Abstract
Combined use of an opioid with a psychostimulant is popular among drug abusers. Such “polydrug use” may increase drug effects or attenuate adverse effects of either drug alone. We proposed that a combination of methamphetamine (meth) and morphine may change physical opioid withdrawal symptoms. Adult male rats were chronically injected with cumulative subcutaneous (s.c.) doses of morphine, meth or a combination of both drugs within five days. On day six, a challenge dose of the same drug was injected. Two hours later, precipitated withdrawal symptoms were scored within 30 minutes after naloxone (1mg/kg, i.p.) injection. Both frequency and incidence of jumping significantly increased in combined treated animals (P<0.05). The sole emergent symptom in combined treated animals was digging which we consider as another escaping behavior in addition to jumping. Our findings imply that combined use of meth and morphine may exacerbate averseness of morphine withdrawal which may cause more intense opioid dependence.
Keywords: Polydrug Use, Morphine, Meth, Naloxone, Withdrawal Syndrome, Rat
1. Introduction
Some drug users administer a combination of popular addictive substances possibly to intensify rewarding effects, experience a unique effect or attenuate side effects (Leri, Bruneau, & Stewart, 2003a; Guzman & Ettenberg, 2004). Such “polydrug use” may increase mortality rate (Uemura, Sorimachi, Yashik, & Yoshida, 2003) and complicate the treatment process. Combination of a psycho stimulant with an opiate known as “speedball” is very popular especially involving cocaine and heroin (Trujillo, Smith, & Guaderrama, 2011). However, recent reports indicate an increase in world prevalence of amphetamine-type stimulant abuse (UNODC, World Drug Report 2012; Vocci & Ling, 2005). Considering worldwide prevalence of opioid abuse, (UNODC, World Drug Report 2012) high prevalence of combined abuse of meth and opioid is plausible.
Repeated drug administration leads to molecular and even morphological adaptations in drug target areas in the brain (Nestler, 1997). These adaptations can cause severe somatic symptoms and/or motivational effects after drug cessation that forces the subject to seek for the drug. While physical withdrawal symptoms are severe in opioid and alcohol dependence, they do not occur during cocaine or amphetamine abstinence. However, emotional and motivational symptoms are expressed in any kind of drug abstinence (Hyman, Malenka, & Nestler, 2006).
Most of research on polydrug use has been focused on cocaine and heroin co-administration (Trujillo, Smith, & Guaderrama, 2011). Previous studies revealed that psychostimulants and opioids have stronger effects in combined administration (Masukawa, Suzuki, & Misawa, 1993; Trujillo, Smith, & Guaderrama, 2011; Ito, Mori, Namiki, Suzuki, & Sawaguchi, 2007; Mori, Ito, Narita, Suzuki, & Sawaguchi, 2004). These studies mostly explore the acute behavioral and motivational effects of polydrug use. It is unclear how physical symptoms of opioid withdrawal differ in polydrug use. Moreover, recent human studies demonstrate that opioid antagonist naltrexone administration can be effective in treatment of meth dependence (Jayaram-Lindstrom et al., 2008; Jayaram-Lindstrom, Hammarberg, Beck, & Franck, 2008). Although these studies reported no physical adverse effect in human subjects, controlled animal experiments are necessary to confirm these evidences.
The aim of the current study is to determine whether chronic morphine and meth co-administration changes naloxone precipitated morphine withdrawal symptoms. The results may be clinically important since the treatment of polydug users is not straight due to the combined effects of drugs.
2. Methods
2.1. Animals
43 male Wistar rats weighting 250-350 were used in this experiment. They were housed in groups of four per cage in a colony house with food and water supply ad libitum. Twelve hours light/dark cycle and a controlled temperature of 25 ± 2°C were set for the colony room throughout the experiments. This experiment was performed in accordance with ethical guidelines for animal subject care and use at Neuroscience Research Center, Shahid Beheshti Medical University.
2.2. Drugs
Morphine sulfate (Temad, Tehran, Iran) and methamphetamine HCl (anti-narcotics police, Tehran, Iran) were dissolved in physiological saline and were injected subcutaneously in a volume of 1.0 ml/kg in all dose combinations. Naloxone (Sigma Aldrich, USA) was also dissolved in physiological saline and was injected intraperitoneally on test day. Drug solutions were diluted to contain their respective doses and kept in 4°C. To make the experiment blind, drug solutions were kept in identical containers with their first labels (e.g. Mor, Meth, Mor+Meth, Sal) covered with a blank label. The containers were then shuffled and second labels marked with numbers 1-7.
2.3. Experimental Procedure
The rats were randomly assigned to each drug by sorting rat labels (1-43) against random drug labels (1-7). Morphine assigned rats were injected subcutaneously with cumulative doses of 5, 10, 20, 30 and 40 mg/kg per day within five days. Each dose-per-day was divided into two identical injections with 12 hour intervals. On day six, rats were injected by a single dose of morphine (40 mg/kg) and were then returned to their cages. Two hours after morphine injection, they received 1 mg/kg naloxone intraperitoneally and were placed into a cylindrical test chamber. Naloxone induced morphine withdrawal signs were monitored for 30 minutes. Similarly, another group of rats received meth cumulative doses of 1, 2, 4, 6, and 8 mg/kg per day. On day six, rats were injected by a single dose of meth (8 mg/kg). Naloxone (1 mg/kg) was injected after 2 hours and behavioral signs were monitored. Meth and morphine combinations were made by mixing corresponding doses in each day within the 5-day period and were injected subcutaneously. On day six, a combination of 8 mg/kg meth and 40 mg/kg morphine was injected and naltrexone (1 mg/kg) induced withdrawal signs were monitored for 30 minutes. In addition to a saline group, 3 further groups were added where the above mentioned drug doses were made half in all corresponding days (2.5, 5, 10, 15 and 20 mg/ kg morphine and 0.5, 1, 2, 3 and 4 mg/kg meth).
2.4. Data Analysis
Withdrawal behavioral signs were scored separately. Percent of incidence of each symptom as well as the average total number of each symptom was compared among the groups using chi-square and Kruskal–Wallis followed by Dunn's tests respectively. Data are presented by contingency plots and mean ± SEM.
3. Results
3.1. Mortality Rate
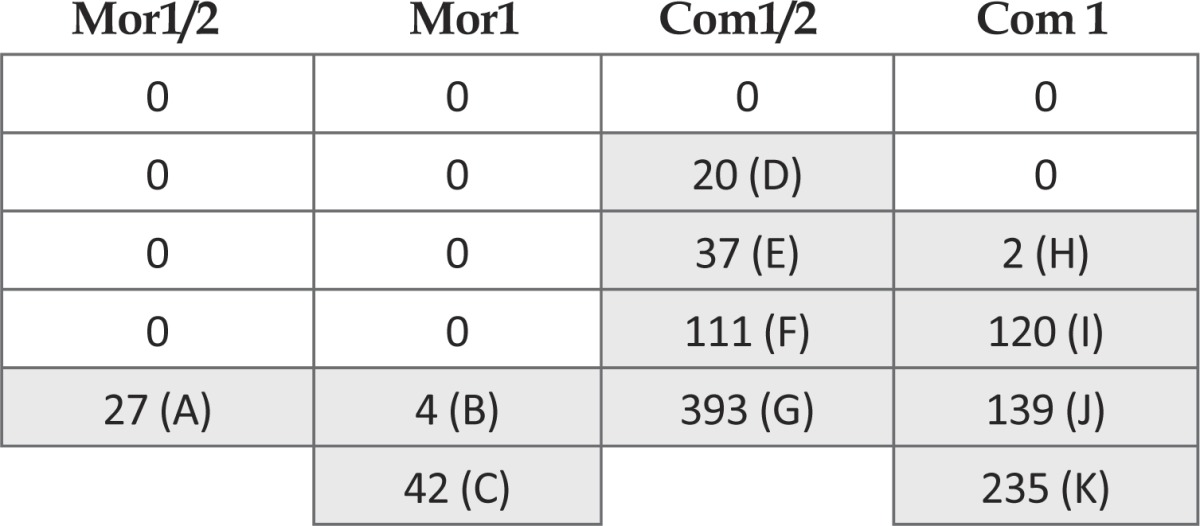
Out of 43 rats, 4 died after a high drug does injection and one died after naloxone injection. Combined, higher dosage of morphine (20 mg/kg) and meth (4 mg/kg) injection, caused mortality of 2 rats out of 13. However, two out of 7 animals died after injection of 20 mg/kg morphine alone. In higher does combinations (morphine 40 mg/kg+meth 5mg/kg) two out of 7 animals went into a deep coma 1-2 hours after dosing and recovered by naloxone injection, then were observed for behavioral signs. From these 7 rats, which were assigned to the higher dose combination of morphine plus meth, one rat (K, Table 1) died 20 min after naloxone injection. It was the only case in 43 rats which died after naloxone injection.
Table 1.
Number of jumps is presented for morphine alone and combined treated animals. Capital letters tag each rat for further reference (Table 2).
3.1. Naloxone Induced Jumping
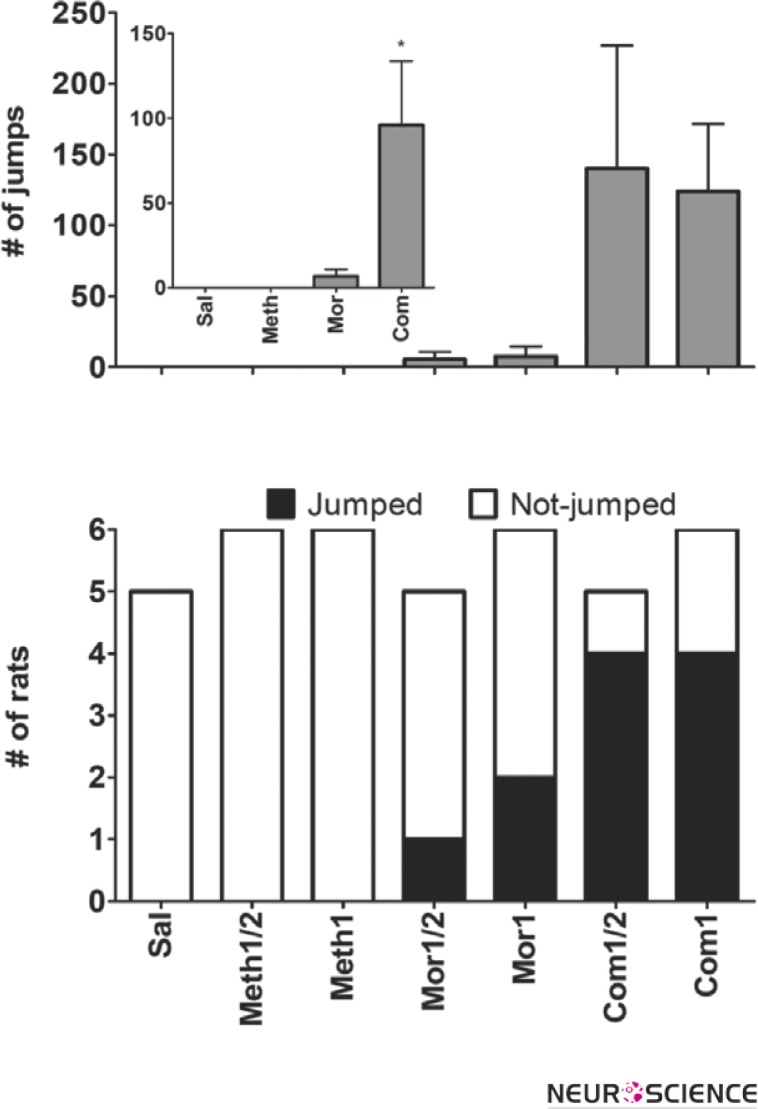
Jumping behavior was strongly dependent on the drug treatment (Fig. 1). Neither saline treated rats nor meth alone treated ones jumped after naloxone injection. In lower dose morphine treatment, one out of 5 and in higher dose 2 out of 6 rats jumped after naloxone injection. Combined treated rats showed a higher incidence and frequency of jumping. In lower dose 4 out of 5 and in higher dose 4 out of 6 animals jumped.
Figure 1.
Naloxone induced jumping. Upper: jumping frequency (mean ± SEM). Inset shows grouping of drug treatments regardless of drug dose. Asterisk mark indicates significant difference of combined (Com) versus saline (Sal) and meth groups (Kruskal–Wallis followed by Dunn's tests, P<0.05). Lower: contingency plot represents a significant trend in the incidence of jumping across groups (qui-square test for trend, P<0.001).
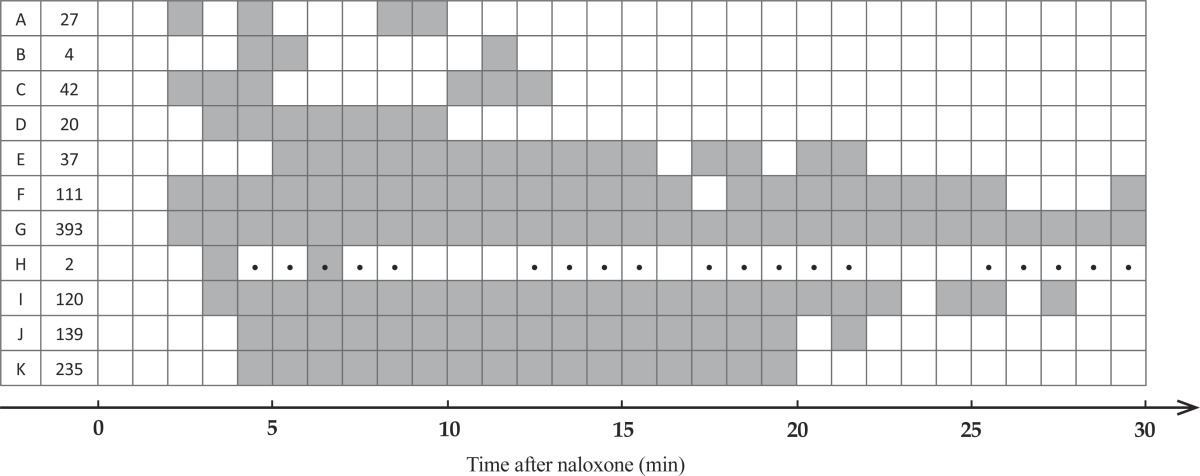
The maximum number of jumps within one observing session was 393 in a morphine plus methamphetamine treated rat (G in Table 1) while maximum number of jumps in morphine treated groups was 42 (C in Table 1). Rat H also expressed digging behavior that we consider as “escaping behavior” in addition to the jumping. None of other rats expressed digging after naloxone injection. Jumping expression in morphine alone treated animals was limited to minutes 3-13 with a biphasic pattern (Table 2). However, combined treated animals expressed jumping more continuous and in a longer timeframe. Overall, the incidence of naloxone induced jumping were significantly increased in combined treated animals compared to saline (qui-square test for trend; P<0.001). Kruskal–Wallis test revealed a significant difference in frequency of jumping among groups. However, due to high variation within groups, paired comparisons showed no significant difference. Therefore, regardless of drug dose we mixed animals into four groups; 1) Morphine, 2) Meth, 3) Combined (morphine plus meth) and 4) Saline. Here, Dunn's post tests revealed significantly higher frequency of jumping in combined treated rats compared to the saline and meth treated ones (P<0.05).
Table 2.
Temporal expression of jumping in morphine (A-C) and combined (D-K) treated rats (Table 1). Second column represents number of jumps for each rat. Next columns indicate the time from naloxone injection in one minute steps. Gray cells show the incidence of jumping while the asterisk marks represent the incidence of digging.
3.1. Other Behavioral Signs
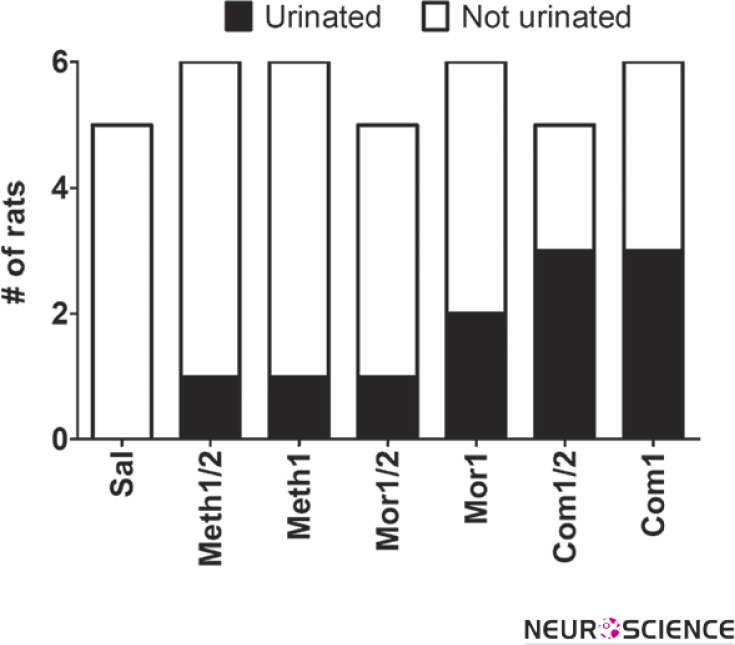
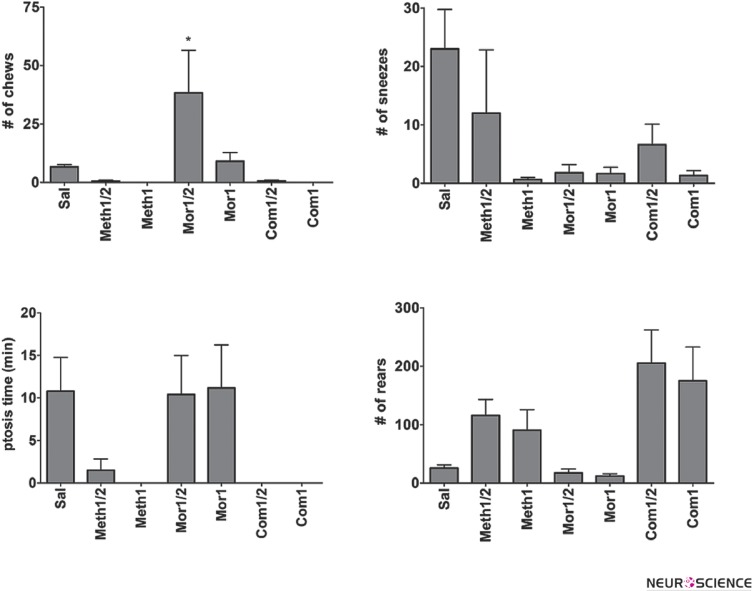
We quantified other observable behaviors like chewing, ptosis, rearing, urination, and sneezing. Among these behaviors, urination showed a trend more similar to the jumping (Fig. 2, Qui-square test for trend, P<0.05). However, we were unable to quantify frequency of urination. On the other hand, chewing, sneezing, ptosis and rearing are quantified with frequency (Fig. 3). It worth to state that animals treated by meth alone expressed no emergent behavior after naloxone injection.
Figure 2.
The incidence of urination has a significant rising trend across treated groups (qui-square test for trend, P<0.05).
Figure 3.
Further behavioral signs. Mean frequency of chewing is significantly higher in morphine ½ treated rats (* versus P<0.05). Other signs are not compared statistically due to the confounding factors (see the discussion).
4. Discussion
Our results indicate that chronically administered combination of morphine and meth increase both incidence and frequency of naloxone induced jumping. Interestingly, higher number of jumps was in lower dose treatment, confirming an emergent or synergistic effect of drug combination.
We chose a cumulative dosing paradigm to expose animals maximally with drugs. However, we concerned that possible high mortality rates may limit data collection. Thus, three further groups were added in which drug doses were made half of the original.
Jumping behavior is considered as an attempt to escape from the test chamber during withdrawal (Liu, Rockhold, & Ho, 1999). Therefore, we can assume that this behavior implies anxiety and stress during antagonist induced opioid withdrawal. Jumping expression in rats is highly variable and context dependent (Azizi, Ranjbar-Slamloo, & Semnanian, 2012). Nevertheless, the involvement of brain stress system in vertical jumping behavior leads us to conclude that jumping may be a result of drug induced maladaptation in noradrenergic and dopaminergic systems in rat brain (Nishikawa, Tanaka, Kohno, Tsuda, & Nagasaki, 1981). On the other hand, our results did not show a significant increase or emergence of other symptoms except urination. Then, we propose two possibilities; combined administration of morphine and meth may alleviate some of the symptoms such as diarrhea, scratching or chewing, while exacerbate escaping attempts. Another possibility is that the behavior is a compulsive motor action induced by naloxone in combined treated animals. Meth itself caused motor hyperactivity and compulsive behaviors in higher doses in some rats. However, none of meth treated rats showed an escape behavior such as jumping and digging. Two further evidences can support the former interpretation. First, one of the combined treated rats expressed digging corners of the chamber in a very frequent manner; like a helpless animal trying to find a way outside an aversive environment. Second, two of the rats expressed writing, another prominent withdrawal sign, back in their home cage. Thus, it is credible to argue that other symptoms are masked with escaping attempts caused by place aversion. In contrast to our results, Sprague and Takemori showed that meth plus morphine treatment is not able to increase naloxone induced jumping in mice (Sprague & Takemori, 1978). This controversy may result from their acute paradigm to induce physical dependence compared to our chronic paradigm.
Other behavioral signs are not consistently changed across different treated groups. Rearing, for example is confounding since meth treated animals expressed rearing repeatedly, after each dosing. Rearing is also an exploratory normal behavior which is also expressed in saline treated group even more than morphine treated ones. Ptosis was also confounding since it was expressed during resting phases of saline treated rats as well as in withdrawal phase of morphine treated ones. Chewing frequency was significantly higher in morphine treated animals (Figure 3).
Mortality rate did not increase with drug combination. Apparently, 20 mg/kg morphine dose alone induced maximum mortality rate (%28). However, the sample size is too small for a contingency analysis. Furthermore, tolerance and cross-tolerance (Sprague & Takemori, 1978) make the mortality analysis less reliable.
We conclude that morphine and meth chronic co-administration might have caused more intense escaping behaviors in rats, reflecting severe place aversion or helplessness. According to our predictions, future studies can reveal place aversion with robust methods like conditional place aversion in lower dose combinations. Additionally, spontaneous withdrawal paradigms can be used to quantify escaping behaviors along with other symptoms. Further extension of our study may help to establish the involvement of physical and psychological withdrawal in polydrug use.
Acknowledgements
This work was supported by the grants from Neuroscience Research Center, Baqiyatallah University of Medical Science and Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- Azizi, H., Ranjbar-Slamloo, Y., & Semnanian, S. (2012). Heightdependent difference in the expression of naloxone-induced withdrawal jumping behavior in morphine dependent rats. Neuroscience Letters, 515, 174–176 [DOI] [PubMed] [Google Scholar]
- Cornish, J., Lontos, J., Clemens, K., & McGregor, I. (2005). Cocaine and heroin (‘speedball’) selfadministration:the involvement of nucleus accumbens dopamine and mu-opiate,but not delta-opiate receptors. Psychopharmacology (Berl), 180, 21–32 [DOI] [PubMed] [Google Scholar]
- Guzman, D., & Ettenberg, A. (2004). Heroin attenuates the negative consequences of cocaine in a runway model of selfadministration. Pharmacol Biochem Behav, 79, 317–24 [DOI] [PubMed] [Google Scholar]
- Hyman, S., Malenka, R., & Nestler, E. (2006). Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annu Rev Neurosci, 29, 565–98 [DOI] [PubMed] [Google Scholar]
- Ito, S., Mori, T., Namiki, M., Suzuki, T., & Sawaguchi, T. (2007). Complicated interaction between psychostimulants and morphine in expression of phenotype of behavior in the dopaminergic system of BALB/c mice. J Pharmacol Sci, 105, 326–33 [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom, N., Hammarberg, A., Beck, O., & Franck, J. (2008). Naltrexone for the Treatment of Amphetamine Dependence: A Randomized, Placebo-Controlled Trial. Am J Psychiatry, 165, 1442–1448 [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom, N., Konstenius, M., Eksborg, S., Beck, O., Hammarberg, A., & Franck, J. (2008). Naltrexone Attenuates the Subjective Effects of Amphetamine in Patients with Amphetamine Dependence. Neuropsychopharmacology, 33, 1856–1863 [DOI] [PubMed] [Google Scholar]
- Leri, F., Bruneau, J., & Stewart, J. (2003a). Understanding polydrug use: review of heroin and cocaine co-use. Addiction, 98, 7–22 [DOI] [PubMed] [Google Scholar]
- Liu, N., Rockhold, R. W., & Ho, I. K. (1999). Electrical stimulation of nucleus paragigantocellularis induces opioid withdrawal-like behaviors in the rat. Pharmacol. Biochem. Behav, 62, 263–271 [DOI] [PubMed] [Google Scholar]
- Masukawa, Y., Suzuki, T., & Misawa, M. (1993). Differential modification of the rewarding effects of methamphetamine and cocaine by opioids and antihistamines. Psychopharmacology (Berl), 111, 139–43 [DOI] [PubMed] [Google Scholar]
- Mori, T., Ito, S., Narita, M., Suzuki, T., & Sawaguchi, T. (2004). Combined effects of psychostimulants and morphine on locomotor activity in mice. J Pharmacol Sci, 96, 450–8 [DOI] [PubMed] [Google Scholar]
- Nestler, E. (1997). Molecular Mechanisms Underlying Opiate Addiction: Implications for Medications Development. Seminars in NEUROSCIENCE, 9, 84–93 [Google Scholar]
- Nishikawa, T., Tanaka, M., Kohno, Y., Tsuda, A., & Nagasaki, N. (1981). Involvement of noradrenergic and dopaminergic neurons in shock-induced jumping in rats. Europian Journal of Pharmacology, 71, 429–436 [DOI] [PubMed] [Google Scholar]
- Sprague, G. L., & Takemori, A. E. (1978). Effect of methamphetamine on the development of acute morphine tolerance and dependence in mice. Life Sciences, 2491–2498 [DOI] [PubMed] [Google Scholar]
- Trujillo, K. A., Smith, M. L., & Guaderrama, M. M. (2011). Powerful behavioral interactions between methamphetamine and morphine, Pharmacology. Biochemistry and Behavior, 99, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura, K., Sorimachi, Y., Yashik, i. M., & Yoshida, K. (2003). Two fatal cases involving concurrent use of methamphetamine and morphine. J Forensic Sci, 48, 1179–81 [PubMed] [Google Scholar]
- Vocci, F., & Ling, W. (2005). Medications development: successes and challenges. Pharmacol Ther, 108, 94–108 [DOI] [PubMed] [Google Scholar]
- World Drug Report 2012. Vienna: United Nations [Google Scholar]