the present study was to investigate the effects of riluzole on harmaline-induced tremor in rat.
Methods
Four groups of Wistar rats weighing 80–100 g were injected with harmaline (30 mg/ kg i.p.) for inducing experimental tremors. The rats in group 1 served as control, whereas the animals in groups 2, 3 and 4 were also given riluzole intraperitonealy at doses of 2, 4 and 8 mg/ kg 30 min before and 90 min after harmaline administration. The onset latency, intensity and duration of tremor were recorded.
Results
The results of this study demonstrated that riluzole could significantly increase latency period, and reduce duration and intensity of tremor.
Discussion
It is concluded that pretreatment of riluzole can ameliorate harmaline-induced tremor in rats.
Keywords: Harmaline, Tremor, Riluzole, Rat
1. Introduction
Essential tremor (ET) is regarded as one of the most common neurological disorders of adults, with a prevalence similar to or greater than that of stroke, Alzheimer disease, migraine and lumbosacral pain syndromes and is as much as 20 times more prevalent than Parkinson's disease. The incidence of ET increases with advancing age, but it is fairly common in all age groups and almost equal in men and women. Although ET is often viewed as a benign problem, almost all the patients with ET are disabled to some extent (e.g., difficulty with or inability to perform daily activities such as writing, feeding, or dressing) and around 15% are sufficiently motorically impaired due to continuous high amplitude shaking that they are unable to continue to work (Tariq et al., 2002; Bain et al., 1994). Essential tremors result from both physiologic and pathologic processes in the nervous system, and always involve the interaction of central and peripheral nervous systems. Due to lack of understanding of the basic mechanism and origin of tremors, it is difficult to develop pharmacological agents with selective and specific antitremor activity.
The cerebellum is generally accepted to be involved in the control and integration of motor processes as well as of cognitive functions. Several studies suggested an important role of this structure in pathological processes underlying different forms of tremor, schizophrenia, attention deficit, and Parkinson's disease (PD). Abnormal activation of climbing glutamatergic fibers, arising from the inferior olive, which induces synchronous firing of Purkinje cells of the cerebellar cortex, assumed to be a “pacemaker” responsible for development of essential tremor (DeLong, 1978).
Harmaline, a derivative of β-carboline is a well-known tremorgenic compound suggested as a model for essential tremor in animals (Miwa, 2007). Harmaline induces the action and postural tremor in several animal species which is manifested by the tremor of fore and hind limbs, head tremor or generalized tremor of the whole body (Miwa, 2007; Milner et al., 1995; Wang, & Fowler, 2001; Kolasiewicz et al., 2009). Oscillation frequency in this tremor decreases with increasing the weight of an animal and is equal to 11 – 14 Hz in mice, 10 – 12 Hz in rats and 8 – 10 Hz in monkeys (Miwa, 2007). However, synchronous activation of the olivo-cerebellar pathway and release of glutamate in the cerebellum which acts at NMDA and AMPA receptors is suggested to be a primary cause of the harmaline-induced tremor (Miwa, 2007; Beitz & Saxon, 2004; Paterson, et al., 2009).
Much attention focused on neuroprotection as a strategy in therapies for neurodegenerative diseases as means of preserving the synaptic and/or intrinsic properties of neurons. Riluzole (2-amino-6-trifluoromethoxybenzothiazole), a Ca2+-dependent K+ channel opener with antiglutamatergic activity can slow the progression of disease in patients with amyotrophic lateral sclerosis and is approved for treatment of this disorder in several countries (Bensimon et al., 1994; Lacomblez et al., 1996). Riluzole is neuroprotective in animal models of acute and chronic neurodegenerative disease (Stutzmann & Doble, 1994; Mary et al., 1995), including rat and primate models of PD (Benazzouz et al., 1995) and 3-acethyl pyridine model of ataxia in rats (Janahmadi et al., 2009).
Riluzole inhibits the presynaptic release of excitatory amino acids both in vitro (Martin et al., 1993) and in vivo (Cheramy et al., 1992). This mechanism proposed as the one responsible for the drug's neuroprotective effects. However, riluzole is shown to have modulatory effects on the membrane ion channels.
Thus, the beneficial neuroprotective action of riluzole on the motor diseases and its antiglutamatergic effect, prompted us to examine the role of riluzole on harmaline-induced tremor in rats.
2. Methods
2.1. Drugs and Chemicals
Harmaline HCl and Riluzole were purchased from Sigma-Aldrich, Germany. All compounds were prepared freshly on the day of the experiment. Harmaline HCl and riluzole dissolved in normal saline and solution of 0.1NHCl, respectively.
2.2. Animals
Male Wistar rats (weighing 80-100 g) were purchased from Pastur Institue of Iran. The animals were kept under standard laboratory conditions with a 12-h light/dark cycle and given ad libitum access to food and water throughout the experiments. The experimental procedures approved by the Animal Ethics Committee of Damghan University.
2.3. Tremor Induction
On the day of experimentation, the animals were transferred to the testing room and left 1 h for acclimatization. They were randomly assigned to experimental groups and experimental tremors produced in four groups (ten rats each) of animals by a single injection of harmaline (30 mg/kg) intraperitoneally as previously described (Krahl et al., 2004). The rats in group 1 served as control (harmaline only), whereas, animals in group 2, 3, and 4 were given riluzole (i.p) at doses of 2, 4 and 8 mg/kg 30 min before and 90 min after harmaline administration. Riluzole dosage was selected on the basis of earlier reports which demonstrated its neuroprotective effects in ataxic rat (Janahmadi et al., 2009). The occurrence of tremors was rated by an observer blinded to treatment protocol. The period between the injection of harmaline and the appearance of the first symptoms of tremors was recorded as the time of onset of tremors. The duration of tremors was recorded as the time between onset and complete disappearance of tremors. The intensity of tremors at tremor onset and then every 30 min after harmaline administration over a 240-min period (until the tremors completely subsided and the animals became normal) were recorded. The clinical grading of tremor intensity was done according to Arshaduddin et al. method (2004) as follows: 0: no tremor, 1: mild tremor, 2: moderate intermittent tremor, 3: moderate persistent tremor and 4: pronounced severe tremor (Arshaduddin et al., 2004).
2.4. Statistical Analysis
The results expressed as the means ± S.E.M. The analysis of intensity, duration, and latency period were undertaken using one-way ANOVA followed by Dunnett's multiple comparison tests. Differences with p-value <0.05 were considered significant.
3. Results
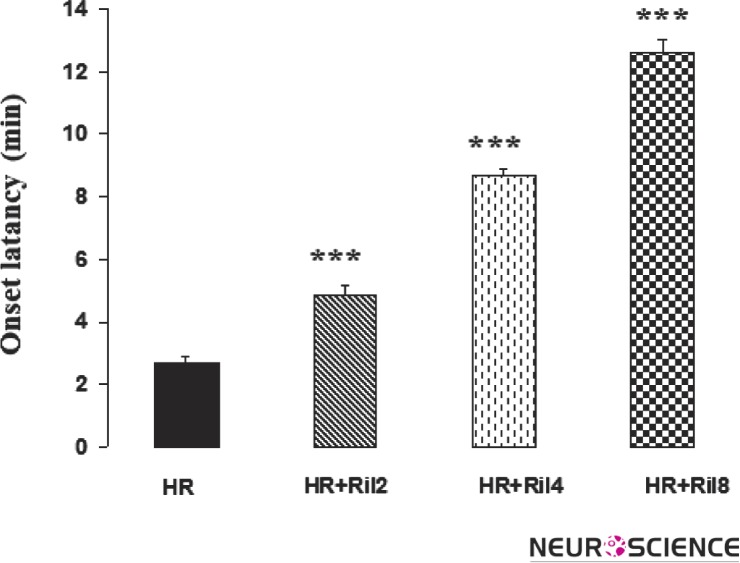
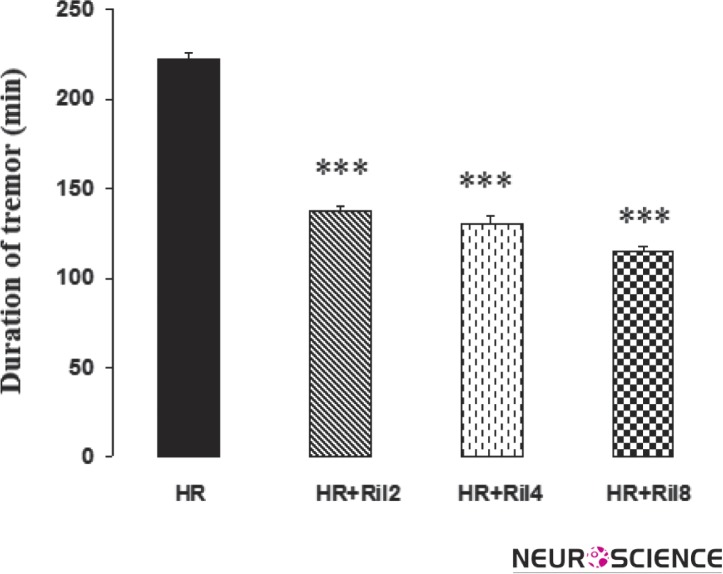
Harmaline administration to rats induced the characteristic pattern of tremors starting within 2.67±0.23 min following administration and lasted for more than 4 h. The tremor intensity at 5 min following harmaline administration was 3.1±0.11. The data showed that treatment of rats with riluzole (2, 4 and 8 mg/kg) produced significant decrease (137.5±3.35, 130.5±3, 115±2.7 min; p < 0.01, dose 2, 4 and 8 mg/kg, respectively) in the duration of harmaline-induced tremor (222±3.4 min). Riluzole also increased duration of onset of tremor (p < 0.01) in harmaline treated rats (Figs. 1, 2 and 3).
Figure 1.
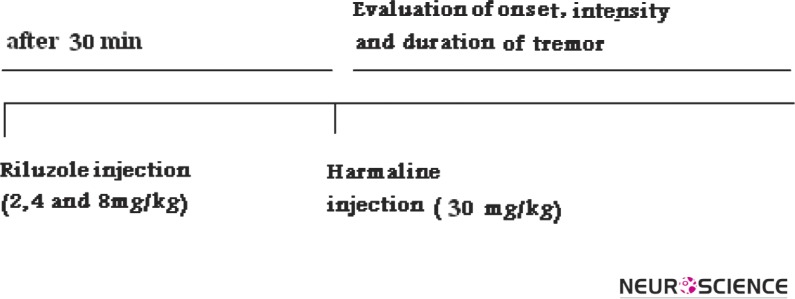
Timeline of the behavioral procedures.
Figure 2. Effect of riluzole on onset latency of harmaline induced tremor.
Riluzole significantly reduced onset latency of tremor than harmaline treated group. The values are mean ± SEM. HR, Harmaline; Ril2, Riluzole 2mg/kg; Ril4, Riluzole 4mg/kg; Ril8, Riluzole 8 mg/kg.
Figure 3. Effect of riluzole on duration of harmaline induced tremor.
Riluzole significantly reduced duration of tremor in comparision to harmaline group. The values are mean ± SEM. HR, Harmaline; Ril2, Riluzole 2mg/kg; Ril4, Riluzole 4mg/kg; Ril8, Riluzole 8 mg/kg.
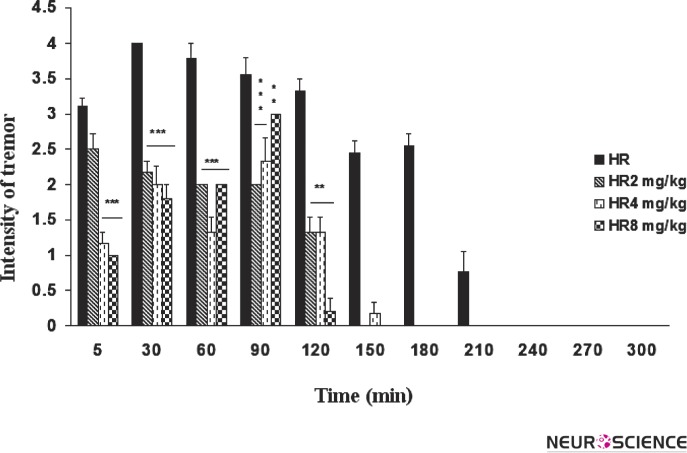
The intensity of the tremor at 30, 60 and 120 min following harmaline remained unchanged throughout this period. Treatment with riluzole in the doses of 2, 4 and 8 mg/kg resulted in a significant reduction in the intensity of the tremor at 30 min (p < 0.001), 60 min (p < 0.001), 90 min (p < 0.001, dose 2 and 4 mg/kg; p < 0.01, dose 8 mg/kg, respectively), 120 min (p < 0.001) and 150 min (p < 0.001, dose 4 mg/kg) (Fig. 4).
Figure 4. Effect of riluzole on tremor intensity (total score over the 210 min period in 30 min bins) after harmaline administration (30 mg/kg, i.p.).
The values are mean ± SEM, **P < 0.01 and ***P < 0.001 as compared with harmaline treated animals. HR, Harmaline; Ril2, Riluzole 2mg/kg; Ril4, Riluzole 4mg/kg; Ril8, Riluzole 8 mg/kg.
4. Discussion
The results of this study showed significant reduction of harmaline-induced tremors by riluzole. Reduction of harmaline-induced tremors was evident from delay in onset, decreased duration and severity of tremors in the rats treated with riluzole (Figs. 2, 3 and 4).
Administration of harmaline to rats induces severe tremor as previously reported beginning within a few minutes, and lasted for at least 3.5 h (Arshaduddin et al., 2004). The tremor was more pronounced upon movement and when the animals were not leaning against the wall of the cage. The motor activity was reduced and urination and defecation were increased. Harmaline produces tremors in the 8–12 Hz frequency range that is believed to be due to an enhancement of neuronal synchrony and rhythmicity in the inferior olive (Wilms et al.,1999). Among other experimental models of essential tremor, the harmaline model is one of particular interest since it presents behavioral, metabolic imaging and pharmacological similarities to essential tremor. Furthermore, β-carbolines related to harmaline, are detected in patients suffering from essential tremor and induce severe tremors in humans (Pennes & Hoch, 1957).
Riluzole in the doses of 2, 4 and 8 mg/kg produced a highly significant reduction in the duration and intensity of harmaline induced tremor (Figs. 3 and 4). Our results are in agreement with earlier investigator who observed beneficial effects of memantine (NMDA receptor antagonist) on the harmaline model of transient action tremor (Iseri et al., 2011).
It is thought that genetic factors, age, ethnicity, and several toxic agents (such as b-carboline alkaloids and lead) are the risk factors for the occurrence of ET (Louis, 2005); however, the underlying mechanisms are not clearly identified. A widely accepted hypothesis for its etiology is the functional disturbance of olivo-cerebellar pathways (Wills et al., 1995; Deuschl and Elble, 2000). This hypothesis was derived from clinical and animal studies (treated by b-carbolines) showing over activity of cerebellar cortex and deep nuclei (Wills et al., 1995; Wilms et al., 1999). On the other hand, the results of recent studies (e.g., swelling of purkinje cell axons and purkinje cell loss) supported the idea that ET may have neurodegenerative features (Louis, 2010).
Experimental and clinical evidences implicated the role of glutamatergic system in ET (Eblen et al., 1996; Málly et al., 1996). Harmaline preferentially enhances synaptic activity of climbing fibers which originate in the inferior olive (Ryder et al., 2006). The activation of the climbing fiber system increases the level of excitatory amino acids, nitric oxide, and cGMP in the cerebellum that is believed to be responsible for molecular mechanism underlying harmaline-induced tremor, increased cerebellar blood flow, and neurotoxicity (O'Hearn and Molliver, 1997; Yang and Iadecola, 1998; Zhang et al., 2003; Beitzand Saxon, 2004).
As excessive synchrony among olivo-cerebellar ensembles could underlie ET, we hypothesized that glutamate release enhancement resulting of olivo-cerebellar synchrony following of harmaline administration can probably underlie tremor. Therefore, we hypothesized that glutamate content reduction by inhibitor of glutamatergic neurotransmission may suppress tremor.
Riluzole (2-amino-6-trigluoromethoxy benzothiazole) has neuroprotective, anticonvulsant, anxiolytic and anesthetic qualities. These effects are mediated by blockade of glutamate transmission, stabilizing of sodium channels and blockade of g-aminobutyric acid (GABA) reuptake. Riluzole-induced neuroprotection was observed in various animal models of injury (Malgouris et al., 1989; Stutzmann et al., 1997) and in neurodegenerative pathologies, such as ALS and Parkinson's disease (Estevez & Stutzmann, 1995; Jacquin & and Gruol, 1999; Noh et al., 2000). Janahmadi et al. (2009), reported that combined riluzole (4 mg/kg) and 3-AP treatment can relatively improve 3-Ap induced ataxia in rat (Janahmadi et al., 2009).
Also, there are several reports that riluzole can inhibit voltage gated Na+ channels (Herbert et al., 1994; Wang et al., 2008). Na+ channel blockage is proposed to be responsible for prevention of epilepsy and cellular death induced by this neuroprotective agent (Herbertet al., 1994).
Our previous study demonstrated that riluzole had therapeutic effects on harmaline-induced tremor and ataxia in rats. Riluzole could significantly reduce cerebellar glutamate content and Purkinje cell loss in harmalinetreated rat (Rahimi Shoormasti et al., 2012). In the present study, antiglutamatergic action of riluzole may presumably counteract the neurotoxic glutamatergic effect of harmaline and following tremor.
In conclusion, we suggest that riluzole might be a potentially useful choice in the treatment of essential tremor. The protective effect of riluzole probably related to its inhibitory effect on glutamatergic neurotransmission or its modulatory effect on ion channels. Therefore, the mechanism of action of riluzole in harmaline-induced tremor should be further investigated in more details.
Acknowledgment
We acknowledge Damghan University, Damghan, Iran for supporting this work.
References
- Arshaduddin, M., Al Kadasah, S., Biary, N., Al Deeb, S., Al Moutaery, K., & Tariq, M. (2004). Citalopram, a selective serotonin reuptake inhibitor augments harmaline-induced tremor in rats. Behav Brain Res, 153, 15–20 [DOI] [PubMed] [Google Scholar]
- Bain, P.G., Findley, L.J., Thompson, P.D., Gresty, M.A., Rothwell, J.C., Harding, A.E., Marsolen, C.D. (1994). A study of hereditary essential tremor. Brain, 117, 805–824 [DOI] [PubMed] [Google Scholar]
- Beitz, A.J., & Saxon, D. (2004). Harmaline-induced climbing fiber activation causes amino acid and peptide release in the rodent cerebellar cortex and a unique temporal pattern of Fos expression in the olivo-cerebellar pathway. J Neurocytol, 33, 49–74 [DOI] [PubMed] [Google Scholar]
- Benazzouz, A., Boraud, T., Dubedat, P., Boireau, A., Stutzmann, J.M., & Gross, C. (1995). Riluzole prevents MPTP-induced Parkinsonism in the rhesus monkey: a pilot study. Eur J Pharmacol, 284, 299–307 [DOI] [PubMed] [Google Scholar]
- Bensimon, G., Lacomblez, L., & Meininger, V. (1994). A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/ Riluzole Study Group. N Engl J Med, 330, 585–591.10 [DOI] [PubMed] [Google Scholar]
- Cheramy, A., Barbeito, L., Godeheu, G., & Glowinski, J. (1992). Riluzole inhibits the release of glutamate in the caudate nucleus of the cat in vivo. Neurosci Lett, 147, 209–12 [DOI] [PubMed] [Google Scholar]
- DeLong, M.R. (1978). Possible involvement of central pacemakers in clinical disorders of movement. Fed Proc, 37, 2171–2175 [PubMed] [Google Scholar]
- Deuschl, G., & Elble, R.J. (2000). The pathophysiology of essential tremor. Neurology, 54, 14–20 [PubMed] [Google Scholar]
- Eblen, F., Loschmann, P.A., Wullner, U., Turski, L., & Klockgether, T. (1996). Effects of 7 nitroindazole, NG-nitro-L-arginine, and D-CPPene on harmaline-induced postural tremor, Nmethyl-D-aspartate-induced seizures, and lisuride-induced rotations in rats with nigral 6-hydroxydopamine lesions. Eur J Pharmacol, 28, 9–16 [DOI] [PubMed] [Google Scholar]
- Estevez, A.G., & Stutzmann, J.M. (1995). Barbeito L. Protective effect of riluzole on excitatory amino acid-mediated neurotoxicity in motoneuron-enriched cultures. Eur J Pharmacol, 280, 47–53 [DOI] [PubMed] [Google Scholar]
- Herbert, T., Drapeau, P., Pradier, L., & Dunn, R.J. (1994). Block of the rat brain IIA sodium channel alpha subunit by the neuroprotective drug riluzole. Mol Pharmacol, 45, 1055–60 [PubMed] [Google Scholar]
- Iseri, P.K., Karson, A., Gullu, K. M., Akman, O., Kokturk, S., Yardýmoglu, M., Erturk, S., & Ates, N. (2011). The effect of memantine in harmaline-induced tremor and neurodegeneration. Neuropharmacology, 61(4), 715–23 [DOI] [PubMed] [Google Scholar]
- Jacquin, T.D., & Gruol, D.L. (1999). Ca2+ regulation of a large conductance K+ channel in cultured rat cerebellar Purkinje neurons. Eur J Neurosci, 11, 735–739 [DOI] [PubMed] [Google Scholar]
- Janahmadi, M., Goudarzi, I., Kaffashian, M.R., Behzadi, G., Fathollahi, Y., & Hajizadeh, S. (2009). Co-treatment with riluzole, a neuroprotective drug, ameliorates the 3-acetylpyridine-induced neurotoxicity in cerebellar Purkinje neurones of rats: behavioural and electrophysiological evidence. Neurotoxicology, 30, 393–402 [DOI] [PubMed] [Google Scholar]
- Kolasiewicz, W., Kuter, K., Wardas, J. and Ossowska, K. (2009). Role of the metabotropic receptor subtype 1 in the harmaline-induced tremor in rats. J Neural Transm, 116, 1059–1063 [DOI] [PubMed] [Google Scholar]
- Krahl, S.E., Martin, F.C., & Handforth, A. (2004). Vagus nerve stimulation inhibits harmaline-induced tremor. Brain Research, 1011, 135–138 [DOI] [PubMed] [Google Scholar]
- Lacomblez, L., Bensimon, G., Leigh, P.N., Guillet, P., & Meininger, V. (1996). Dose-ranging study of riluzole in amyotrophic lateral sclerosis: amyotrophic lateral sclerosis/riluzole study group II. Lancet, 374, 1425–1431.11 [DOI] [PubMed] [Google Scholar]
- Louis, E.D., & Ferreira, J.J. (2010). How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord, 25(5), 534–541 [DOI] [PubMed] [Google Scholar]
- Louis, E.D. (2005). Essential tremor. Lancet Neurol, 4, 100–110 [DOI] [PubMed] [Google Scholar]
- Malgouris, C., Bardot, F., Daniel, M., Pellis, F., Rataud, J., Uzan, A., Blanchard, J.C., & Laduron, P.M. (1989). Riluzole, a novel antiglutamate, prevents memory loss and hippocampal neuronal damage in ischemic gerbils. J Neurosci, 9, 3720–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málly, J., Baranyi, M., & Vizi, E.S. (1996). Change in the concentrations of amino acids in CSF and serum of patients with essential tremor. J Neural Transm, 103, 555–560 [DOI] [PubMed] [Google Scholar]
- Mary, V., Wahl, F., & Stutzmann, J.M. (1995). Effect of riluzole on quinolate-induced neural damage in rats: comparison with blockers of glutamate neurotransmission. Neurosci Lett, 201, 92–96 [DOI] [PubMed] [Google Scholar]
- Milner, T.E., Cadoret, G., Lessard, L., & Smith, A.M. (1995). EMG analysis of harmaline-induced tremor in normal and three strains of mutant mice with Purkinje cell degeneration and the role of the inferior olive. J Neurophysiol, 73, 2568–2577 [DOI] [PubMed] [Google Scholar]
- Miwa, H. (2007). Rodent models of tremor. Cerebellum, 6, 66–72 [DOI] [PubMed] [Google Scholar]
- Noh, K.M., Hwang, J.Y., Shin, H.C., & Koh, J.Y. (2000). A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol Dis, 7, 375–383 [DOI] [PubMed] [Google Scholar]
- O'Hearn, E., & Molliver, M.E. (1997). The olivocerebellar projection mediates ibogaine induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci, 17, 8828–8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, N.E., Malekiani, S.A., Foreman, M.M., Olivier, B., & Hanania, T. (2009). Pharmacological characterization of harmalineinduced tremor activity in mice. Eur J Pharmacol, 616, 73–80 [DOI] [PubMed] [Google Scholar]
- Pennes, H.H., & Hoch, P.H. (1957). Psychotomimetics, clinical and theoretical considera-tions: harmine, WIN-2299 and Nalline. Am J Psychiatry, 113, 887–892 [DOI] [PubMed] [Google Scholar]
- Rahimi Shoormasti, F., Goudarzi, I., Lashkarbolouki, T., Abrari, K, Elahdadi Salmani, M., & Goudarzi, A. (2012). Effects of riluzole on harmaline induced tremor and ataxia in rats: Biochemical,histological and behavioral studies. European Journal of Pharmacology, 69, 40–47 [DOI] [PubMed] [Google Scholar]
- Ryder, J.W., Falcone, J.F., Manro, J.R., Svensson, K.A., & Merchant, K.M. (2006). Pharmacological characterization of cGMP regulation by the biarylpropylsulfonamide class of positive, allosteric modulators of alpha-amino-3hydroxy 5-methyl-4-isoxazolepropionic acid receptors. J Pharmacol Exp Ther, 319, 293–298 [DOI] [PubMed] [Google Scholar]
- Stutzmann, J.M., & Doble, A. (1994). Blockade of glutaminergic transmission and neuroprotection: the strange case of riluzole, in Neurodegenerative Disease. Jolles G., & Stutzmann J. M., eds, (pp. 205–214). Academic Press, San Diego [Google Scholar]
- Stutzmann, J.M., Wahl, F., Pratt, J., Mary, V., Reibaud, M., Tecoult, E., & Rataud, J. (1997). Neuroprotective profile of riluzole in vivo models of acute neurodegenerative diseases. CNS Drugs Rev, 3, 83–101 [Google Scholar]
- Tariq, M., Arshaduddin, M., Biary, N., Al Moutaery, K., & Al Deeb, S. (2002). 2 -Deoxy-D-glucose attenuates harmaline induced tremors in rats. Brain Research, 945, 212–218 [DOI] [PubMed] [Google Scholar]
- Wang, G., & Fowler, S.C. (2001). Concurrent quantification of tremor and depression of locomotor activity induced in rats by harmaline and physostigmine. Psychopharmacology, 158, 273–80 [DOI] [PubMed] [Google Scholar]
- Wang, Y.J., Lin, M.W., Lin, A.A., & Wu, S.N. (2008). Riluzoleinduced block of voltage-gated Na+ current and activation of BKCa channels in cultured differentiated human skeletal muscle cells. Life Sci, 82, 1–20 [DOI] [PubMed] [Google Scholar]
- Wills, A.J., Jenkins, I.H., Thompson, P.D., Findley, L.J., & Brooks, D.J. (1995). A positron emission tomography study of cerebral activation associated with essential and writing tremor. Arch Neurol, 52, 299–305 [DOI] [PubMed] [Google Scholar]
- Wilms, H., Sievers, J., & Deusc hl, G. (1999). Animal models of tremor. Mov Disord, 14, 557–571 [DOI] [PubMed] [Google Scholar]
- Yang, G., & Iadecola, C. (1998). Activation of cerebellar climbing fibers increases cerebellar blood flow: role of glutamate receptors, nitric oxide, and cGMP. Stroke, 29, 499–507 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Forster, C., Milner, T.A., & Iadecola, C. (2003). Attenuation of activity-induced increases in cerebellar blood flow by lesion of the inferior olive. Am J Physiol Heart Circ Physiol, 285, H1177–H82 [DOI] [PubMed] [Google Scholar]