Abstract
Introduction
Inflammatory pain is a common sign of chronic diseases. Some brain regions such as locus coeruleus (LC) of the brainstem nor-epinephrine (NE) system have a key role in The mechanisms of the pain modulation and dependence. Bupropion synthesized as an antidepressant, but it is using for smoke cessation. It can change morphine withdrawal signs such as pain related behaviors. This study tested the acute effect of intra-LC microinfusion of bupropion on the formalin-induced pain behavior in rats.
Methods
Wistar male rats were divided into 6 groups (control-naïve, control-operated, shamoperated, and 3 treated groups with 10-2, 10-3, 10-4 mol/µl intra-LC of bupropion). The injection guide cannulae were implanted into LC nuclei bilaterally by stereotaxic coordinated surgery under sterile condition. The sham group received normal saline as drug vehicle but control groups had no intra-LC injections. Formalin (50 µl, 2.5%) was injected subcutaneously in plantar region of the right hindpaw in all animals (30 min after drug administration in treated animals). Nociceptive signs were observed continuously and registered on-line each minute. Common pain scoring was used for pain assessment.
Results
The analysis of data by one-way ANOVA showed that bupropion can reduce pain behavior scores significantly. Bupropion reduced total pain score in the phase 01 (60%) and phase 02 (52%) of maximal behavior compared to the sham group, dose dependently and significantly. The pain scores of controls and sham groups had no significant difference.
Discussion
The results showed that bupropion has analgesic effects on LC neurons and can alter the neurochemical involvement of LC in pain process. Bupropion has different and significant effect on early and late phases of formalin test.
Keywords: Bupropion, Locus Coeruleus, Formalin test, Pain, Analgesia, Antidepressant, Rat
1. Introduction
Pain is a complex phenomenon with broad mechanisms that is expressed as acute or chronic states. The animal models of pain have developed for the discovery of physiological mechanisms underlying acute and chronic pain in human. The most common animal model for persistent pain is the formalin test in which low concentrations of formalin solution (0.5 to 5%, diluted by 0.9% NaCl) are injected into the dorsal surface of one of the hind paws intradermally (Le Bars, Gozariu, & Cadden, 2001). A four level of painful behavior, flinching/shaking, or the licking/biting of the injected paw is assessed in a timeline up to 60 or 90 min (Dubuisson & Dennis, 1977; Tjolsen, Berge, Hunskaar, Rosland, & Hole, 1992). The number of paw licking or twitching per unit of time and cumulative time that is spent for biting/ licking the paw can also be used (Sufka, Watson, Nothdurft, & Mogil, 1998; Wheeler-Aceto & Cowan, 1991). In a 45 to 60 minute timescale, the biphasic pain behavior appeared in which a transient early phase is followed by a tonic late phase. The first or initial phase is related to direct peripheral nociceptive afferents but the second phase depends upon prolonged changes in central nervous system function (central sensitization) (Abbadie, Taylor, Peterson, & Basbaum, 1997; Cadet, Aigouy, & Woda, 1995; Huang et al., 2006; Lebrun, Manil, & Colin, 2000; Tjolsen et al., 1992; Vaccarino & Chorney, 1994).
One of the important and well-known neurotransmitter systems involved in pain modulation is the brain monoamine systems. The brain monoamine systems are involved in the neural mechanisms underlying the central regulation of cardiovascular dynamics, the endocrine control and affective behavior, awareness and sleep. The locus coereleus (LC), as a part of these systems, has several roles in pain modulation. The LC is a crucial component of ascending and descending pain pathways and is a major site for analgesic agents (G. J. Liu & Wang, 1988; Nygren & Olson, 1977), which facilitates the development and maintenance of neuropathic pain and contributes to pain facilitation (Brightwell & Taylor, 2009).
Bupropion can alleviate some withdrawal signs of nicotine and is being used as a smoking cessation agent despite its classification (Ascher et al., 1995; Hays & Ebbert, 2003). The smoking cessation and antidepressive mechanisms of bupropion have not been completely clarified (Balfour, 2001, 2002; Clayton, 2007). Bupropion mainly inhibits nicotinic acetylcholine receptors (nAChRs), but can also inhibit the synaptic dopamine transporter (DAT) and noradrenalin transporter (NET). Bupropion increases extracellular NE and DA concentrations in the hippocampus of freely moving rats. The combined effect of bupropion in inhibiting these transporters and antagonizing of nAChRs makes it a good pharmaceutical drug for the treatment of depression and smoke cessation (Dwoskin, Rauhut, King-Pospisil, & Bardo, 2006). Recent studies have shown that bupropion can attenuate the brain reward activity in successfullytreated smokers (Weinstein et al., 2009). The evidence showed that bupropion can change the dopamine receptor affinity indirectly or by binding with its residues directly (Bischoff, Bittiger, Krauss, Vassout, & Waldmeier, 1984).
Our recent studies showed that microinjection of bupropion in the ventral tegmental area (VTA) can alter the occurrence and pattern of the pain and opioid withdrawal behaviors (Ghaderi Pakdel, Naderi, & Zare, 2011). IntraVTA bupropion can also alter the aggressive behaviors in a non-dose dependent manner (Mokhtari hashtjin, Zare, Ghaderi Pakdel, & Heysieattalab, 2010). The present study was designed to evaluate the effect of intra-LC microinjection of bupropion on formalin-induced pain behaviors in healthy male rats.
2. Methods
All procedures and experiments were approved by the local ethical committee and were in accordance with the guidelines for the Care and Use of Experimental Animals outlined by the Laboratory Animal Center of Urmia University of Medical Sciences. All experimental procedures and protocols were approved by the Urmia Medical Science Research Ethics Committee (UMSREC) and performed in accordance with the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals. The animals were housed in groups of 5 per cage prior to surgery and singly afterwards. Animals were housed at 12h light/dark cycle (7:00am-7:00pm) and controlled temperature (22±2 °C) with food and water ad libitum. All experiments were done after surgery recovery and adaptation (10 days).
2.1. Animal Preparation and Stereotaxic Brain Cannulation
Wistar healthy adult male rats (Pasteur Institute, TehranIran, weighing 230-280 g) were divided into 6 groups: control-naive, control-operated, sham-operated, and 3 groups with the microinfusion of bupropion (10-2, 10-3, and 10-4 mol, 1 µl, intra-LC, n=8 in each group). The rats were anesthetized with a mixture of ketamine and xylazine (80mg/kg and 10mg/kg respectively) and were secured in a stereotaxic frame (Steolting, USA). The 25-gauge stainless steel guide cannulae (SUPA, Iran) were implanted into the LC bilaterally (AP= -0.6, DV= 6.2, ML= ±1.3 mm with respect to the bregma zerozero plate and interaural axis) according to Paxinos and Watson rat brain stereotaxic atlas under sterile condition (Paxinos & Watson, 2005). Cannulae were secured on the skull with screws and the dental cement (Acropars, Marlic, Iran). The control-naive and control-operated groups had no intra-LC injections. The control-naive group received normal sterile saline but the control-operated group received formalin in the dorsal surface of the right hind paws. The sham-operated group received an intra-LC drug vehicle and formalin in the right hind paw. The tips of the guide cannulae were located near the top surface of the LC and were blocked by obturators until drug injection.
2.2. Drugs, Materials and Chemicals
Drugs and chemicals which were used in this study include: bupropion, formalin, Brilliant Sky Blue, fast cresyl violet (purchased from Sigma-Aldrich, USA), sodium acetate and sodium chloride (Merck, Darmstadt, Germany), ketamine (Parke-Davies, Freiburg, Germany), xylazine (Kepro B.V., The Netherlands), polyethylene microtubes (A-M system, USA), Hamilton microsyringes (Hamilton Bonaduz AG, Switzerland).
2.3. Formalin Test
After acclimation (30 min) each rat received 50µl of formalin solution (2.5% in normal saline) subcutaneously into the dorsal surface of the right hind paw using a microsyringe with a 26-gauge needle. In an open Plexiglas observation chamber (30×30×30cm), the pain score of the injected paw was acquired on-line by an observer. A mirror was placed at an angle of 45° under the transparent floor to clear observation. The Dubuisson and Dennis procedure of the pain rating method was applied (Dubuisson & Dennis, 1977). The score of four nociceptive behavior categories was registered each minute up to 90 min. Briefly, in this method the categories were: 0= normal behavior of the hind limbs to support the body; 1= slight touching of the injected paw on the glass surface to lightly support or not support the body; 2= total withdrawal of the injected paw; and 3=licking, biting or shaking of the injected paw (Dubuisson & Dennis, 1977). In this research the data between 0 and 10 min after the formalin injection were represented as phase one (early phase) and between 16 and 90 min were represented as phase two (late phase). In this study the pain score was recorded and expressed in 5-minute bins. In the bupropion microinjected groups, the formalin test was done 30 min after intra-LC bupropion administration. All the animals were used only once and experiments were carried out at the same time of the day (09.00 to 14.00).
2.4. Drug Microinjection
All the animals were adapted to the restraint devices. For microinjection in the intra-LC in the rat which was awake the animal was gently restrained, and 30-G injection cannula was inserted into the implanted guide cannulae. The injection cannula was longer by 1.0 mm from the tip of the guide cannula within the brain. The external tip of the injection cannula was connected to Hamilton micro syringes via a polyethylene microtube. 1.0µl of the solution was microinjected over 1-2 min via the injection cannula during an experiment which was left for 3.00 min in place to allow the diffusion of the drug and to minimize the backflow along the cannulae tracts.
2.5. Histological Verification
To evaluate the diffused region of the drug and histological verification, at the end of the experiments all the animals received 1µl of Brilliant Sky Blue (dissolved in 0.5M sodium acetate).The animals were deeply anesthetized, killed and the brains were removed and fixed in the 10% phosphate buffered formalin solution. Coronal 40µm sections were taken on a microtome (SLEE, London) and stained with cresyl violet. The trajectory path and location of guide cannulae tips were observed under light microscopy for verification. The misinjected rats were excluded from the analysis.
2.6. Data Analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by the post-hoc Tukey's test. The statistical significance was p<0.05. Results are expressed as the means ± SEM. The GB-Stat ver. 5.0 statistical software was used for statistical analysis and Microsoft Excel ver. 2003 was used for the graphical presentation of the data.
3. Results
The analysis of the of pain score between control-naive, control-operated and sham-operated groups showed that there were no significant differences between their data. However, the analysis of the data between the sham and two control groups has not been shown here.
3.1. Bilateral Intra-LC Microinjection of Bupropion Decreases the Formalin-Induced Nociceptive Score
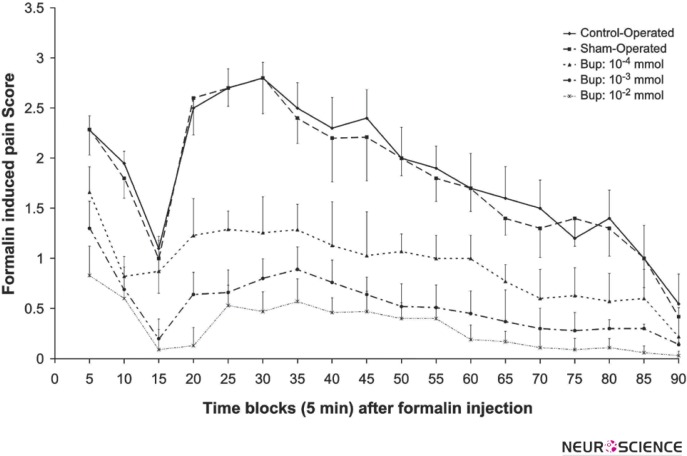
Figure 1 shows the pain scores in the sham-operated and 3 doses of bupropion that were microinjected intra- LC. The data analysis using repeated-measure one-way ANOVA showed a significant difference between the formalin-induced pain behavior within bupropion microinjected and the sham-operated groups. The microinjection of bupropion (10-2, 10-3, and 10-4 mol) in the LC decreased the pain score in phase 01 and 02 significant and dose dependently.
Figure 1.
The mean of the formalin-induced pain scores in 5 min bin time blocks during phase 01 (0-10 min) and phase 02 (16- 90 min) in the control-operated (n=8), sham-operated (n=12) and 10-4, 10-3, 10-2 mol of intra-LC bupropion groups (n=8 in each group). The drug or vehicle was microinfused bilaterally in Locus Coeruleus 30 min before the formalin injection. Values are the means ± SEM. The data of the control and sham-operated groups had no significant difference.
3.2. The Effect of Intra-LC Microinjection of Bupropion on the Average of the Formalin-Induced Pain Score
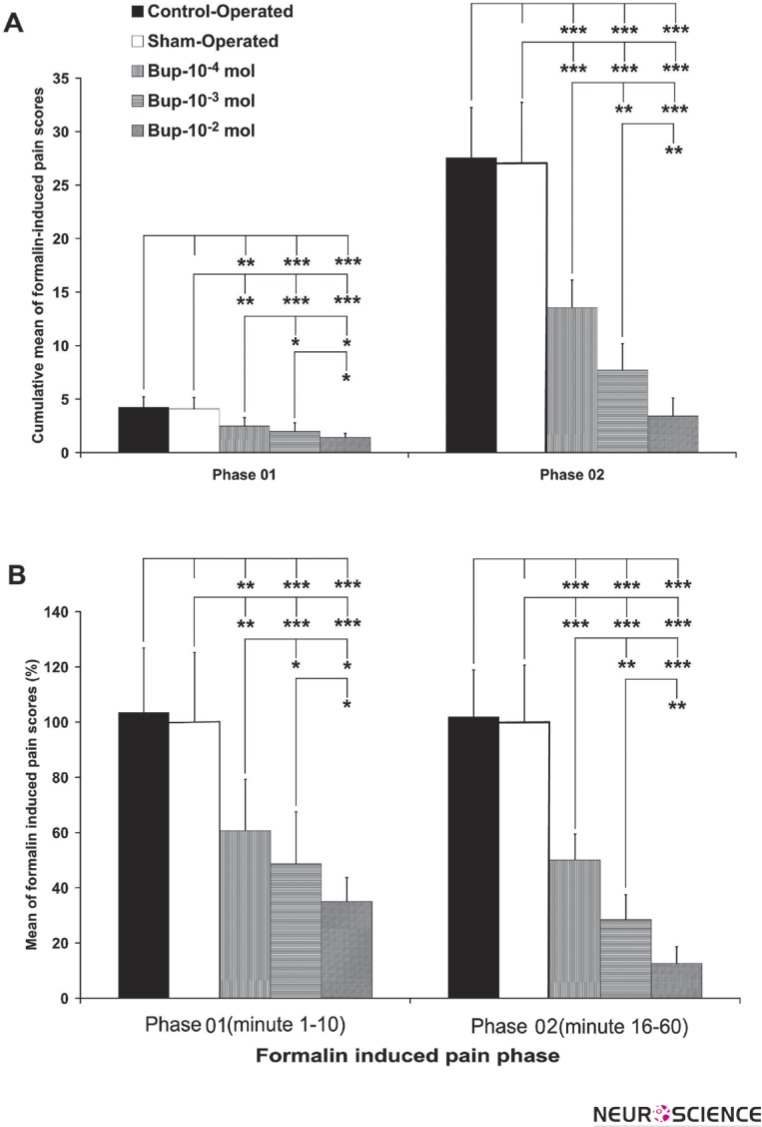
Figure 2-A shows the effect of intra-LC bupropion on average of the formalin-induced pain scores in all groups. The analysis of the average of the pain scores in phase 01 showed that the pain scores between the groups were significantly different (F7, 3= 55.20372, p<0.001). Tukey's protected post hoc t-test showed that there is no significant difference between the pain scores of the sham and dose 10-4 mol of intra-LC bupropion group. The analysis of the average of the pain scores in phase 02 showed that the pain scores between groups were significantly different (F42, 3 = 34.50914 and p<0.0001). Tukey's protected post hoc t-test showed that there is no significant difference between the pain scores of the sham and dose 10-4 mol of intra-LC of the bupropion.
Figure 2.
The mean of formalin-induced pain scores (part A is cumulative absolute and part B is percentage) in the total duration of phase 01 (0-10 min) and phase 02 (16-90 min) in control operated (n=8), sham-operated (n=12) and 10-4, 10-3, 10-2 mol of intra-LC bupropion groups (n=8 in each group). The drug or vehicle was microinfused bilaterally in Locus Coereleus 30 min before the formalin injection. The sham-operated values indicated as %100 in part B. Values are the means ± SEM.
(Repeated-measure one-way ANOVA.* p<0.05, * p<0.01, and *** p<0.001)
Figure 2-B shows the effect of intra-LC bupropion on the average percent of the formalin-induced pain score in phase 01 and 02. In phase 01 and phase 02, intra-LC microinjection of bupropion can decrease pain score percentages which are dose dependent. The analysis of data showed that, intra-LC of bupropion microinfusion decreased the percentage of pain score up to 60% in phase 01 and 58% in phase 02 in comparison with the shamoperated group scores.
3.3. The Effect of Intra-LC Microinjection of Bupropion on Licking/Biting Duration
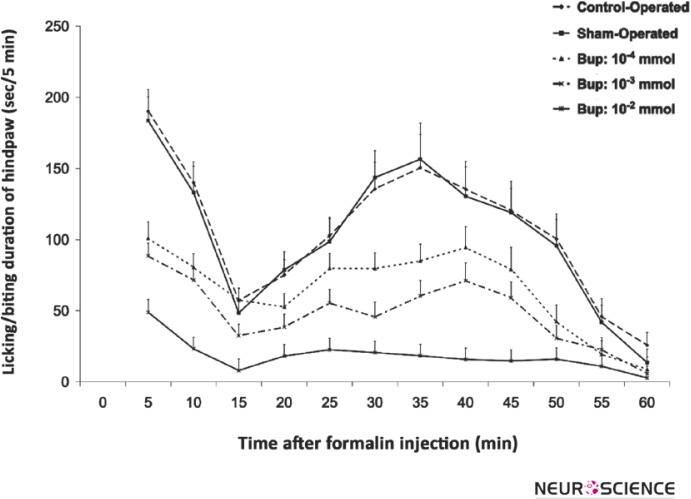
Figure 3 shows the hindpaw licking/biting duration curve in control-operated, the sham-operated and different doses of intra-LC microinjected bupropion. The curve indicates that the licking/biting time duration of the hindpaw with the formalin injection is different in the intra-LC bupropion microinjected according to the control and sham-operated groups. The analysis of licking/biting duration in 5-minute time blocks by repeated-measured one-way ANOVA showed that there is no significant difference between the control and sham-operated groups but the duration of licking/biting duration decreased in intra-LC microinjected bupropion groups. The point-to-point analysis of 5-minute time blocks showed that there is a significant difference in licking/ biting duration among all time blocks of intra-LC bupropion microinjected groups except the last blocks.
Figure 3.
The mean of formalin-induced licking/biting duration in 5 min time blocks during phase 01 (0-10 min) and phase 02 (16-60 min) in the control-operated (n=8), sham-operated (n=12) and 10-4, 10-3, 10-2 mol of intra-LC bupropion groups (n=8 in each group). The drug or vehicle was microinfused bilaterally in Locus Coeruleus 30 min before the formalin injection. Values are means ± SEM. The data of the control and sham-operated groups had no significance.
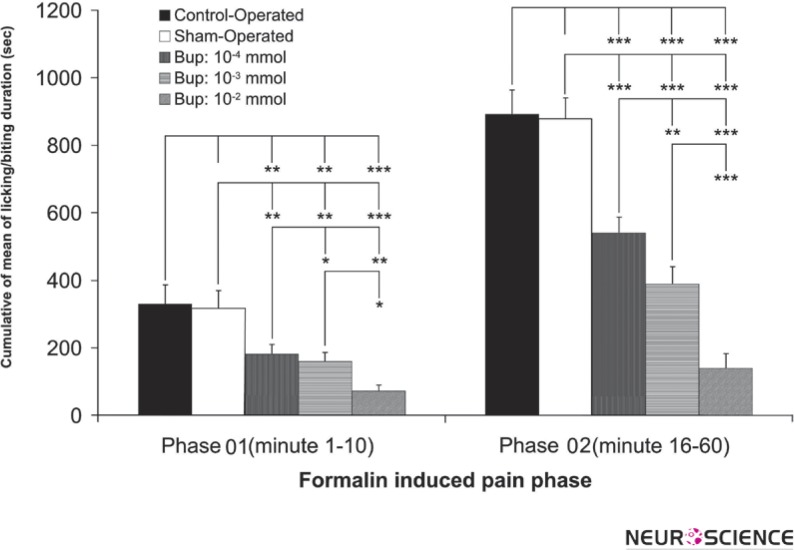
Figure 4 shows the cumulative means of licking/biting duration of the formalin injected hindpaw in phases 01 and 02 in all groups. There was no difference between the control and sham-operated groups but the groups with intra-LC microinjection of bupropion showed a significant difference between themselves and the control/sham groups. Bupropion decreased the licking/biting duration dose dependently. The difference of cumulative means of licking/biting duration is markedly decreased in the second phase of the formalin test.
Figure 4.
The mean percent of formalin-induced licking/biting duration in phase 01 (0-10 min) and phase 02 (16-60 min) in the control operated (n=8), sham-operated (n=12) and 10-4, 10-3, 10-2 mol of intra-LC bupropion groups (n=8 in each group). The drug or vehicle was microinfused bilaterally in Locus Coereleus 30 min before the formalin injection. The sham-operated values indicated as %100 in each phase. Values are the means ± SEM.
(Repeated-measure one-way ANOVA.* p<0.05, ** p<0.01, and *** p<0.001)
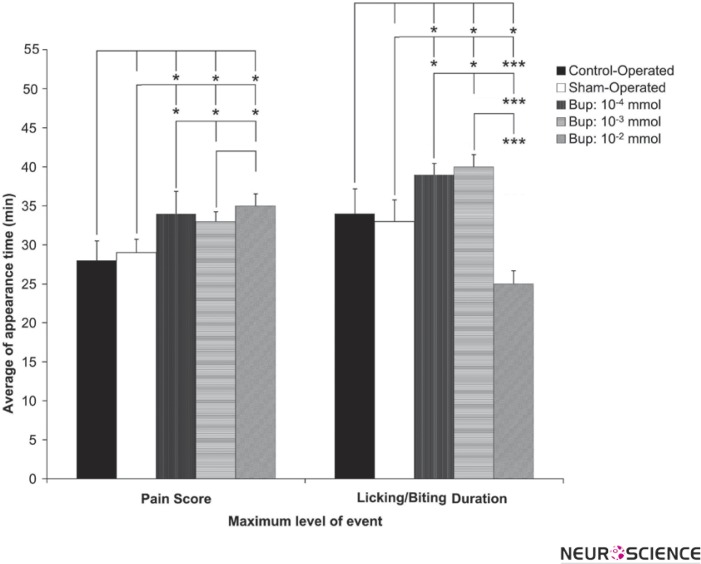
3.4. The Effect of Intra-LC Microinjection of Bupropion on Appearance of Maximum Pain Score and Time of Licking/Biting Behavior
The appearance of the maximum pain score and licking/biting time duration in the animal groups in the second phase revealed that there is a significant difference. Figure 5 shows the time of the appearance of the maximum pain and the licking/biting time. This figure shows the microinjection of bupropion in the LC nuclei can prolong the appearance of pain behavior. The licking/biting appearance time was prolonged by doses of 10-3 and 10-4 but appeared very earlier than the one 10-2 mol of bupropion. The differences were statistically significant.
Figure 5.
The mean of the appearance time of the maximum pain score and licking/biting duration in the phase 02 (16-60 min) of the control-operated (n=8), sham-operated (n=12) and 10-4, 10-3, 10-2 mol of intra-LC bupropion groups (n=8 in each group). The drug or vehicle was microinfused bilaterally in Locus Coeruleus 30 min before the formalin injection. The sham-operated values indicated as %100. Values are means ± SEM.
(Repeated-measure one-way ANOVA.* p<0.05, ** p<0.01, and *** p<0.001)
The overall results showed that intra-LC microinjection of bupropion can alleviate the formalin-induced persistent pain behavior. The decrease of pain behavior is dose-dependent
4. Discussion
Antidepressants have been used as the pain reliever for over 40 years. Although the mechanism of the action of antidepressants leads to an increase in the availability of NE and/ or serotonin, the mechanism of the action underlying their analgesic effects remains unknown. The direct and indirect effects of antidepressants on other neurotransmission systems such as the opioid system have also been proposed. The present study demonstrated that intra-LC microinjection of bupropion can decrease the formalin-induced pain behavior. The pain killing effects of antidepressants have been totally studied and reviewed (Thaler et al., 2012), but the antinociceptive mechanisms of antidepressants are not well known. The formalin-induced pain is very similar to the chronic pain in human. The neuropathic pain is a clinical chronic pain and the sustained application of bupropion in patients with neuropathic pain can relieve it significantly (Semenchuk, Sherman, & Davis, 2001; Shah & Moradimehr, 2010). The serotonin, dopamine and norepinephrine reuptake inhibition is the common mechanism for the antinociception actions of antidepressants with different efficacy and sensitivity. It seems that the pain relief of antidepressants may be achieved at doses lower than those used in the treatment of depression and with a different mechanism (Gallagher, 2006; Jackson & St Onge, 2003; Sharp & Keefe, 2005). Therefore, the most common mechanism of bupropion is an inhibition of noradrenaline and dopamine reuptake. Along with bupropion, some other antidepressants also have an antinociceptive effect (Hawley, 2009; Miller & RabeJablonska, 2005; Sansone & Sansone, 2008). The antinociceptive action of antidepressants in acute pain of the animal models has not provided strong evidence to be used for human clinical pain (Blackburn-Munro, 2004). On the other hand, many antidepressants are effective in the treatment of chronic pain with different degrees. TriCyclic Antidepressants (TCAs) are the most-studied antidepressants for the treatment of chronic pain such as neuropathic pain (Jackson & St Onge, 2003). Compared to TCAs, selective serotonin reuptake inhibitors (SSRIs) are less effective in the management of neuropathic pain (Colombo, Annovazzi, & Comi, 2006; Gallagher, 2006; Sullivan & Robinson, 2006). In a double-blind crossover study of patients with various forms of neuropathic pain, at doses of 300 mg per day, bupropion SR was similar in efficacy to TCAs (Wolfe & Trivedi, 2004). In a chronic neuropathic animal model of pain, bupropion, as a combined reuptake inhibitor of dopamine and noradrenaline, showed greater anti-nociceptive effect than a single action antidepressant such as reboxetine (Pedersen, Nielsen, & Blackburn-Munro, 2005). A recent study on bupropion nociceptive effect showed a surprisingly high efficacy of this drug in peripheral neuropathic pain (Semenchuk et al., 2001).
The monoaminergic system is the predominant biological substrate related to both depression and pain. Its key role belongs to serotonin and noradrenaline (Gormsen, Jensen, Bach, & Rosenberg, 2006; Robinson et al., 2009).
The Locus Coeruleus as A5, A6 aminergic cell group is a major source of noradrenergic projections to the dorsal horn and has a well-established role in pain modulation (L. Liu, Tsuruoka, Maeda, Hayashi, & Inoue, 2007; L. Liu et al., 2008; Maeda, Tsuruoka, Hayashi, Nagasawa, & Inoue, 2009; Ossipov, Dussor, & Porreca, Voisin, Guy, Chalus, & Dallel, 2005). The pain modulation by LC nucleus is mediated by massive projection to dorsal horn, intermediolateral and also ventral horn of spinal cord (Kwiat & Basbaum, 1992; Nuseir & Proudfit, 2000; Tavares & Lima, 1994; Tavares, Lima, & Coimbra, 1996; Westlund, 1992). The projections and interneurons of spinal dorsal horn receive adrenergic synapses that alpha-2 adrenoceptors control the release of nociceptive primary afferent fibers (Coggeshall & Carlton, 1997; Millan, 1992). Electrical stimulation of LC nucleus elicits robust antinociception (W. L. West, Yeomans, & Proudfit, 1993) that is mediated by non alpha-2 adrenoceptors (W. L. West et al., 1993; Zhao & Duggan, 1988).
According to the present data, intra-LC bupropion has same effects on pain processing. It seems that these effects are achieved by NE reuptake inhibition and the increase of the NE in the LC, but the increase of NE in the LC can decrease the firing rate of LC neurons. This is a paradoxical effect, but recent studies of neuronal activity of LC nucleus, adjacent to some of the antidepressants, revealed that they can decrease the firing rate of LC neurons despite their antinociceptive effects (Grant & Weiss, 2001; C. H. West, Ritchie, Boss-Williams, & Weiss, 2009). In contrast to antinociception, LC lesions can reduce tonic behavioral responses to intraplantar formalin injection (Martin, Gupta, Loo, Rohde, & Basbaum, 1999; Taylor, Roderick, & Basbaum, 2000). These data showed that LC plays a complex role in nociception.
It is supposed that the elevation of NE in the spinal cord causes nociception, but its elevation in the LC nucleus causes anti-nociception by decreasing the NE secretion via alpha-2 autoreceptors. The present results showed that intra-LC bupropion can decrease the nociceptive responses despite its action on NE reuptake inhibition. Many researches have concluded that LC neuronal firing rate can increase in pain modulation via neuronal pathway to inhibit central pain nuclei (L. Liu et al., 2007; Tsuruoka, Arai, Nomura, Matsutani, & Willis, 2003; Tsuruoka, Matsutani, Maeda, & Inoue, 2003). Sustained bupropion administration (s.c.) produces a dose-dependent attenuation of the mean spontaneous firing of LC neurons (7.5 mg/kg per day: 15%; 15 mg/kg per day: 61%; 30 mg/kg per day: 80%). This attenuation is reversed by alpha 2-adrenoceptor antagonist idazoxan. Sustained bupropion administration decreased the firing rate of NE neurons due to an increased activation of their inhibitory somatodendritic alpha 2-adrenoceptors. This effect of the bupropion treatment would be attributable mainly to an enhancement of NE release and not to reuptake inhibition (Dong & Blier, 2001).
Although several researches have showed that the neuronal firing rate of LC neurons enhances in acute pain (tail pinch, footshock, heat) (Aston-Jones & Bloom, 1981; Cedarbaum & Aghajanian, 1978; Hajos, Engberg, & Elam, 1986) and chronic pain (Chapman, Suzuki, & Dickenson, 1998; Pertovaara, Kontinen, & Kalso, 1997; Viisanen & Pertovaara, 2007), Alba-Delgado and coworkers have showed that the LC neuronal firing rate did not change in Chronic Constriction Injury (CCI) as a model of neuropathic pain (Alba-Delgado et al., 2012). On the other hand, NE reuptake inhibitor desipramine, elevates endogenous NE and attenuates the firing rate of LC neurons (C. H. West et al., 2009). Rosenberg et al. studied the pain-stimulated and pain-depressed behaviors of various antidepressants. The results of this study suggest the application of some DA/NE/5-HT reuptake inhibitors as antinociceptive agents in some circumstances (Rosenberg, Carroll, & Negus, 2013).
Projection of LC to spinal cord (descending pain pathway), exerts inhibitory influences on pain threshold. Furthermore, projections from LC nucleus to spinal cord control the release of serotonin and adrenaline at the level of the spinal cord. As a general rule, when these monoamines increase in synaptic cleft within the spinal cord there is a decrease in the pain threshold. However, it should be noted that serotonin can both dampen and enhance the sensation of pain, depending on the receptor subtypes activated. On the other hand, antidepressants are the most effective treatment to deal with chronic pain of diverse origins, with or without co-existing depression (Campbell, Clauw, & Keefe, 2003; Mico, Berrocoso, Ortega-Alvaro, Gibert-Rahola, & Rojas-Corrales, 2006). At the supraspinal level, these compounds increase NE and 5-HT levels in the synapse while simultaneously enhancing the activity of the descending inhibitory bulbospinal pathways, thereby producing analgesia.
Another mechanism that is involved in analgesic effects of antidepressants is the activation of the opioid system. The opioid and monoaminergic systems appear to share common molecular mechanisms in mediating nociception (Berrocoso & Mico, 2009; Berrocoso, Sanchez-Blazquez, Garzon, & Mico, 2009). Administration of antidepressants increases opioid receptor density in brain areas, hence the analgesic effects appear dominantly (Ortega-Alvaro et al., 2004). The increase of the opioid receptor depends on treatment duration, dose, and the brain region (Baamonde et al., 1992).
In addition to the monoaminergic and opioid systems, other lesser-known mechanisms have been proposed. There is some evidence in support of the involvement of ionic channels (such as calcium, potassium, and sodium) and neurotransmitter receptors (gamma-aminobutyric acid or GABA, N-methyl-D-aspartate, or NMDA, nicotinic AcethyCholine, or nACh and substance P) in the analgesic mechanism of antidepressants (Antkiewicz-Michaluk, Romanska, Michaluk, & Vetulani, 1991; Galeotti, Ghelardini, & Bartolini, 2001; Pandhare et al., 2012).
The results of this study suggest that the acute inhibition of NE transportation in the vicinity of the LC neurons increases the concentration of the NE in the LC. Although the increase of the NE in the LC can decrease the firing rate of the LC neurons, the projection of LC to other brain regions can contribute to analgesic action of bupropion.
Acknowledgments
This research was part of the MSc dissertation of M. Jahanbani which was supported by the Pakdel's Research Lab, Faculty of Medicine, and Urmia University of Medical Sciences. The authors thanks to Mr Majid Ghaderi Pakdel for final grammatically editing.
Conflict of Interest
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors have no conflicts of interest to declare regarding the study presented in this paper and preparation of the manuscript.
References
- Abbadie, C., Taylor, B. K., Peterson, M. A., & Basbaum, A. I. (1997). Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain, 69(1-2), 101–110 [DOI] [PubMed] [Google Scholar]
- Alba-Delgado, C., Borges, G., Sanchez-Blazquez, P., Ortega, J. E., Horrillo, I., Mico, J. A., et al. (2012). The function of alpha-2-adrenoceptors in the rat locus coeruleus is preserved in the chronic constriction injury model of neuropathic pain. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk, L., Romanska, I., Michaluk, J., & Vetulani, J. (1991). Role of calcium channels in effects of antidepressant drugs on responsiveness to pain. Psychopharmacology (Berl), 105(2), 269–274 [DOI] [PubMed] [Google Scholar]
- Ascher, J. A., Cole, J. O., Colin, J. N., Feighner, J. P., Ferris, R. M., & Fibiger, H. C., et al. (1995). Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry, 56(9), 395–401 [PubMed] [Google Scholar]
- Aston-Jones, G., & Bloom, F. E. (1981). Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci, 1(8), 887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baamonde, A., Dauge, V., Ruiz-Gayo, M., Fulga, I. G., Turcaud, S., & Fournie-Zaluski, M. C., et al. (1992). Antidepressant-type effects of endogenous enkephalins protected by systemic RB 101 are mediated by opioid delta and dopamine D1 receptor stimulation. Eur J Pharmacol, 216(2), 157–166 [DOI] [PubMed] [Google Scholar]
- Balfour, D. J. (2001). The pharmacology underlying pharmacotherapy for tobacco dependence: a focus on bupropion. Int J Clin Pract, 55(1), 53–57 [PubMed] [Google Scholar]
- Balfour, D. J. (2002). The neurobiology of tobacco dependence: a commentary. Respiration, 69(1), 7–11 [DOI] [PubMed] [Google Scholar]
- Berrocoso, E., & Mico, J. A. (2009). Cooperative opioid and serotonergic mechanisms generate superior antidepressant-like effects in a mice model of depression. Int J Neuropsychopharmacol, 12(8), 1033–1044 [DOI] [PubMed] [Google Scholar]
- Berrocoso, E., Sanchez-Blazquez, P., Garzon, J., & Mico, J. A. (2009). Opiates as antidepressants. Curr Pharm Des, 15(14), 1612–1622 [DOI] [PubMed] [Google Scholar]
- Bischoff, S., Bittiger, H., Krauss, J., Vassout, A., & Waldmeier, P. (1984). Affinity changes of rat striatal dopamine receptors in vivo after acute bupropion treatment. Eur J Pharmacol, 104(1-2), 173–176 [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro, G. (2004). Pain-like behaviours in animals - how human are they?. Trends Pharmacol Sci, 25(6), 299–305 [DOI] [PubMed] [Google Scholar]
- Brightwell, J. J., & Taylor, B. K. (2009). Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience, 160(1), 174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet, R., Aigouy, L., & Woda, A. (1995). Enhanced nociceptive behaviour following conditioning injection of formalin in the perioral area of the rat. Brain Res, 676(1), 189–195 [DOI] [PubMed] [Google Scholar]
- Campbell, L. C., Clauw, D. J., & Keefe, F. J. (2003). Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry, 54(3), 399–409 [DOI] [PubMed] [Google Scholar]
- Cedarbaum, J. M., & Aghajanian, G. K. (1978). Activation of locus coeruleus neurons by peripheral stimuli: modulation by a collateral inhibitory mechanism. Life Sci, 23(13), 1383–1392 [DOI] [PubMed] [Google Scholar]
- Chapman, V., Suzuki, R., & Dickenson, A. H. (1998). Electrophysiological characterization of spinal neuronal response properties in anaesthetized rats after ligation of spinal nerves L5-L6. J Physiol, 507(Pt 3), 881–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, A. H. (2007). Extended-release bupropion: an antidepressant with a broad spectrum of therapeutic activity?. Expert Opin Pharmacother, 8(4), 457–466 [DOI] [PubMed] [Google Scholar]
- Coggeshall, R. E., & Carlton, S. M. (1997). Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res Brain Res Rev, 24(1), 28–66 [DOI] [PubMed] [Google Scholar]
- Colombo, B., Annovazzi, P. O., & Comi, G. (2006). Medications for neuropathic pain: current trends. Neurol Sci, 27Suppl 2, S183–189 [DOI] [PubMed] [Google Scholar]
- Dong, J., & Blier, P. (2001). Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl), 155(1), 52–57 [DOI] [PubMed] [Google Scholar]
- Dubuisson, D., & Dennis, S. G. (1977). The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain, 4(2), 161–174 [DOI] [PubMed] [Google Scholar]
- Dwoskin, L. P., Rauhut, A. S., King-Pospisil, K. A., & Bardo, M. T. (2006). Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev, 12(3-4), 178–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti, N., Ghelardini, C., & Bartolini, A. (2001). Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology, 40(1), 75–84 [DOI] [PubMed] [Google Scholar]
- Gallagher, R. M. (2006). Management of neuropathic pain: translating mechanistic advances and evidence-based research into clinical practice, Clin J Pain, 22 (1 Suppl), S2–8 [DOI] [PubMed] [Google Scholar]
- Ghaderi Pakdel, F., Naderi, S., & Zare, S. (2011). The opposite effect of intra-VTA bupropion on chewing and escape attendance behaviors of morphine withdrawal syndrome in rat. Urmia Med J, 21(5), 415–422 [Google Scholar]
- Gormsen, L., Jensen, T. S., Bach, F. W., & Rosenberg, R. (2006). [Pain and depression]. Ugeskr Laeger, 168(20), 1967–1969 [PubMed] [Google Scholar]
- Grant, M. M., & Weiss, J. M. (2001). Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biol Psychiatry, 49(2), 117–129 [DOI] [PubMed] [Google Scholar]
- Hajos, M., Engberg, G., & Elam, M. (1986). Reduced responsiveness of locus coeruleus neurons to cutaneous thermal stimuli in capsaicin-treated rats. Neurosci Lett, 70(3), 382–387 [DOI] [PubMed] [Google Scholar]
- Hawley, P. (2009). Nontricyclic antidepressants for neuropathic pain #187. J Palliat Med, 12(5), 476–477 [DOI] [PubMed] [Google Scholar]
- Hays, J. T., & Ebbert, J. O. (2003). Bupropion sustained release for treatment of tobacco dependence. Mayo Clin Proc, 78(8), 1020–1024; quiz 1024 [DOI] [PubMed] [Google Scholar]
- Huang, J., Chang, J. Y., Woodward, D. J., Baccala, L. A., Han, J. S., & Wang, J. Y., et al. (2006). Dynamic neuronal responses in cortical and thalamic areas during different phases of formalin test in rats. Exp Neurol, 200(1), 124–134 [DOI] [PubMed] [Google Scholar]
- Jackson, K. C. 2nd, & St Onge, E. L. (2003). Antidepressant pharmacotherapy: considerations for the pain clinician. Pain Pract, 3(2), 135–143 [DOI] [PubMed] [Google Scholar]
- Kwiat, G. C., & Basbaum, A. I. (1992). The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosens Mot Res, 9(2), 157–173 [DOI] [PubMed] [Google Scholar]
- Le Bars, D., Gozariu, M., & Cadden, S. W. (2001). [Acute pain measurement in animals. Part 1]. Ann Fr Anesth Reanim, 20(4), 347–365 [DOI] [PubMed] [Google Scholar]
- Lebrun, P., Manil, J., & Colin, F. (2000). Formalin-induced central sensitization in the rat: somatosensory evoked potential data. Neurosci Lett, 283(2), 113–116 [DOI] [PubMed] [Google Scholar]
- Liu, G. J., & Wang, S. (1988). Effects of nucleus raphe magnus and locus coeruleus in descending modulation of the habenula on pain threshold and acupuncture analgesia]. Zhongguo Yao Li Xue Bao, 9(1), 18–22 [PubMed] [Google Scholar]
- Liu, L., Tsuruoka, M., Maeda, M., Hayashi, B., & Inoue, T. (2007). Coeruleospinal inhibition of visceral nociceptive processing in the rat spinal cord. Neurosci Lett, 426(3), 139–144 [DOI] [PubMed] [Google Scholar]
- Liu, L., Tsuruoka, M., Maeda, M., Hayashi, B., Wang, X., & Inoue, T. (2008). Descending modulation of visceral nociceptive transmission from the locus coeruleus/subcoeruleus in the rat. Brain Res Bull, 76(6), 616–625 [DOI] [PubMed] [Google Scholar]
- Maeda, M., Tsuruoka, M., Hayashi, B., Nagasawa, I., & Inoue, T. (2009). Descending pathways from activated locus coeruleus/subcoeruleus following unilateral hindpaw inflammation in the rat. Brain Res Bull, 78(4-5), 170–174 [DOI] [PubMed] [Google Scholar]
- Martin, W. J., Gupta, N. K., Loo, C. M., Rohde, D. S., & Basbaum, A. I. (1999). Differential effects of neurotoxic destruction of descending noradrenergic pathways on acute and persistent nociceptive processing. Pain, 80(1-2), 57–65 [DOI] [PubMed] [Google Scholar]
- Mico, J. A., Berrocoso, E., Ortega-Alvaro, A., Gibert-Rahola, J., & Rojas-Corrales, M. O. (2006). The role of 5-HT1A receptors in research strategy for extensive pain treatment. Curr Top Med Chem, 6(18), 1997–2003 [DOI] [PubMed] [Google Scholar]
- Millan, M. J. (1992). Evidence that an alpha 2A-adrenoceptor subtype mediates antinociception in mice. Eur J Pharmacol, 215(2-3), 355–356 [DOI] [PubMed] [Google Scholar]
- Miller, A., & Rabe-Jablonska, J. (2005). The effectiveness of antidepressants in the treatment of chronic non-cancer pain--a review]. Psychiatr Pol, 39(1), 21–32 [PubMed] [Google Scholar]
- Mokhtari hashtjin, M., Zare, S., Ghaderi Pakdel, F., & Heysieattalab, S. (2010). The effect of intra-VTA injection of Bupropion on submissive defensive aggressive behavior induced by electrical foot shock of rat. Pharmaceutical Sciences, 16(3), 125–130 [Google Scholar]
- Nuseir, K., & Proudfit, H. K. (2000). Bidirectional modulation of nociception by GABA neurons in the dorsolateral pontine tegmentum that tonically inhibit spinally projecting noradrenergic A7 neurons. Neuroscience, 96(4), 773–783 [DOI] [PubMed] [Google Scholar]
- Nygren, L. G., & Olson, L. (1977). A new major projection from locus coeruleus: the main source of noradrenergic nerve terminals in the ventral and dorsal columns of the spinal cord. Brain Res, 132(1), 85–93 [DOI] [PubMed] [Google Scholar]
- Ortega-Alvaro, A., Acebes, I., Saracibar, G., Echevarria, E., Casis, L., & Mico, J. A. (2004). Effect of the antidepressant nefazodone on the density of cells expressing mu-opioid receptors in discrete brain areas processing sensory and affective dimensions of pain. Psychopharmacology (Berl), 176(3-4), 305–311 [DOI] [PubMed] [Google Scholar]
- Ossipov, M. H., Dussor, G. O., & Porreca, F.. Central modulation of pain, J Clin Invest, 120(11), 3779–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandhare, A., Hamouda, A. K., Staggs, B., Aggarwal, S., Duddempudi, P. K., & Lever, J. R., et al. (2012). Bupropion binds to two sites in the Torpedo nicotinic acetylcholine receptor transmembrane domain: a photoaffinity labeling study with the bupropion analogue [(125)I]-SADU-3-72. Biochemistry, 51(12), 2425–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos, G., & Watson, C. (2005). The Rat Brain in Stereotaxic Coordinates (5th ed.). San Diego, CA: Academic Press Inc. [Google Scholar]
- Pedersen, L. H., Nielsen, A. N., & Blackburn-Munro, G. (2005). Anti-nociception is selectively enhanced by parallel inhibition of multiple subtypes of monoamine transporters in rat models of persistent and neuropathic pain. Psychopharmacology (Berl), 182(4), 551–561 [DOI] [PubMed] [Google Scholar]
- Pertovaara, A., Kontinen, V. K., & Kalso, E. A. (1997). Chronic spinal nerve ligation induces changes in response characteristics of nociceptive spinal dorsal horn neurons and in their descending regulation originating in the periaqueductal gray in the rat. Exp Neurol, 147(2), 428–436 [DOI] [PubMed] [Google Scholar]
- Robinson, M. J., Edwards, S. E., Iyengar, S., Bymaster, F., Clark, M., & Katon, W. (2009). Depression and pain. Front Biosci (Landmark Ed), 14, 5031–5051 [DOI] [PubMed] [Google Scholar]
- Rosenberg, M. B., Carroll, F. I., & Negus, S. S. (2013). Effects of monoamine reuptake inhibitors in assays of acute painstimulated and pain-depressed behavior in rats. J Pain, 14(3), 246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone, R. A., & Sansone, L. A. (2008). Pain, pain, go away: antidepressants and pain management. Psychiatry (Edgmont), 5(12), 16–19 [PMC free article] [PubMed] [Google Scholar]
- Semenchuk, M. R., Sherman, S., & Davis, B. (2001). Doubleblind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology, 57(9), 1583–1588 [DOI] [PubMed] [Google Scholar]
- Shah, T. H., & Moradimehr, A. (2010). Bupropion for the Treatment of Neuropathic Pain. Am J Hosp Palliat Care. [DOI] [PubMed] [Google Scholar]
- Sharp, J., & Keefe, B. (2005). Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep, 7(3), 213–219 [DOI] [PubMed] [Google Scholar]
- Sufka, K. J., Watson, G. S., Nothdurft, R. E., & Mogil, J. S. (1998). Scoring the mouse formalin test: validation study. Eur J Pain, 2(4), 351–358 [DOI] [PubMed] [Google Scholar]
- Sullivan, M. D., & Robinson, J. P. (2006). Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am, 17(2), 381–400, vi-vii [DOI] [PubMed] [Google Scholar]
- Tavares, I., & Lima, D. (1994). Descending projections from the caudal medulla oblongata to the superficial or deep dorsal horn of the rat spinal cord. Exp Brain Res, 99(3), 455–463 [DOI] [PubMed] [Google Scholar]
- Tavares, I., Lima, D., & Coimbra, A. (1996). The ventrolateral medulla of the rat is connected with the spinal cord dorsal horn by an indirect descending pathway relayed in the A5 noradrenergic cell group. J Comp Neurol, 374(1), 84–95 [DOI] [PubMed] [Google Scholar]
- Taylor, B. K., Roderick, R. E., & Basbaum, A. I. (2000). Brainstem noradrenergic control of nociception is abnormal in the spontaneously hypertensive rat. Neurosci Lett, 291(3), 139–142 [DOI] [PubMed] [Google Scholar]
- Thaler, K. J., Morgan, L. C., Van Noord, M., Gaynes, B. N., Hansen, R. A., & Lux, L. J., et al. (2012). Comparative effectiveness of second-generation antidepressants for accompanying anxiety, insomnia, and pain in depressed patients: a systematic review. Depress Anxiety, 29(6), 495–505 [DOI] [PubMed] [Google Scholar]
- Tjolsen, A., Berge, O. G., Hunskaar, S., Rosland, J. H., & Hole, K. (1992). The formalin test: an evaluation of the method. Pain, 51(1), 5–17 [DOI] [PubMed] [Google Scholar]
- Tsuruoka, M., Arai, Y. C., Nomura, H., Matsutani, K., & Willis, W. D. (2003). Unilateral hindpaw inflammation induces bilateral activation of the locus coeruleus and the nucleus subcoeruleus in the rat. Brain Res Bull, 61(2), 117–123 [DOI] [PubMed] [Google Scholar]
- Tsuruoka, M., Matsutani, K., Maeda, M., & Inoue, T. (2003). Coeruleotrigeminal inhibition of nociceptive processing in the rat trigeminal subnucleus caudalis. Brain Res, 993(1-2), 146–153 [DOI] [PubMed] [Google Scholar]
- Vaccarino, A. L., & Chorney, D. A. (1994). Descending modulation of central neural plasticity in the formalin pain test. Brain Res, 666(1), 104–108 [DOI] [PubMed] [Google Scholar]
- Viisanen, H., & Pertovaara, A. (2007). Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience, 146(4), 1785–1794 [DOI] [PubMed] [Google Scholar]
- Voisin, D. L., Guy, N., Chalus, M., & Dallel, R. (2005). Nociceptive stimulation activates locus coeruleus neurones projecting to the somatosensory thalamus in the rat. J Physiol, 566(Pt 3), 929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, A., Greif, J., Yemini, Z., Lerman, H., Weizman, A., & Even-Sapir, E. (2009). Attenuation of cue-induced smoking urges and brain reward activity in smokers treated successfully with bupropion. J Psychopharmacol. [DOI] [PubMed] [Google Scholar]
- West, C. H., Ritchie, J. C., Boss-Williams, K. A., & Weiss, J. M. (2009). Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol, 12(5), 627–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, W. L., Yeomans, D. C., & Proudfit, H. K. (1993). The function of noradrenergic neurons in mediating antinociception induced by electrical stimulation of the locus coeruleus in two different sources of Sprague-Dawley rats. Brain Res, 626(1-2), 127–135 [DOI] [PubMed] [Google Scholar]
- Westlund, K. N. (1992). Anatomy of noradrenergic pathways modulating pain. In Besson J. M. & Guilbaud G. (Eds.), Towards the Use of Noradrenergic Agonists for the Treatment of Pain (pp. 91–118). Amsterdam: Elsevier [Google Scholar]
- Wheeler-Aceto, H., & Cowan, A. (1991). Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology (Berl), 104(1), 35–44 [DOI] [PubMed] [Google Scholar]
- Wolfe, G. I., & Trivedi, J. R. (2004). Painful peripheral neuropathy and its nonsurgical treatment. Muscle Nerve, 30(1), 3–19 [DOI] [PubMed] [Google Scholar]
- Zhao, Z. Q., & Duggan, A. W. (1988). Idazoxan blocks the action of noradrenaline but not spinal inhibition from electrical stimulation of the locus coeruleus and nucleus Kolliker-Fuse of the cat. Neuroscience, 25(3), 997–1005 [DOI] [PubMed] [Google Scholar]