Abstract
Introduction
Temporal lobe epilepsy (TLE) is a long lasting neurological disorder in which patients suffer from spontaneous seizures. New treatments with novel mechanisms of action are needed to help those patients whose seizures are resistant to available drugs. In this study, we investigated the possible neuroprotective effect of berberine in an intrahippocampal kainate model of TLE in rat.
Methods
In the present study, the anticonvulsant and antioxidant effects of intraperitoneal administration of berberine (25, 50 and 100 mg/kg), was evaluated in intrahippocampal kainate (4µg)-induced TLE in rats.
Results
The results showed that the kainate rats exhibit acute and spontaneous seizures in 24 hours and two weeks after intrahippocampal kainic acid injection. Administration of berberine, significantly decreased the Racine score and rate of incidence of seizure in kainate rats (P<0.05). On the other hand, berberine ameliorated the lipid peroxidation (P<0.001) and nitrite (P<0.001) level, but had no effect on SOD activity.
Discussion
These data suggest that berberine pretreatment could attenuate spontaneous recurrent seizures. Since, administration of berberine decreased lipid peroxidation in kainate rats, it seems that berberine favorable effect is due to its effectiveness in lessening of oxidative stress in rat.
Keywords: Kainic Acid, Berberine, Seizure, Hippocampus, Oxidative Stress, Rat
1. Introduction
One of the most well-known epilepsy in humans is temporal lobe epilepsy (TLE). TLE is related to a visible abnormal structural change in the hippocampus which is known as hippocampal sclerosis. This condition is characterized by a specific pattern of neuronal loss (Jokeit and Schacher, 2004). The most susceptible neurons are in the CA1 region and in the hilus of the dentate gyrus (Sloviter, 2005).
Neuronal death in the hippocampal region causes seizures. Because brain damage is necessary for developing of temporal lobe epilepsy (Sharma et al. 2007); therefore, hippocampus injury must be involved in most models (Jokeit and Schacher, 2004). Kainic acid (KA)-induced brain damage was used as a model for temporal lobe epilepsy and other excitotoxic neurodegenerative disorders (Sperk, 1994). Accumulating evidence indicates that hippocampal oxidative stress might be involved in KA-induced neurotoxicity in vivo (Floreani et al., 1997; Kim et al., 2000a, b; Shin et al., 2008) and in vitro (Kim et al., 2008). Activation of KA receptors results in intracellular signaling cascades including nitric oxide synthase (NOS) activation, free radical formation, and mitochondrial dysfunction, which in turn, result in inflammatory responses, cytokine expression and oxidative stress via reactive oxygen or nitrogen species (Carrasco et al., 2000; Lehtimäki et al., 2003). Because of the central nervous system high lipid content, it is highly susceptible to free-radical-mediated damage and inflammatory reactions that play an important role in the neuronal death processes (Perry et al., 2002; Migliore et al., 2005; Ashrafi et al., 2007).
With excessive stimulation of excitatory amino acid receptors, the development of radical scavengers and the maintenance of low ROS levels for neuroprotection are unavoidable (Sumanont et al., 2006). Thus, agents with antioxidant and anti-inflammatory properties are suggested to be useful in this condition.
Berberine is an isoquinoline alkaloid that is found in some plants principally berberis. It has some beneficial effect on anxiety, nociception, inflammation, psychosis, depression, and amnesia (Imanshahidi and Hosseinzadeh, 2008; Kulkarni and Dhir, 2008; Kulkarni and Dhir, 2010). Previous studies indicated that berberine could attenuate neuronal damage in ischemia-reperfusion model (Yoo et al., 2006), and in model mice of autoimmune encephalomyelitis (Ma et al., 2010). In addition, berberine showed neuroprotective effects on stroke (Zhou et al., 2008) and focal cerebral ischemia models (Xiao et al., 2007). It was shown that berberine intensify neuronal cell survival and differentiation in hippocampus and other sites of rat brain (Lim, 2008). Some studies showed that berberine exerts an antioxidant action on corpus cavernosum smooth muscle cells (Tan et al., 2007).
Accordingly, this study was designed to determine the possible protective effect of berberine against kainate model of epilepsy in rats by determining some biochemical parameters of stress oxidative and status epilepticus and recurrent seizure.
2. Methods
2.1. Animals
Male albino Wistar rats (Pasteur's Institute, Tehran, Iran) weighing 250–280 g (10–12 weeks old) were housed in an air-conditioned colony room on a light/ dark cycle (21–23 °C and a humidity of 30–40%) and supplied with standard pelleted diet and tap water ad. The procedures were made to minimize the number of animals used and their suffering.
2.2. Experimental Procedure
The rats (n=63) were randomly allocated and unequally grouped into seven groups: Sham-operated (SH; n=7); vehicle-treated SH (n=7); berberine (100 mg/Kg)-treated SH (n=7); kainate (n=12); berberine (25 mg/Kg)-treated kainate (n=10); berberine (50 mg/Kg)-treated kainate (n=10) and berberine (100 mg/Kg) -treated kainate (n=10) rats. For stereotaxic surgery, the rats were anesthetized with a combination of ketamin (100 mg/Kg, i.p.) and xylazine (5 mg/Kg, i.p.), placed in a Stoelting stereotaxic apparatus (incisor bar -3.3 mm, ear bars positioned symmetrically). The scalp was cleaned with iodine solution and incised on the midline, and a burr hole drilled through the skull. The animals in kainate group were unilaterally injected in the dorsal hippocampus with 5 µl of normal saline containing 0.4 µg/µl kainic acid (Sigma Chemicals, USA). Berberine (Sigma Chemicals, USA) was dissolved in propylene glycol and administered intraperitoneally daily for one week before surgery. The vehicle-treated SH rats were infused with an equivalent volume of normal salin in the same stereotaxic coordinates and received daily propylene glycol (i.p.) for one week.
The progression of kainate-induced seizures was scored according to Racine's standard classification: stage 0, no reaction; stage 1, stereotype mounting, eye blinking, and/or mild facial clonus; stage 2, head nodding and/ or several facial clonus; stage 3, myoclonic jerks in the forelimbs; stage 4, clonic convulsions in the forelimbs with rearing; and stage 5, generalized clonic convulsions associated with loss of balance (Racine, Okujava, & Chipashvili, 1972).
2.3. Behavioral Monitoring
In the first 24 hours post-surgery, all animals were evaluated for status epilepticus. At third week post-surgery, the animals were also assessed for behavioral progression of kainate-induced seizures 5 h/day for five consecutive days to record the chronic phases of seizures.
2.4. Determination of Hippocampal MDA Concentration
After evaluating of seizure behavior, the rats were anesthetized with ketamine (100 mg/kg) and decapitated. Hippocampi were isolated and blotted dry, and then weighed and prepared as a 5% tissue homogenate in ice-cold 0.9% saline solution. After centrifugation (1000g, 4 °C, 10 min), the supernatant was aliquoted and stored at -80°C until assayed. The concentration of malondialdehyde (MDA) was used as a marker of lipid peroxidation index and calculated by measuring thiobarbituric acid reactive substances (TBARS) in the supernatant as described previously (Roghani & Baluchnejadmojarad, 2009).
Briefly, trichloroacetic acid and TBARS reagent were added to aliquots of the supernatant, which subsequently mixed and incubated at 100 _C for 80 min. After cooling on ice, the samples were centrifuged at 1000g for 10 min, and the absorbance of the supernatant was read at 532 nm. The results of TBARS measurements were expressed as MDA equivalents, using tetraethoxypropane as standard.
2.5. Measurement of Hippocampal SOD Activity
The supernatant of hippocampal homogenate was obtained as described above. Superoxide dismutase (SOD) activity was measured as previously reported (Roghani & Baluchnejadmojarad, 2011). Briefly, supernatant was incubated with xanthine and xanthine oxidase in potassium phosphate buffer (pH 7.8, 37 °C) for 40 min, and then nitroblue tetrazolium (NBT) was added. Thereafter, blue formazan was monitored spectrophotometrically at 550 nm. The amount of protein that inhibited NBT reduction to 50% maximum was defined as 1 nitrite unit (NU) of SOD activity.
2.6. Assay of Hippocampal Nitrite Concentration
Supernatant nitrite (NO2-) content was assayed by the Griess Method (Roghani & Baluchnejadmojarad, 2011). The compound of NO has a short half-life and is rapidly converted to the stable end products nitrate (NO2- and NO3-). In the assay used in this study, NO3- is converted to NO2- by cadmium, and this is followed by color development with Griess reagent (sulfanilamide and N-naphthyl ethylenediamine) in acidic medium. The absorbance was determined using a spectrophotometer at 540 nm.
2.7. Protein Assay
The protein content of the supernatant was measured by the Bradford method, using bovine serum albumin (Sigma Chemical, St. Louis, MO) as the standard (Bradford, 1976).
2.8. Statistical Analysis
All statistical analysis was performed using SigmaStat software (version 3.5). The values were expressed as means±SEM. To compare the experimental groups, non-behavioral data were analyzed using one-way ANOVA followed by Tukey's post-hoc test. Seizure-related behavioral data were analyzed using the nonparametric Kruskal–Wallis test. Percentage of rats with spontaneous seizure was examined by x2 test. In all calculations, a difference at p<0.05 was regarded as significant.
3. Results
3.1. Behavior Observation
According to Racine's standard classification, the seizures reached class 5 during the acute period (24 hours after kainic acid administration) in 66.6% (8 of 12) of rats treated with kainic acid. Administration of berberine at doses of 25, 50 and 100 mg/kg decreased the class 5 to 40% and 10%, respectively (Table 1). Behavioral data showed that berberine could significantly decrease the number of spontaneous seizures incidence two weeks after surgery (Table 2). The sham, vehicle-treated SH and berberine -treated SH rats showed no acute or spontaneous seizures.
Table 1.
Numbers and rates of status epilepticus in each group
| Group | Number | Rate (%) |
|---|---|---|
| Sham(n=7) | 0 | 0 |
| Berberine (10 mg/Kg)-treated SH(n=7) | 0 | 0 |
| Kainate (n=12) | 8 | 66.6 |
| Berberine (25 mg/kg)-Kainate (n=10) | 4 | 40* |
| Berberine (50 mg/kg)-Kainate (n=10) | 4 | 40* |
| Berberine (100 mg/kg)-Kainate (n=10) | 1 | 10** |
χ2 test
P<0.01
P<0.005 compared with Kainate group; Seizure activity was observed for five hours during the first day post-surgery.
Table 2.
Numbers and rates of spontaneous seizures in each group
| Group | Number | Rate (%) |
|---|---|---|
| Sham(n=7) | 0 | 0 |
| Berberine (10 mg/Kg)-treated SH(n=7) | 0 | 0 |
| Kainate (n=12) | 5 | 41.6 |
| Berberine (25 mg/kg)- Kainate (n=10) | 5 | 50* |
| Berberine (50 mg/kg)- Kainate (n=10) | 3 | 30** |
| Berberine (100 mg/kg)- Kainate (n=10) | 2 | 20** |
χ2 test
P<0.05
P<0.01 compared with Kainate group; Two weeks post-surgery, seizure activity was observed for five hours/per day during five days.
3.2. Markers of Oxidative Stress
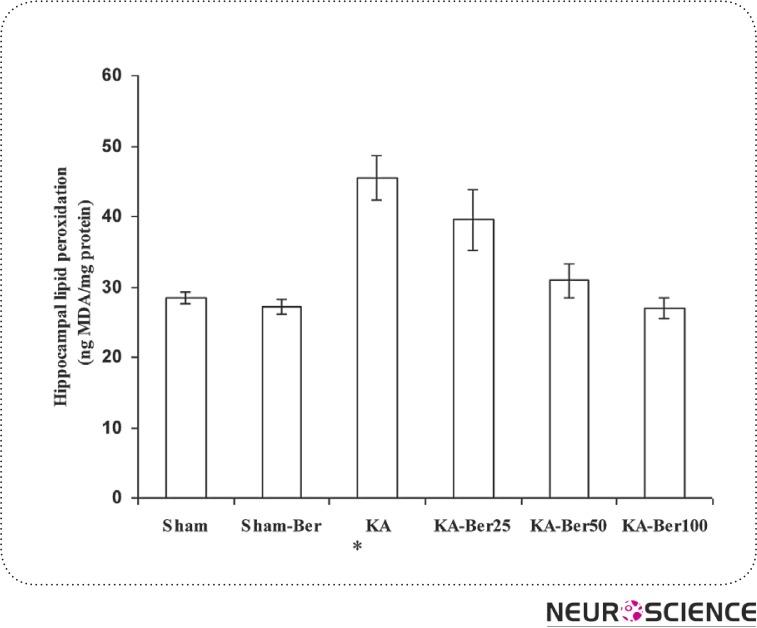
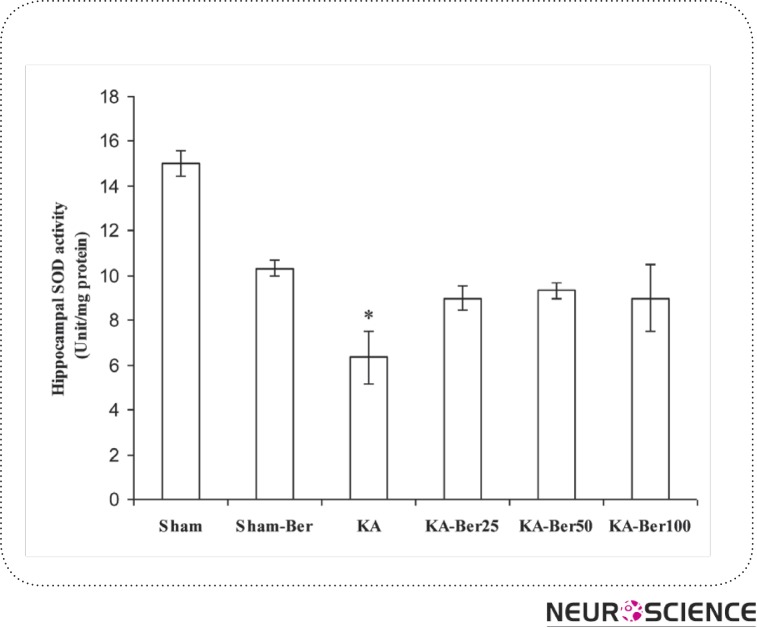
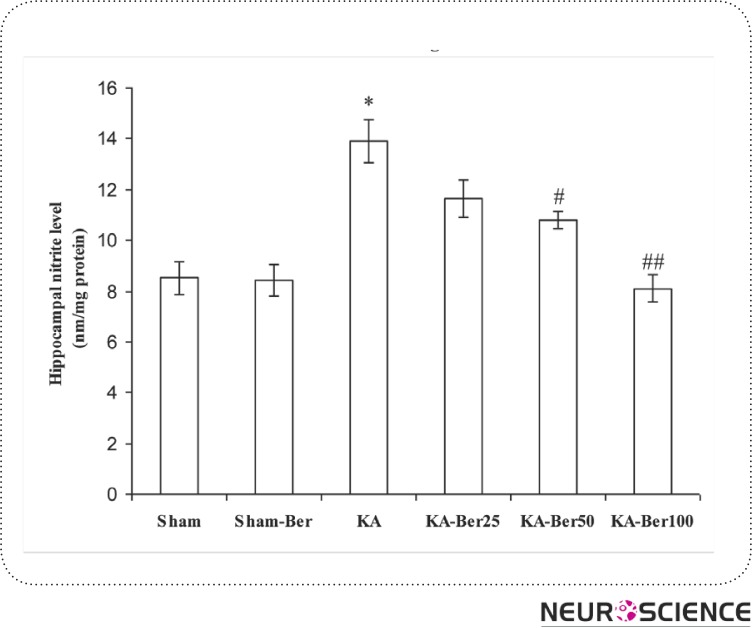
Kainic acid injection caused a considerable elevation in the levels of MDA (45.51 ± 3.22 ng/mg protein; p<0.01), nitrite (13.92 ± 0.86 nmol/ mg protein; p<0.001), and a significant reduction in SOD activity (6.33 ± 1.2 unit/mg protein; p<0.001) in hippocampal tissue (Figs. 1–3) compared to the sham-operated group (MDA, 28.43 ± 0.78 ng/mg protein; nitrite, 8.53 ± 0.65 nmol/mg protein; SOD 15 ± 0.57 unit/mg protein). Pretreatment of epileptic rats with 25, 50 and 100 mg/kg berberine significantly lowered the increased MDA content at all three doses (p<0.01- p<0.001). But, the content of nitrite decreased only with 100 mg/kg berberine and pretreatment of kainic acid injected rats with berberine has no effect on SOD activity. Meanwhile, berberine pretreatment of sham group did not cause a significant change in hippocampal level of MDA and nitrite and activity of SOD compared to sham group.
Figure 1.
The effect of berberine on malondialdehyde (MDA) concentration in hippocampal homogenate from different groups. The animals were treated with berberine at doses of 25, 50 and 100 mg/kg/day before intrahippocampal kainate injection. *p<0.01 (in comparison with sham); #p<0.05, ##p<0.01 (in comparison with kainate). (means±SEM)
Figure 3.
The effect of berberine on superoxide dismutase (SOD) activity in hippocampal homogenate from different groups. The animals were treated with berberine at doses of 25, 50 and 100 mg/kg/day before intrahippocampal kainate injection. *p<0.005 (in comparison with sham). (means±SEM)
Figure 2.
The effect of berberine on nitrite content in hippocampal homogenate from different groups. The animals were treated with berberine at doses of 25, 50 and 100 mg/kg/day before intrahippocampal kainate injection. *p<0.01 (in comparison with sham); #p<0.005, ##p<0.001 (in comparison with kainate). (means±SEM)
4. Discussion
This study reveals that intrahippocampal administration of kainic acid was followed with acute and chronic spontaneous seizures, intensifying of MDA and nitrite level and weakening of SOD activity. Pretreatment of kainate rats with berberine improved spontaneous seizures and oxidative stress at a dose-dependent manner.
Since, in the KA-induced epileptic model, the basis of activation of KA ionotropic glutamate receptors is similar to human TLE, so it seems that this model is an advantageous procedure for determination of the involved mechanisms of epileptic discharge in the limbic system including the hippocampus and amygdale that have key role in its acute and chronic spontaneous seizures.
Some studies showed that KA administration caused the level of protein oxidation, lipid peroxidation and 8-hydroxy-2-deoxyguanosine (8-OHdG), as oxidative marker for DNA damage, to be increased in the hippocampus and cerebral cortex (Kim et al., 1997; Tang et al. 1998). In addition, in some region of hippocampus including CA1, CA3 and the dentate hilus KA with activation of ionotropic glutamate receptors could conduct neurons to inadequate O2 utilization, reduced ATP production, inordinate production of ROS, NO, and peroxynitrite with consequent impairment of cell component including lipids, proteins, and DNA. On the other hand, some evidences demonstrated that KA also caused a decrease in reduced form of glutathione (GSH) levels in the hippocampus (Shin et al., 2008), so that, GSH administration ensure hippocampus against KA-induced neuronal loss (Saija et al., 1994). It appears that excessive activation of glutamate receptors and oxidative stress represent factors that make neurons impressionable to harm (Coyle & Puttfarcken, 1993). Since mitochondrial malfunction, lipid peroxidation and decreased GSH may be in front of neuronal death in susceptible brain regions (Frantseva et al., 2000); therefore, agents with antioxidant properties are recommended to be useful in the clinical setting of neuronal damage. Since berberine exerts favorable effect against oxidative stress-induced apoptosis, it is suggested that it has powerful antioxidant activity and could be used as a therapeutic agent in oxidative stress-related disorders (Zhu et al., 2013). The potential antioxidant effects of berberine were shown in a number of clinical and preclinical studies (Bhutada et al., 2011). In addition, berberine with increasing cholinergic neuronal system activity could show antiamnesic effect (Peng et al., 1997). Berberine has also useful effects on cognitive disturbance and can protect neurons in various brain disorders (Bhutada et al., 2011). Also, it was shown that berberine has functional effect in a transgenic mouse model of Alzheimer's disease (Durairajan et al., 2012).
Berberine could protect neurons from hydrogen peroxide-induced apoptosis through its antioxidant activity (Zhu et al., 2013). Since berberine could inhibit apoptosis in some conditions (Hu et al., 2012), it is possible that it could survive neurons in a kainic acid-induced apoptosis model. In support of this idea, it is revealed that berberine could protect hippocampal neurons against STZ-induced apoptosis (Sherin et al., 2012; Hong et al., 2012). There are some evidences that berberine can modulate nitric oxide synthesis (Kulkarni and Dhir, 2007). Many studies revealed that nitric oxide is either proconvulsant (Mülsch, 1994) or anticonvulsant (Marangoz,1994), so that, if the level of nitric oxide be reduced before glutamate receptor (NMDA) activation, it decreases epileptiform activity, otherwise nitric oxide content is ineffective on epileptic condition (De Sarro, 1991). Also, it was shown that L-argenine intensifies epileptiform activity induced by kainic acid (De Sarro, 1993) and pretreatment with 7-nitroendazole, selective NOS inhibitor, not only it decreases nitric oxide production, but also attenuates epileptiform activity (Mülsch, 1994). Some studies reported that nitric oxide level increase in experimental models of epilepsy (Kato, 2005). With regards to these controversial results, it seems that berberine could decrease seizure incidence by modulation of nitric oxide production. In addition, berberine could also block potassium channels of hippocampal CA1neurons (Wang et al., 2004) and this blockade leads to the suppression of apoptosis and a substantial increase in the rate of cell survival (Zarch et al., 2009). The therapeutic importance of berberine as anticonvulsant was tested in different animal models such as chemical kindling and maximal electroshock (MES)-induced seizures in swiss albino mice. In MES induced seizure model, the anticonvulsant effect of berberine was comparable to that of phenytoin. Phenytoin showed anticonvulsant effect by blocking the voltage-gated sodium channels (Kohl & Dannhardt, 2001). It was reported that berberine reduces NMDA receptor binding and inhibits NMDA receptor channel current in brain. In addition, berberine protects neuronal cells from brain ischemia such as NMDA receptor antagonists (Cui et al., 2009). On the other hand, it also assessed the effects of berberine against convulsions induced by PTZ, an agent that induce animal model of seizure by inhibition of GABAergic neurotransmission (Katzung, 2004). Since berberine did not influence convulsions induced by PTZ, therefore, berberine has no effect on GABAergic neuro-transmission. According to our results, part of beneficial effect of berberine could be attributed to attenuation of oxidative stress and glutamate receptors activity in those brain structures involved in temporal lobe epilepsy.
One of the limitations of our work was that a positive control group like valproate and/or diazepam with known and potent anticonvulsant effect was not considered. This may be considered in future studies.
5. Conclusion
In conclusion, our results suggest that berberine pretreatment could prevent kainic acid-induced acute and chronic seizures partially via its antioxidant activity.
Acknowledgements
This work was funded and supported by Tehran University of Medical Sciences Tehran, Iran (grant no. 90-01-30-13113).
References
- Ashrafi, M.R., Shams, S., Nouri, M., Mohseni, M., Shabanian, R., & Yekaninejad, M.S., et al. (2007). A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia, 48, 1750–1755 [DOI] [PubMed] [Google Scholar]
- Baluchnejadmojarad, T., & Roghani, M. (2011). Chronic epigal-locatechin-3- gallate ameliorates learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress. Behavioral Brain Research, 224, 305–310 [DOI] [PubMed] [Google Scholar]
- Bhutada, P., Mundhada, Y., Bansod, K., Tawari, S., Patil, S., & Dixit, P., et al. (2011). Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behavioral Brain Research, 220, 30–41 [DOI] [PubMed] [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254 [DOI] [PubMed] [Google Scholar]
- Carrasco, J., Penkowa, M., Hadberg, H., Molinero, A., & Hidalgo, J. (2000). Enhanced seizures and hippocampal neurodegeneration following kainic acid-induced seizures in metallothionein-I + II-deficient mice. European Journal Neuroscience, 12(7), 2311–22 [DOI] [PubMed] [Google Scholar]
- Coyle, J.T., & Puttfarcken, P. (1993). Oxidative stress, glutamate, and neurodegenerative disorders. Science, 262, 689–695 [DOI] [PubMed] [Google Scholar]
- Cui, H., Matsumoto, K., Murakami, Y., Hori, H., Zhao, Q., & Obi, R. (2009). Berberine Exerts Neuroprotective Actions against in Vitro Ischemia-Induced Neuronal Cell Damage in Organotypic Hippocampal Slice Cultures: Involvement of B-Cell Lymphoma 2 Phosphorylation Suppression. Biological & pharmaceutical bulletin, 32, 79–85 [DOI] [PubMed] [Google Scholar]
- De Sarro, G., Di Paola, E.D., De Sarro, A., & Vidal, M.J. (1991). Role of nitric oxide in the genesis of excitatory amino acid-induced seizures from the deep prepiriform cortex. Fundamental and Clinical Pharmacology, 5, 503–511 [DOI] [PubMed] [Google Scholar]
- De Sarro, G., Di Paola, E.D., De Sarro, A., & Vidal, M.J. (1993). L-arginine potentiates excitatory amino acid-induced seizures elicited in the deep prepiriform cortex. European Journal of Pharmacology, 230(2), 151–158 [DOI] [PubMed] [Google Scholar]
- Durairajan, S.S., Liu, L.F., Lu, J.H., Chen, L.L., Yuan, Q., & Chung, S.K., et al. (2012). Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiology of Aging, 33, 2903–2919 [DOI] [PubMed] [Google Scholar]
- Floreani, M., Skaper, S. D., Facci, L., Lipartiti, M., & Giusti, P. (1997). Melatonin maintains glutathione homeostasis in kainic acid-exposed rat brain tissues. FASEB Journal, 11(14), 1309–15 [DOI] [PubMed] [Google Scholar]
- Frantseva, M.V., Velazquez, J.L., Hwang, P.A., & Carlen, P.L. (2000). Free radical production correlates with cell death in an in vitro model of epilepsy. European Journal of NeuroScience, 12, 1431–1439 [DOI] [PubMed] [Google Scholar]
- Hong, Y.J., Kim, N., Lee, K., Hee Sonn, C., Eun Lee, J., & Tae Kim, S., et al. (2012). Korean red ginseng (Panax ginseng) ameliorates type 1 diabetes and restores immune cell compartments. Journal of Ethnopharmacology, 144(2), 225–33 [DOI] [PubMed] [Google Scholar]
- Hu, J., Chai, Y., Wang, Y., Kheir, M.M., Li, H., & Yuan, Z., et al. (2012). PI3K p55γ promoter activity enhancement is involved in the anti-apoptotic effect of berberine against cerebral ischemia-reperfusion. European Journal of Pharmacology, 674(2-3), 132–42 [DOI] [PubMed] [Google Scholar]
- Imanshahidi, M., & Hosseinzadeh, H. (2008). Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytotherapy Research, 22, 999–1012 [DOI] [PubMed] [Google Scholar]
- Jokeit, H., & Schacher, M. (2004). Neuropsychological aspects of type of epilepsy and etiological factors in adults. Epilepsy Behavior,, 5Suppl 1, S14–20 [DOI] [PubMed] [Google Scholar]
- Kato, N., Sato, S., & Yokoyama, H. (2005). Sequential changes of nitric oxide levels in the temporal lobes of kainic acid-treated mice following application of nitric oxide synthase inhibitors and phenobarbital. Epilepsy Research, 65, 81–91 [DOI] [PubMed] [Google Scholar]
- Katzung, B.G. (2004). Basic and Clinical Pharmacology. (9th ed.), Boston, London, Toronto: McGraw-Hill Inc [Google Scholar]
- Kim, E.J., Won, R., Sohn, J.H., Chung, M.A., Nam, T.S., & Lee, H.J., et al. (2008). Anti-oxidant effect of ascorbic and dehydroascorbic acids in hippocampal slice culture. Biochemical and biophysical research communications, 366(1), 8–14 [DOI] [PubMed] [Google Scholar]
- Kim, H.C., Jhoo, W.K., Bing, G., Shin, E.J., Wie, M.B., & Kim, W.K., et al. (2000a). Phenidone prevents kainate-induced neurotoxicity via antioxidant mechanisms. Brain Research, 874(1), 15–23 [DOI] [PubMed] [Google Scholar]
- Kim, H.C., Jhoo, W.K., Kim, W.K., Suh, J.H., Shin, E.J., & Kato, K., et al. (2000b). An immunocytochemical study of mitochondrial manganese-superoxide dismutase in the rat hippocampus after kainate administration. Neuroscience Letter, 281(1), 65–8 [DOI] [PubMed] [Google Scholar]
- Kim, H.C., Choi, D.Y., Jhoo, W.K., Lee, D.W., Koo, C.H., & Kim, C. (1997). Aspalatone, a new antiplatelet agent, attenuates the neurotoxicity induced by kainic acid in the rat. Life Science, 61, PL383–PL381 [DOI] [PubMed] [Google Scholar]
- Kohl, B.K., & Dannhardt, G. (2001). The NMDA receptor complex: a promising target for novel antiepileptic strategies. Current Medicinal Chemistry, 8, 1275–89 [DOI] [PubMed] [Google Scholar]
- Kulkarni, S.K., & Dhir, A. (2008). On the mechanism of antide-pressant-like action of berberine chloride. European Journal of Pharmacology, 589, 163–172 [DOI] [PubMed] [Google Scholar]
- Kulkarni, S.K., & Dhir, A. (2010). Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phytotherapy Research, 24, 317–324 [DOI] [PubMed] [Google Scholar]
- Kulkarni, S.K., & Dhir, A. (2007). Possible involvement of L-arginine-nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling pathway in the antidepressant activity of berberine chloride. European Journal of Pharmacology, 569(1-2), 77–83 [DOI] [PubMed] [Google Scholar]
- Lehtimäki, KA., Peltola, J., Koskikallio, E., Keränen, T., & Honkaniemi, J. (2003). Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain research Molecular brain research, 110(2), 253–60 [DOI] [PubMed] [Google Scholar]
- Lim, J., Kim, H., Choi, Y.S., Kwon, H., Shin, K.S., & Joung, I., et al. (2008). Neuroprotective effects of berberine in neurode-generation model rats induced by ibotenic acid. Animal Cells and Systems, 12, 203–209 [Google Scholar]
- Ma, X., Jiang, Y., Wu, A., Chen, X., Pi, R., & Liu, M., et al. (2010). Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS One, 5, e13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoz, C., Ayyildiz, M., & Agar, E. (1994). Evidence that sodium nitroprusside possesses anticonvulsant effects mediated through nitric oxide. Neuro Report, 5, 2454–2456 [DOI] [PubMed] [Google Scholar]
- Migliore, L., Fontana, I., Colognato, R., Coppede, F., Siciliano, G., & Murri, L. (2005). Searching for the role and the most suitable biomarkers of oxidative stress in Alzheimer's disease and in other neurodegenerative diseases. Neurobiology Aging, 26, 587–595 [DOI] [PubMed] [Google Scholar]
- Mülsch, A., Busse, R., Mordvintcev, P.I., Vanin, A.F., Nielsen, E.O., & Scheel-Krüger, J., et al. (1994). Nitric oxide promotes seizure activity in kainate-treated rats. Neuro Report, 5, 2325–2328 [DOI] [PubMed] [Google Scholar]
- Peng, W.H., Hsieh, M.T., & Wu, C.R. (1997). Effect of long-term administration of berberine on scopolamine-induced amnesia in rats. Japanese journal of pharmacology, 74, 261–266 [DOI] [PubMed] [Google Scholar]
- Perry, G., Nunomura, A., Hirai, K., Zhu, X., Perez, M., & Avila, J., et al. (2002). Is oxidative damage the fundamental pathogenic mechanism of Alzheimer's and other neurodegenerative disease? Free Radic. Biology and Medicine, 33, 1475–1479 [DOI] [PubMed] [Google Scholar]
- Racine, R., Okujava, V., & Chipashvili, S. (1972). Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalography and clinical neurophysiology, 32, 295–299 [DOI] [PubMed] [Google Scholar]
- Roghani, M., & Baluchnejadmojarad, T. (2009). Chronic epigallocatechin-gallate improves aortic reactivity of diabetic rats: Underlying mechanisms. Vascular Pharmacology, 51(2–3), 84–89 [DOI] [PubMed] [Google Scholar]
- Saija, A., Princi, P., Pisani, A., Lanza, M., Scalese, M., & Aramnejad, E., et al. (1994). Protective effect of glutathione on kainic acid-induced neuropathological changes in the rat brain. General Pharmacology, 25, 97–102 [DOI] [PubMed] [Google Scholar]
- Sharma, A.K., Reams, R.Y., Jordan, W.H., Miller, M.A., Thacker, H.L., & Snyder, P.W. (2007). Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicologic Pathology, 35, 984–999 [DOI] [PubMed] [Google Scholar]
- Sherin, A., Anu, J., Peeyush, K.T., Smijin, S., Anitha, M., & Roshni, B.T., et al. (2012). Cholinergic and GABAergic receptor functional deficit in the hippocampus of insulin-induced hypoglycemic and streptozotocin-induced diabetic rats. Neuroscience, 202, 69–76 [DOI] [PubMed] [Google Scholar]
- Shin, E.J., Ko, K.H., Kim, W.K., Chae, J.S., Yen, T.P., & Kim, H.J., et al. (2008). Role of glutathione peroxidase in the ontogeny of hippocampal oxidative stress and kainate seizure sensitivity in the genetically epilepsy-prone rats. Neurochemistry international, 52, 1134–1147 [DOI] [PubMed] [Google Scholar]
- Sloviter, R.S. (2005). The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. Comptes Rendus Biologies, 328(2), 143–53 [DOI] [PubMed] [Google Scholar]
- Sperk, G. (1994). Kainic acid seizures in the rat. Progress in Neurobiology, 42(1), 1–32 [DOI] [PubMed] [Google Scholar]
- Sumanont, Y., Murakami, Y., Tohda, M., Vajragupta, O., Watanabe, H., & Matsumoto, K. (2006). Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell damage in the rat hippocampus by manganese complexes of curcumin and diacetylcurcumin. Life Science., 78, 1884–1891 [DOI] [PubMed] [Google Scholar]
- Tan, Y., Tang, Q., Hu, B.R., & Xiang, J.Z. (2007). Antioxidant properties of berberine on cultured rabbit corpus cavernosum smooth muscle cells injured by hydrogen peroxide. Acta pharmacologica Sinica, 28, 1914–1918 [DOI] [PubMed] [Google Scholar]
- Tang, L., Reiter, R.J., Li, Z.R., Ortiz, G.G., Yu, B.P., & Garcia, J.J. (1998). Melatonin reduces the increase in 8-hydroxy-deoxyguanosine levels in the brain and liver of kainic acid-treated rats. Molecular and cellular biochemistry, 178, 299–303 [DOI] [PubMed] [Google Scholar]
- Wang, F., Zhao, G., Cheng, L., Zhou, H.Y., Fu, L.Y., & Yao, W.X. (2004). Effects of berberine on potassium currents in acutely isolated CA1 pyramidal neurons of rat hippocampus. Brain Research, 999, 91–97 [DOI] [PubMed] [Google Scholar]
- Xiao, B., Bi, F.F., Hu, Y.Q., Tian, F.F., Wu, Z.G., & Mujlli, H.M., et al. (2007). Edaravone neuroprotection affected by suppressing the gene expression of the Fas signal pathway following transient focal ischemia in rats. Neurotoxicity Research, 12, 155–162 [DOI] [PubMed] [Google Scholar]
- Yoo, K.Y., Hwang, I.K., Lim, B.O., Kang, T.C., Kim, D.W., & Kim, S.M., et al. (2006). Berberry extract reduces neuronal damage and N-Methyl-D-aspartate receptor 1 immunoreactivity in the gerbil hippocampus after transient forebrain ischemia. Biological and pharmaceutical bulletin, 29, 623–628 [DOI] [PubMed] [Google Scholar]
- Zarch, A.V., Toroudi, H.P., Soleimani, M., Bakhtiarian, A., Katebi, M., & Djahanguiri, B. (2009). Neuroprotective effects of diazoxide and its antagonism by glibenclamide in pyramidal neurons of rat hippocampus subjected to ischemiareperfusion-induced injury. International Journal of Neuroscience, 119, 1346–1361 [DOI] [PubMed] [Google Scholar]
- Zhou, X.Q., Zeng, X.N., Kong, H., & Sun, X.L. (2008). Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neuroscience Letter, 447, 31–36 [DOI] [PubMed] [Google Scholar]
- Zhu, X., Guo, X., Mao, G., Gao, Z., Wang, H., & He, Q., et al. (2013). Hepatoprotection of berberine against hydrogen peroxide-induced apoptosisby upregulation of sirtuin 1. Phytotherapy Research, 27(3), 417–21 [DOI] [PubMed] [Google Scholar]