Abstract
Introduction
Some studies showed that magnesium has anticonvulsive effect in some animal models. Despite of the availability of well-studied anticonvulsant drugs, this evaluation was not carried on new kind of magnesium supplement, magnesium oxide nanoparticles (nMgO). According to the association between magnesium and convulsion and high prevalence of seizure and epilepsy in diabetics, this study was designed to evaluate the effect of nMgO compared to conventional MgO (cMgO) on strychnine-induced convulsion model in diabetic and non-diabetic mice.
Methods
Healthy male albino mice were divided into 10 groups. Diabetes mellitus was induced by streptozotocin in 5 groups. Conventional and nanoparticle MgO (5 and 10mg/kg) were administered to diabetic and non-diabetic mice, then strychnine were injected and onset of convulsions and time of death measured after strychnine administration.
Results
There were no significant differences between normal and diabetic groups in onset of convulsions and time of death. Pretreatment of cMgO did not have anticonvulsant effect in strychnine-induced convulsion in normal and diabetic mice. But nMgO significantly changed convulsion onset and death time after strychnine administration in normal and diabetic status (p < 0.05).
Discussion
According to our results, it seems that acute administration of nMgO may be important in prevention of convulsion and is more effective than its conventional form in showing anticonvulsive effect that probably is related to the physicochemical properties of nMgO, especially in diabetic subjects, a point that need further investigations.
Keywords: MgO Nanoparticles, Strychnine, Convulsion, Mice
1. Introduction
Epilepsy is a prevalent neurological disorder associated with significant morbidity and mortality (Goldberg & Coulter, 2013). It is also one of the chronic and serious neurological disorders in the world and around 50 million people worldwide suffer from this disease (Ngugi et al., 2010). Several factors influence epilepsy. Diabetes, dietary and lifestyle factors may have an influence on seizures, and magnesium is one such potential factor) Yuen & Sander, 2012, McCorry et al., 2006). Magnesium is the fourth most common mineral and the second intracellular cation after potassium in the human body. Magnesium as an important ion has a limited level in the serum and those bound mainly to albumin and complexes to anions (Fawcett et al., 1999). Equilibrium between tissue pools is reached slowly with a half-life for the majority of radiolabelled magnesium varying between 41 and 181 days (Elin, 1994).
The main physiological role of magnesium is related to its function in enzyme systems and its influence on membrane properties. Magnesium appears to play an important role in conduction in nervous system (Fawcett et al., 1999). Magnesium modulates the seizure, and mechanism of action of magnesium in treating seizure by antagonizing the excitatory calcium influx through the N-methyl-D-aspartate (NMDA) receptors. Magnesium enters via the NMDA receptor channel and blocks the passage of more permeable ions, such as Ca 2+, in a voltage-dependent manner (Cotton et al., 1993). It is indicated that low extracellular magnesium can reduce surface charge of neuronal membrane (produced by sialic acid, phosphates, charged lipids, charged amino acids and etc), thereby increases neuronal hyper excitability (Isaev et al., 2012). Magnesium salts have an anticonvulsant effect and hypomagnesemia is related to epilepsy (Spasov et al., 2007).
There is a significant association between diabetes and idiopathic generalized epilepsy. In a study, a group of adults with epilepsy were found to have a four-fold higher prevalence of type 1 diabetes compared to the general population (McCorry, 2006). Since diabetics are prone to seizure and epilepsy thus their drug therapy is more important than non-diabetics.
Conventional drugs often face with the major limitation of adverse effects, the result of the non-specificity of their action, and are little effective due to improper or ineffective dosages, e.g., in cancer chemotherapy and anti-diabetic therapy (Chekman, 2008). Nanotechnology offers the possibility of designing new drugs with greater cell specificity and drug-release systems acting selectively on specific targets. Nanotechnology by producing new materials that, are attractive from many aspects, especially when entering to biological systems, introduced as a leader technology in overall the world (Colvine, 2003). The same properties leading to the technical advantages of nanotechnology also lead to unique biological effects (Dreher, 2004). These new materials have longer halftime and higher ability in living systems to interaction with cellular components and can open a new way to the treatment of human diseases and disorders (Murthy, 2007; Dreher, 2004). Nanomedicine is a new branch of nanotechnology using nanoparticles for therapeutic targets such as drug delivery specially into central nervous system, imaging and diagnosis, treatment of tissue damages and etc (Cho& Borgens, 2012; Chandra, Barick & Bahadur, 2011; Kreuter, 2005).
This allows the administration of smaller but more effective doses, minimizing adverse effects. Nanotechnology can also be used to optimize drug formulations, increasing drug solubility and altering the pharmacokinetics to sustain the release of the drug, and thereby prolonging its bioavailability (Ghrab et al., 2009).
According to the physiochemical properties of nMgo and little investigations about its anticonvulsive effects, the aim of the current exploratory study was to examine nMgO anticonvulsive effect in comparison with its conventional form in a convulsion model at diabetic and non-diabetic mice.
2. Methods
2.1. Experimental Animals and Groups
This study was conducted in Shahid Chamran University of Ahvaz, Iran in 2011. Adult male mice (NMRI strain) with 29 ± 3 gram weight were obtained from Animal house of Ahvaz Joundi Shapoor University of Medical Sciences (AHUMS) and kept in controlled room with a 12:12 h light/dark cycle and 20-24 °C temperature. All the animals had freely access to food and water, unless during the test time. All the tests were done between 9AM-13PM in light phase of the cycle. In every group, 7 animals were used and each animal once only. To study the effect of magnesium supplements on strychnine-induced convulsive diabetic and nondiabetic male mice, the animals were divided into 10 groups: Normal groups, which were non-diabetic mice and received saline or 5 and/or 10 mg/kg (IP) of cMgO or nMgO before convulsion induced by strychnine; and diabetic groups that by STZ and received saline or 5 and/ or 10 mg/kg (IP) of cMgO or nMgO before convulsion induced by strychnine.
Experimental protocol was approved by Institutional Animal Ethical Committee (IAEC) of Shahid Chamran University of Ahvaz, Iran.
2.2. Experimental Design and Drugs
Diabetes mellitus was induced by single dose injection of streptozocin (Sigma Aldrich, Germany) (50 mg/kg intraperitoneally) (Enhamre et al., 2012) in 5 groups. Blood glucose was measured by glucometer (Multicare, Arezzo Italia) 3 days after streptozotocin administration. If blood glucose was more than 250 mg/dl; the mice were considered as diabetic. CMgO (Merk-Germany) and nMgO (Iolitec, Germany with 40nm size) were intraperitoneally administered 5 and 10 mg/kg (Ghasemi et al., 2010). All the groups were subcutaneously received strychnine (3 mg/kg) 30 min after (Patil et al., 2011) saline or nano and conventional MgO (Ghasemi et al., 2010) injection. The convulsion parameters such as onset of convulsions and time of death were measured for a 30-min period after injection of strychnine (Honar et al., 2004).
2.3. Statistical Analysis
The mean of data was compared between the groups by SPSS software (version 16, USA) and the statistical significant level was p < 0.05. Graphical data are expressed as means ± SEM. The data analysis was performed by one-way analysis of variance (ANOVA) followed by LSD [least significant difference] and post-hoc test for assessing specific group comparisons.
3. Result
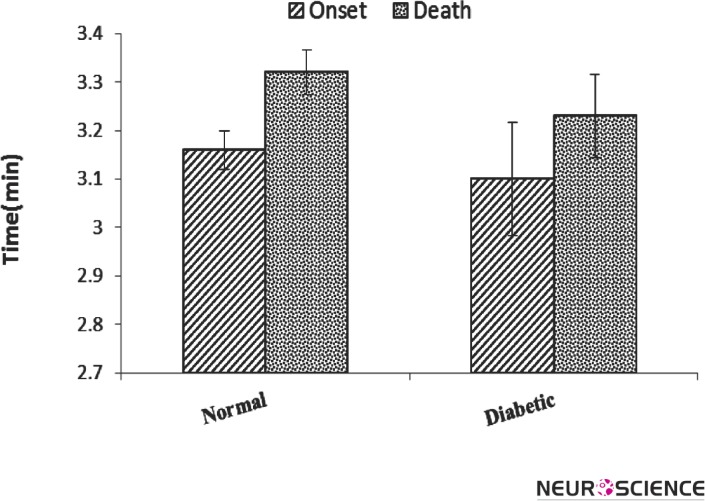
Fig. 1 illustrates the onset of convulsion and death time by strychnine in diabetic mice, compared to nondiabetic (normal) group. Post hoc analysis showed that diabetes did not change both onset of convulsion and death time although the mean of these parameters was decreased in the diabetic group.
Figure 1.
Convulsion onset and death time (Mean ± SEM) after strychnine-induced convulsion in normal and diabetic mice. (n = 7). There was no difference between the groups.
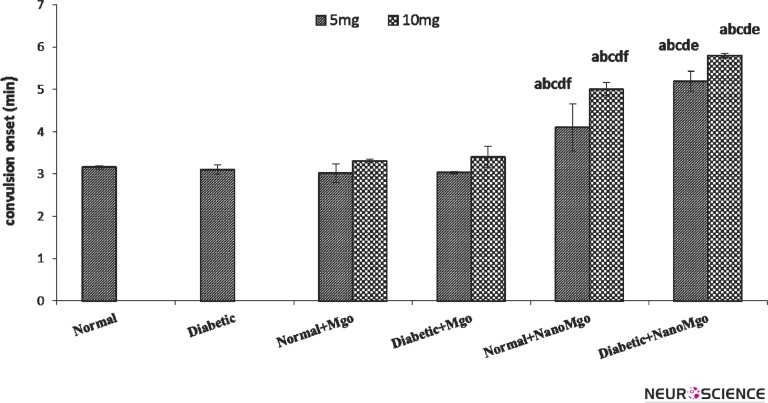
Fig. 2 shows no difference between cMgO (5&10 mg/kg) and saline-treated in convulsion onset. This result showed that cMgO had no effect on strychnine–induced convulsion in normal and diabetic mice.
Figure 2.
Effects of nano and conventional MgO (5&10mg/kg) on convulsion onset (Mean ± SEM) in strychnine-induced convulsion at the normal and diabetic mice. (n = 7). The letters show significant difference between the groups (n = 7 and p < 0.05). a = Normal b = Diabetic c = Normal + cMgO d = Diabetic + cMgO e= Normal + nanoMgO f= Diabetic + nanoMgO
NMgO (5&10mg/kg) increased the onset (p < 0.01) of convulsion in normal groups significantly. Also, pretreatment with nMgO (5&10 mg/kg) significantly increased convulsion onset in diabetic group (p < 0.003, p < 0.001 respectively). This result illustrates nanoparticles have potent anticonvulsant effect in normal and diabetic mice.
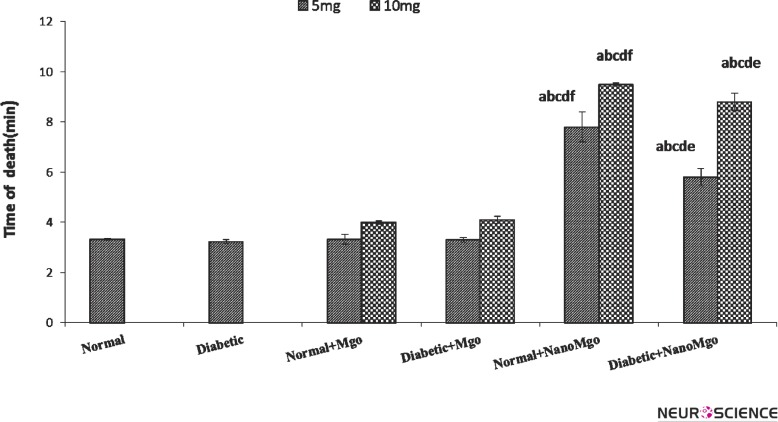
Fig. 3 demonstrates that death time of convulsion was not significantly changed by cMgO (5&10mg/ kg) pretreatment. But it was significantly increased by nMgO (5,10mg/kg) pretreatment (p < 0.001), both in normal and diabetic mice. This effect was significantly greater in normal group (p < 0.001).
Figure 3.
Effects of nano and conventional MgO (5&10mg/kg) on death time (Mean ± SEM) in strychnineinduced convulsion at the normal and diabetic mice. (n = 7). The letters show significant difference between the groups (n = 7 and p < 0.05). a = Normal b = Diabetic c = Normal + cMgO d = Diabetic + cMgO e= Normal + nanoMgO f= Diabetic + nanoMgO
4. Discussion
In this study, we applied the nanoparticle and conventional MgO to compare anticonvulsive effects of them. Our results showed that nano type of MgO had greater anticonvulsant effect than conventional type especially in diabetic mice.
These effects may be due to the small size and different properties of nanoparticles compared to their same conventional form. The small size of nano materials can modify their physicochemical properties as well as giving the opportunity for increased uptake and interaction with biological tissues. ( Zhang, Nelson & beales, 2012). According to the size of nanoparticles of MgO (<60 nm), this effect may be related to greater mobility and more uptake across biological membranes especially in central nervous system (Almeida et al., 2011). Thus, nanoparticles have the potential to improve current disease therapies due to their ability to overcome multiple biological barriers and release a therapeutic load in the optimal dosage range (Alexis et al., 2008). Strychnine – induced convulsions is an experimental model for identification effect and mechanisms of potential anticonvulsive agents in animals (Zhang et al., 2012). Strychnine is a competitive antagonist of the inhibitory neurotransmitter glycine at receptors in the spinal cord, brain stem and higher centers (Khajehpour et al., 2012). It results in increased neuronal activity and excitability, and thereby leads to increased muscular activity (Wood et al., 2002).
Thus, nMgO prevented strychnine–induced convulsions in mice by central or peripheral mechanisms. However, more details of this theory need further investigations. Although cMgO did not decrease convulsion pattern in our study, its other salts such as magnesium sulfate affected convulsion and seizure in animals and human (Euser and Cipolla 2009, Oliveir et al., 2011). Magnesium supplementation was associated with a significant decrease in the number of seizure (Abdelmalik, Politzer & Carlen, 2012). Magnesium sulfate has central anticonvulsant activity on hippocampal seizures, implicating the N-methyl-D-aspartate receptor in eclamptic seizures and in the therapeutic efficacy of magnesium sulfate (Cotton, Janusz & Berman, 1992). Calcium and magnesium have anti-seizure and antiepileptic properties by changing surface charge in rat hippocampus (Isaev et al., 2012). Intraperitoneal injection of magnesium enhanced the efficacy of valproate against pentylenetetrazol-induced seizures (Safar et al., 2010). The effect of magnesium may be dose dependent. Low dose of magnesium sulfate prevented seizures induced by pentylenetetrazole in rats but high dose did not affect (Oliveir et al., 2011). Concentration of magnesium is associated with seizures and low magnesium concentration generates spontaneous epileptiform discharges in rat hippocampal slices (Tancredi, Avoli & Hwa, 1988).
However, it appears that low magnesium concentrations can lead to seizures in the live animal. In a study on rats, dietary Mg deficiency led to decrease the seizure thresholds and latencies; and subsequently oral magnesium supplementation over three weeks, and then led to increase the seizure thresholds and latencies (Spasov et al., 2007). Deficiency of dietary magnesium also increases convulsions and may cause grass tetany in cattle, which can lead to seizures and death (Odette, 2005).
In our finding, cMgO did not alter strychnine induced convulsion. This result also obtained in the other studies. Some studies (Decollogne et al., 1997) reported that the anticonvulsive effect of Mg 2+ is not observed with PTZ-induced convulsions. Dhande et al., (2009) also showed that an oral magnesium oxide failed to produce any significant change in PTZ-induced seizures with especial moderation and low doses. Therefore, it seems this effect may be related to low level of serum magnesium that can be achieved from MgO and insufficiency passing from blood brain barrier that needs further investigations.
In this study, we showed that nMgO has better effect on diabetic. Some researches showed that an anti-seizure drug such as topiramate improves diabetes through improving glycaemic control and body fat (Eliasson et al., 2007). On the other hand, the plasma magnesium level shown to be inversely related to insulin sensitivity. Magnesium supplementation improves insulin sensitivity as well as insulin secretion in patients with type 2 diabetes (de Valk 1999). Furthermore, oral supplementation with MgCl2 solution improves insulin sensitivity and metabolic control in patients with type 2 diabetes and decreased serum magnesium levels (Rodriguez-Mor and Guerrero-Romero 2003). Thus, the better effect of nMgO may be related to its multifunctional properties on diabetes and convulsion.
However, it seems that the nMgO has potential anticonvulsive effect especially in diabetic subjects and could be evaluated in other animal models and clinical trials.
5. Conclusion
According to the present findings, acute administration of nMgO can prevent convulsion onset and dead times in diabetic and non-diabetic mice while cMgO could not induce any effects. The effect of nMgO may be related to greater mobility and high efficacy in central nervous system or peripheral mechanisms.
Acknowledgment
The authors wish to express their gratitude to the research council of Shahid Chamran University of Ahvaz, Iran, for their financial supports (Grant No: 90.302.18672, Date: June 7, 2011).
References
- Abdelmalik, P.A., Politzer, N., & Carlen, P.L. (2012). Magnesium as an effective adjunct therapy for drug resistant seizures. Canadian Journal of Neurological Sciences, 39(3), 323-7 [DOI] [PubMed] [Google Scholar]
- Alexis, F., Pridgen, E., Molnar, L.K., & Farokhzad, O.C. (2008). Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular Pharmaceutical, 5(4), 505-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, J.P., Chen, A.L., Foster, A., & Drezek, R. (2011). In vivo biodistribution of nanoparticles. Nanomedicine (Lond), 6(5), 815-35 [DOI] [PubMed] [Google Scholar]
- Chandra, S., Barick, K.C., & Bahadur, D. (2011). Oxide and hybrid nanostructures for therapeutic applications. Advanced Drug Delivery Reviews, 63(14-15), 1267-1281 [DOI] [PubMed] [Google Scholar]
- Chekman, I.S. (2008). Nanopharmacology: experimental and clinical aspect. Likars'ka sprava, 3-4,104-109 [PubMed] [Google Scholar]
- Cho, Y., & Borgens, R.B. (2012). Polymer and nano-technology applications for repair and reconstruction of the central nervous system. Experiment of Neurology, 233(1), 126–144 [DOI] [PubMed] [Google Scholar]
- Colvin, V. (2003). The potential environmental impact of engineered nanomaterials. Nature Biotechnology, 21(10), 1166-1170 [DOI] [PubMed] [Google Scholar]
- Cotton, D.B., Hallak, M., Janusz, C., Irtenkauf, S.M., & Berman, R.F. (1993). Central anticonvulsant effects of magnesium sulfate on N-methyl-D-aspartate-induced seizures. American Journal of Obstetric Gynecology, 168, 974–978 [DOI] [PubMed] [Google Scholar]
- Cotton, D.B., Janusz, C.A., & Berman, R.F. (1992). Anticonvulsant effects of magnesium sulfate on hippocampal seizures: therapeutic implications in preeclampsia-eclampsia. American Journal of Obstetric Gynecology, 166 (4), 1127-34 [DOI] [PubMed] [Google Scholar]
- Decollogne, S., Tomas, A., Lecerf, C., Adamowicz, E., & Seman, M. (1997). NMDA receptor complex blockade by oral administration of magnesium: comparison with MK-801. Pharmacological Biochemical Behavior, 58, 261-8 [DOI] [PubMed] [Google Scholar]
- De Valk, H.W. (1999). Magnesium in diabetes mellitus. Netherlands Journal of Medicine, 54(4), 139-46 [DOI] [PubMed] [Google Scholar]
- Dhande, P.P., Ranade, R.S., & Ghongane, B.B. (2009). Effect of magnesium oxide on the activity of standard anti-epileptic drugs against experimental seizures in rats. Indian Journal of Pharmacology, 41 (6), 268-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher, K.L. (2004). Health and environmental impact of nanotechnology, Toxicology classmen of manufactured nanoparticles. Toxicological Science, 77(1), 3-5 [DOI] [PubMed] [Google Scholar]
- Eliasson B, Gudbjörnsdottir S, Cederholm J, Liang Y, Vercruysse F., & Smith U. (2007). Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. International Journal of Obesity, 31, 1140–1147 [DOI] [PubMed] [Google Scholar]
- Elin R.J. (1994). Magnesium: the fifth but forgotten electrolyte. American Journal of Clinical Pathology, 102, 616-22 [DOI] [PubMed] [Google Scholar]
- Enhamre, E., Carlsson, A., Grönbladh, A., Watanabe, H., Hallberg, M., & Nyberg, F. (2012). The expression of growth hormone receptor gene transcript in the prefrontal cortex is affected in male mice with diabetes-induced learning impairments. Neuroscience Letter, 523(1), 82-6 [DOI] [PubMed] [Google Scholar]
- Euser, A.G., & Cipolla, M. J. (2009). Magnesium sulfate treatment for the prevention of eclampsia: a brief review. Stroke, 40(4), 1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett, W.J., & Haxby, E.J. (1999). Male DA Magnesium: physiology and pharmacology. British Journal of Anesthesiology, 83(2), 302-320 [DOI] [PubMed] [Google Scholar]
- Ghasemi, A., Saberi, M, Ghasemi, M., Shafaroodi, H., Moezi, L., Bahremand, A., Montaser-Kouhsari, L., Ziai, P., & Dehpour, A.R. (2010). Administration of lithium and magnesium chloride inhibited tolerance to the anticonvulsant effect of morphine on pentylenetetrazole-induced seizures in mice. Epilepsy & Behavior, 19(4), 568-74 [DOI] [PubMed] [Google Scholar]
- Ghrab, B.E., Maatoug, M., Kallel, N., Khemakhem, K., Chaari, M., Kolsi, K., & Karoui, A. (2009). Dose combination of intrathecal magnesium sulphate and morphine improve postoperative section analgesia?, Annales Francaises d. Anesthesie et de Reanimation, 28(5), 459-9 [DOI] [PubMed] [Google Scholar]
- Goldberg, E.M., & Coulter, D.A. (2013). Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nature Review Neuroscience, 14(5), 337-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honar, H., Riazi, K., Homayoun, H., Sadeghipour, H., Rashidi, N., Ebrahimkhani, M.R., Mirazi, N., & Dehpour, A.R. (2004). Ultra-low dose naltrexone potentiates the anticonvulsant effect of low dose morphine on colonic seizures. Neuroscience, 129(3), 733-742 [DOI] [PubMed] [Google Scholar]
- Isaev, D., Ivanchick, G., Khmyz, V., Isaeva, E., Savrasova, A., Krishtal, O., Holmes, G.L., & Maximyuk, O. (2012). Surface charge impact in low-magnesium model of seizure in rat hippocampus. Journal of Neurophysiology, 107(1), 417-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajehpour, L., Najafzadeh, H., Kesmati, M., & Hassanvand, F. (2012). Anticonvulsant effect of progesterone in presence and absence of calcium channel blocker. World Applied Science Journal, 17(6), 704-709 [Google Scholar]
- Kreuter, J. (2005). Application of nanoparticles for the delivery of drugs to the brain. International Congress Series, 1277, 85-94 [Google Scholar]
- McCorry, D., Nicolson, A., Smith, D., Marson, A., Feltbower, RG., & Chadwick, D.W. (2006). An association between type 1 diabetes and idiopathic generalized epilepsy. Annals of Neurology, 59, 204-6 [DOI] [PubMed] [Google Scholar]
- Murthy, S.K. (2007). Nanoparticles in modern medicine: State of the art and future challenges. International Journal of Nano medicine, 2(2), 129–141 [PMC free article] [PubMed] [Google Scholar]
- Ngugi, A.K., Bottomley, C., Kleinschmidt, I., Sander, J.W., & Newton, C.R. (2005). Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia, 51(5), 883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odette, O. (2005). Grass tetany in a herd of beef cows. Canadian Veterinary Journal, 46, 732–734 [PMC free article] [PubMed] [Google Scholar]
- Oliveira, L.D., Oliveira, R.W., Futuro Neto Hde, A., & Nakamura-Palacios, E.M. (2011). The role of magnesium sulfate in prevention of seizures induced by pentylenetetrazole in rats. Arquivos Neuro-psiquiatria, 69(2B), 349-55 [DOI] [PubMed] [Google Scholar]
- Patil, M.S., Patil, C.R., Patil, S.W., & Jadhav, R.B. (2011). Anticonvulsant activity of aqueous root extract of Ficus religiosa. Journal of Ethnopharmacol, 2011, 133(1), 92-6 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mor, M., & Guerrero-Romero, F., (2003). Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects. Diabetes Care, 26, 1147–1152 [DOI] [PubMed] [Google Scholar]
- Safar, M.M., Abdallah, D.M., Arafa, N.M., & Abdel-Aziz, M.T. (2010). Magnesium supplementation enhances the anticonvulsant potential of valproate in pentylenetetrazol-treated rats. Brain Research, 1334, 58–64 [DOI] [PubMed] [Google Scholar]
- Spasov, A.A., Iezhitsa, I.N., Kharitonova, M.V., & Kravchenko, M.S. (2007). Effect of magnesium chloride and magnesium L-aspartate on seizure threshold in rats under conditions of dietary magnesium deficiency. Bulletin of Experimental Biology and Medicine, 144, 214–216 [DOI] [PubMed] [Google Scholar]
- Tancredi, V., Avoli, M., & Hwa, G.G. (1988). Low-magnesium epilepsy in rat hippocampal slices: inhibitory postsynaptic potentials in the CA1 subfield. Neuroscience Letter, 89, 293–298 [DOI] [PubMed] [Google Scholar]
- Wolf, F.I., & Cittadini, A. (2003). Chemistry and biochemistry of magnesium. Molecular Aspects of Medicine, 24, 3-9 [DOI] [PubMed] [Google Scholar]
- Wood, D., Webster, E., Martinez, D., Dargan, P., & Jones, A. (2002). Survival after deliberate strychnine self-poisoning, with toxicokinetic data. Critical Care, 6, 456-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen, A.W., & Sander, J.W. (2012). Can magnesium supplementation reduce seizures in people with epilepsy?. A hypothesis. Epilepsy Research, 100(1-2), 152-6 [DOI] [PubMed] [Google Scholar]
- Zhang, S., Nelson, A., & Beales, P.A. (2012). Freezing or wrapping: the role of particle size in the mechanism of nanoparticle-biomembrane interaction. Langmuir, 28(35), 12831-7 [DOI] [PubMed] [Google Scholar]