Abstract
Introduction
Astrocytes are the most abundant glial cell type. In addition to their neurological roles, astrocytes also have immune functions. They have been involved in antigen presentation in the central nervous system (CNS). Activated astrocytes express adhesion molecules, chemokines and release several inflammatory mediators, pro-inflammatory cytokines, neurotrophic and neuroprotective factors, thus these cells have a dual role within the CNS: neuroinflammation and repair processes.
IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B, and IL-29 are members of the IL-10 family of cytokines. These cytokines have different biological functions in spite of partial amino acid sequences homology. Signal transduction of the IL-10 family of cytokines is through R1-type and R2-type receptors.
Methods
No information has been available about the expression and regulation of IL-19 in mice astrocytes and brain. To investigate the expression of IL-19, we examined its expression in C57BL/6 mice astroglial cells in response to lipopolysaccharide (LPS), using reverse-transcription polymerase chain reaction (RT-PCR) method.
Results
We provide for the first time, evidence that astrocytes can express IL-19 mRNA following LPS stimulation. Furthermore, we have found the expression of IL-19 mRNA in the cortex of adult C57BL/6 mice following intraperitoneal (i.p.) administration of LPS.
Discussion
This finding will contribute to current knowledge on the function and behavior of cells and mediators during inflammatory conditions in the brain.
Keywords: IL-19, Mice Astroglial Cells, brain Cortex, Lipopolysaccharide
1. Introduction
Glial cells play an important role in controlling of CNS inflammation. Astrocytes are the most abundant glial cell type in the brain (Kim, Hong, & Ro, 2011). Astrocytes are multifunctional glial cells that regulate extracellular ion and neurotransmitter concentrations, and are also involved in the immune responses. Astrocytes produce neurotrophic and neuroprotective factors and participate in the CNS repair procedure (Minagar et al., 2002).
When inflammation occurs in the brain, astrocytes are activated and involved in the process of reactive gliosis and the formation of a glial scar (Ledeboer et al., 2002). Astrocytes take part in immune functions by expression of adhesion molecules, chemokines and production of proinflammatory mediators such as IL-1, IL-6, and tumor necrosis factor-α (TNF-α) in response to a variety of stimuli (Dong & Benveniste, 2001).
Astrocytes may participate in the downregulation of T cell autoreactivity in the CNS. Indeed, astrocytes can suppress microglial IL-12 production which is crucial for Th1 differentiation. In addition, Astrocytes produce several immunosuppressive molecules for example prostaglandin E2 (PGE2) or transforming growth factor-β (TGF-β) (Aloisi, Ria, & Adorini, 2000).
Astrocytes represent the non-professional class of CNS-resident antigen presenting cells (APCs) (Constantinescu et al., 2005). These cells can not constitutively express MHC class II molecules; however, MHC class II expression can be induced with Interferon (IFN)-γ, and further modulated by TNF-α (Dong & Benveniste, 2001). In vitro activated astrocytes can stimulate autoreactive T cells, and it has been suggested that astrocytes may promote CNS inflammation (Kort et al., 2006).
The IL-10 family of cytokines has different biological functions and includes IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B, and IL-29 (Sabat et al., 2007; Sabat et al., 2010; Zdanov, 2010). Data have shown that IL-19, IL-20, IL-22, IL-24, and IL-26 have structural homology and constitute the IL-20 subfamily, In fact, IL-10 is an immunosuppressive cytokine but and it seems likely that these cytokines belonging to IL-20 subfamily are proinflammatory (Sa et al., 2007). IL-10, IL-19, IL-20, and IL-24 are primarily secreted by activated macrophages, whereas T cells are the main source of IL-22, IL-26, and IL-28 (Wolk et al., 2010; Gallagher et al., 2000).
The IL-10 family of cytokine binds to heterodimeric transmembrane receptor complexes that are composed of a long α-chain (R1-type; with a long cytoplasmic domain) and a shorter β-chain (R2-type; with a short cytoplasmic domain) (Blumberg et al., 2001).
IL-19 has 21% shared amino acid similarity with IL-10. Previous findings indicated that IL-19 is primarily produced by monocytes, and LPS, IL-4, and granulocyte monocyte-colony stimulating factor (GM-CSF) can induce its expression (Blumberg et al., 2001; Gallagher et al., 2000). Furthermore, keratinocytes and bronchial epithelia have also been reported to express IL-19 in vitro under stimulatory conditions (Sa et al., 2007). IL-19 signaling occurs through a receptor complex composed of the IL-20R1 and IL-20R2 chains and activates monocytes in an autocrine and paracrine fashion (Blumberg et al., 2001).
Concerning the biological effects of IL-19, controversial data exist. Several investigators have demonstrated that long time exposure of T cells to IL-19 plays a role in the appearance of increased numbers of IL-4 and IL-13 producing and fewer IFN-γ producing cells; therefore, they have implicated IL-19 in Th2 immune differentiation. In addition, IL-19 increased IL-10 production in peripheral blood mononuclear cells (PBMCs) (Leng et al., 2011; Jordan et al., 2005; Oral et al., 2006). These observations suggest that IL-19 may have immunoregulatory roles (Liao et al., 2004; Gallagher et al., 2004; Gallagher, 2010).
In contrast, several studies reported that IL-19 has an inflammatory role. For instance, Liao and colleague were the first to report that IL-19 up-regulated the production of IL-6, TNF-α and numerous reactive oxygen species (ROS) and participate in pro-apoptotic functions in monocytes (Huang et al., 2008; Liao et al., 2002), indicating the pro-inflammatory property of this cytokine (Ouyang et al., 2006). Monocyte IL-19 production is downregulated by IFN-γ (Liao et al., 2002). Moreover, nuclear factor (NF)-kB and signal transducer and activator of transcription (STAT) 6 are playing an essential role in IL-17A and IL-13 stimulated IL-19 gene expression, respectively (Huang et al., 2008). This implies that transcription factors involved in inflammatory responses play a key role in IL-19 expression.
Considering the secretion of IL-9 by activated macrophages; we hypothesized that astrocytes which play a similar role in the CNS may also produce this cytokine. We have recently provided evidence concerning mRNA expression of IL-20R1 and IL-20R2 (data are not shown). Since no information is available on this matter, we designed experiments for assessment of IL-19 mRNA expression in astroglial cells and cortex of adult mice following LPS treatment by RT-PCR method.
To the best of our knowledge we are the first to show that IL-19 mRNA expression in LPS-treated astrocyte cultures but not in unstimulated control cells. Furthermore, in the present study, we have detected the expression of IL-19 mRNA in the cortex of adult C57BL/6 mice following i.p administration of LPS.
2. Methods
2.1. Primary Mixed Glial Cell Culture from C57BL/6 Pups Brain
Primary C57BL/6 mice astrocytes were obtained through slight modifications of the McCarthy protocol (Morga et al., 2009). Briefly, cerebral cortices were excised from 1 to 5 day-old C57BL/6 pups and the meninges removed, carefully under a stereo microscope (Nikon). Cortices were cut into small pieces and transferred with the 1.5 ml of serum-free complete Dulbecco’s modified Eagle’s medium (DMEM) to a 50 ml tube containing 13.5 ml HBSS-HEPES, 1.5 ml 1x trypsin and 75 µl DNAase and incubated for 5 min at 37°C.
Small particles of cortical tissue mechanically dissociated into a single cell suspension with a narrow Pasteur pipette by pipetting up and down, and cellular debris was removed by filtration through a 70µm cell strainer and centrifuged at 800 rpm for 8 min. The primary glial cell cultures were resuspended and maintained in DMEM media supplemented with 10% fetal calf serum (FCS), 100U/ml penicillin, 100 µg/ml streptomycin (Invitrogen), and expanded 3×106 cells in poly-L-ornithine (Sigma)– coated 90 mm tissue culture dishes. The culture medium was changed next day and 6 days after and then twice a week with the DMEM supplemented with 10% FCS.
Cells were allowed to grow to confluence (14–18 days) at 37°C under 5% CO2.
The plates were shaken mechanically for 2 h at 150 rpm at 37°C in a temperature controlled orbital shaker (Forma Orbital Shaker, Thermo Electron Corporation) to loosen microglia and oligodendrocytes growing on top of the astrocytic layer from the more adherent astrocytes. Then, microglia and oligodendrocytes were removed. The remaining adherent cells were detached with 0.25% trypsin/0.02% EDTA, resuspended in fresh medium and reseeded in poly-L-lysin–(Sigma) coated tissue culture dishes.
To remove any residual oligodendrocytes and microglial cells, the plates were shaken for 3 times as described above before harvesting.
2.2. Immunocytochemistry Assays for Glial Fibrillary Acidic Protein (GFAP)
The purity of astrocyte cultures was determined by indirect immunocytochemical staining using rabbit antimouse GFAP (Dakopatts) (specific marker for astroglial cells).
Astrocytes were grown on poly-L-lysin-coated coverslips. Cells were washed in phosphate buffer saline (PBS) and fixed in 4% Paraformaldehyde for 30 min. Slides blocked with 10% goat serum in PBS for 1 h, and incubated overnight at 4°C with rabbit anti-GFAP. The next day, cells were washed and incubated with green fluorescence Alexa Fluor 488 dye-labeled goat anti-rabbit IgG antibody (Invitrogen) for 1 h at room temperature. Thereafter, astrocytes were stained with DAPI to visualize the nuclei. The coverslips were mounted onto microscope slides using Mowiol mounting medium (Merck). Observations were carried out with fluorescence and Confocal microscopes.
2.3. RT-PCR Analysis for IL-19, IL-1β and TNF-α mRNA
2.3.1. Preparation of Mononuclear (MN) Cells Suspension from Adult C57BL/6 Mice Spleen
MN cells from mice spleen were used as positive control for expression of IL-19 mRNA in RT-PCR reactions. The Spleen MN cells were isolated as described elsewhere (Babu et al., 2003). Briefly, freshly removed spleens from 8-12 weeks C57BL/6 mice spleen were used for preparation MN cell suspension in DMEM supplemented with 5% FCS. Red blood cells were removed from spleen MN cell suspension by using lysis buffer (NH4Cl).
2.3.2. Treatment of C57BL/6 Mice Astrocytes and MN Cells with LPS
Astrocytes were treated with two doses of LPS from Escherichia coli serotype 0127:B8 (Sigma): (a) 1 µg/ml for 8 h; (b) 10 µg/ml for 24 h. The control groups were not treated. Mice spleen MN cells were treated with 10 µg/ml LPS for 24 h.
2.3.3. Intraperitoneal (i.p.) administration of LPS
A single dose of 1.5 mg/kg of LPS in sterile, pyrogenfree 0.9% saline was randomly administered by i.p. injection to a combination of male and female mice for two time points (24 and 72 h). Control animals received volume-equivalent injections of sterile saline at the same times to rule out effects based upon the stress of the injection procedure itself.
2.3.4. Brain Tissue Preparation for RT-PCR
At the end of each time point, mice were euthanized with i.p. injection of chlorine (200 mg/kg) then they were transcardially perfused with cold sterile PBS. Brains were excised, dissected and frozen on dry ice. The frontal cortex, containing the frontal association, dorsolateral orbital, ventral orbital and prelimbic cortices (first 1 mm of the frontal cortex), was collected and stored at -80°C until total RNA extraction with TRIzol reagent (Invitrogen).
2.3.5. RNA Isolation from Astrocytes, MN cells and Brain Samples
Total RNA was extracted from treated and control groups of astrocytes, MN cells and brain samples using the TRIzol reagent according to manufacturer’s instruction. RNA samples were treated with DNAse1 (Invitrogen) to degrade any traces of contaminating genomic DNA during extraction.
RNA was quantified using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies,Inc.). RNA was stored at -80°C until cDNA synthesis and PCR reaction.
2.3.6. cDNA Synthesis
RNA was reverse transcribed using a RNT cDNA synthesis Kit (Applied Biosystem) to prepare cDNA in accord with instructions of the supplier. PCR amplification, using random primer sets, was conducted at a 25°C, 37°C and 85°C for 10 min, 2 h and 5 min, respectively. The PCR was performed by using a Gradient Thermal Cycler (Eppendorf).
2.3.7. RT-PCR Reactions for IL-19, IL-1β,TNF-α, GFAP and β-actin
Amplifications of the cDNA templates were performed in 25 µl PCR mix containing 2 µl of cDNA, 5 µl PCR buffer, 50 mM dNTPs, 0.25 µl Taq DNA polymerase and 5 µl of Reverse and Forward primers (are listed in Table 1). The astrocytes cDNA were examined for expression of IL-19, IL-1β and TNF-α mRNA. Also, GFAP and β-actin housekeeping gene primers were used as specific marker of astroglial cells and internal control of RT-PCR reactions; respectively. The PCR was carried out using a Gradient Thermal Cycler.
Table 1.
Nucleotide sequences of primer sets and annealing temperature (T) were used for RT-PCR reactions.
| Gene | Forward | Reverse | Annealing Temperature | Product Size (bp) |
|---|---|---|---|---|
| mIL-19 | ATG AAG ACA CAG TGC GCG TC | GTG TCA GGC TGC AGG AG | 65°C | 529 |
| mIL-1β | CTC CAT GAG CTT TGT ACA AGG | TGC TGA TGT ACC AGT TGG GG | 63°C | 240 |
| mTNF-α | ATG AGC ACA GAA ACA TGA TCC GC | CCA AAG TAG ACC TGC CCG GAC TC | 64°C | 692 |
| mGFAP | GGC GCT CAA TGC TGG CTT CA | TCT GCC TCC AGC CTC AGG TT | 54°C | 560 |
| mβ-actin | GGG AAT GGG TCA GAA GGA CT | TTT GAT GTC ACG CAC GAT TT | 58-65°C | 589 |
2.3.8. Electrophoresis
PCR products from each sample were loaded onto 1.5% agarose gels containing 10 µl ethidium bromide, bands were separated by application of 90 V for 30 min. The results were confirmed by at least four independent experiments.
3. Results
3.1. Purity of Enriched Astroglial Cell Cultures
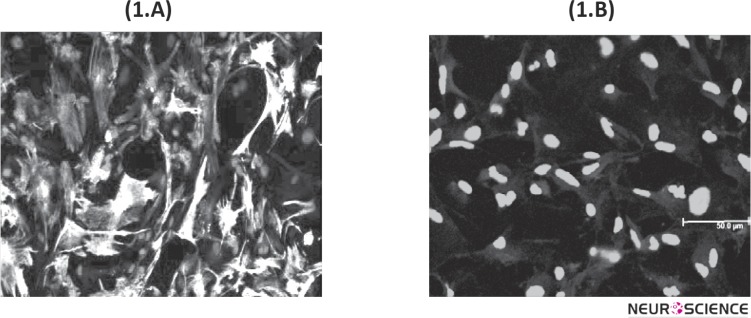
The purity of astrocyte cultures was determined using indirect immunocytochemical staining with antiGFAP antibody. Stained cells with specific marker for astrocytes were counted under fluorescent microscope. Purity of astroglial cells estimated approximately 95% as shown in Fig 1. Cells stained with rabbit anti-GFAP antibody (green) and Nuclei stained with DAPI (blue).
Figure 1.
The purity of astrocyte cultures was determined using indirect immunocytochemical staining with anti-GFAP antibody. (1.A) Confocal Microscopy analysis of primary astroglial cells stained with rabbit anti-GFAP antibody (green). The cell nuclei stained with DAPI (blue). (1.B) Negative control stained with secondary antibody and DAPI. Immunocytochemistry for determination of GFAP, were done for each astrocyte cultures.
3.2. Expression of IL-19, IL-1β and TNF-α mRNA in LPS-treated Astroglial and Mouse Spleen MN Cells
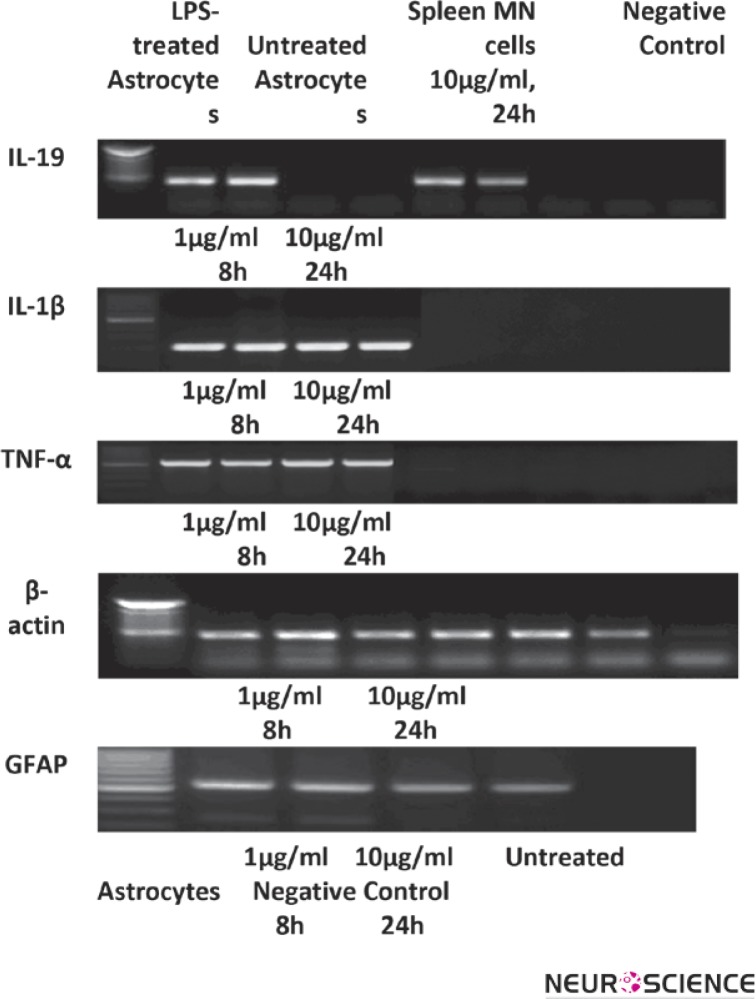
Spleen MN cells are able to produce IL-19, therefore in this study were used as positive control for expression of IL-19. To examine the expression of IL-19 mRNA in the enriched astroglial cells we cultured these cells in the presence or absence of LPS. MN cells were treated with LPS for 24 h. After treatment, IL-19, IL-1β and TNF-α mRNA was expressed in LPS-treated astrocyte cultures but not in control cells. The results are shown in Fig 2.
Figure 2.
The expression of mRNA for IL-19, IL-1β, TNF-α, β-actin and GFAP were carried out using mRNA extracted from LPS-treated and untreated mouse astrocytes cultures and spleen MN cells studied using RT-PCR method. β-actin and GFAP were used as internal control and specific marker of astrocytes, respectively. Experiments were repeated 4 times.
3.3. Intraperitoneal Administration of LPS Induced IL-19, IL-1β and TNF-α mRNA in the Cortex of Adult C57BL/6 Mice
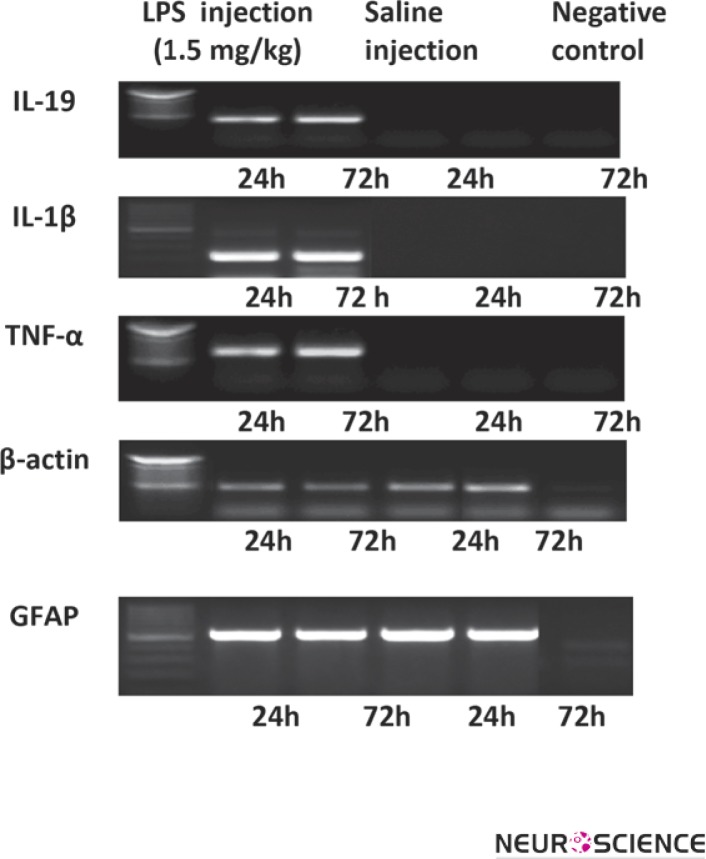
To assess the effects of LPS on IL-19 mRNA expression in the cortex, mice were injected i.p. with 1.5 mg/kg LPS and saline. The findings of present study have demonstrated that mice receiving a single dose of LPS are able to express IL-19, IL-1β and TNF-α mRNA in the cortex as compared with animals receiving only saline. Data are presented in Fig 3.
Figure 3.
RT-PCR Reactions for IL-19, IL-1β, TNF-α, GFAP and β-actin were performed using mRNA isolated from cortex of adult C57BL/6 mice following i.p. administration of LPS. β-actin was used as internal control. Experiments were repeated 4 times. The data represent from at least four independent experiments (independently derived astrocyte cultures).
4. Discussion
Several lines of evidence suggest that astrocyte and astrocyte-derived cytokines initiate and properly coordinate the immune responses of the CNS. Furthermore these cytokines may be a critical component in the mechanisms underlying neurodegeneration. Neuronalglial interactions could be involved in determining the activation threshold of astrocytes to inflammatory cytokines (Aschner, 1998).
Activation of astrocytes is known to be involved in the pathogenesis of neurodegenerative diseases. An inflammatory response leads to neuronal death and brain injury, while activated astrocytes release neurotrophic factors for neuronal survival (Brahmachari, Fung & Pahan, 2006).
The present study focuses on the effect of LPS on IL-19 mRNA synthesis in C57BL/6 mice astroglial cells and cerebral cortex. Considering the potential role of astrocytes in neuroprotection and neurodegeneration we hypothesized that production of certain immunomodulatory and inflammatory cytokines may affect astroglial cell functions to shift towards a desirable or detrimental directions i.e., repair processes in neuropathologic diseases or neurodegeneration in inflammatory conditions.
In this research we have detected the production of IL-19 mRNA by LPS-treated astroglial cells purified from neonatal mice brain and expanded in vitro and after i.p. LPS administration in cerebral cortex.
Also, LPS was injected via the intraperitoneal route for studying the IL-19 mRNA expression in cerebral cortex of adult C57BL/6 mice. The results of this experiment are consistent with the results of in vitro experiments.
Observation by Liao Y. C. et al. showed that IL-19 has proinflammatory activity (Liao et al., 2002). In contrast, other studies have shown that IL-19 is an anti-inflammatory interleukin, as in T cells it stimulates the Th2, rather than the Th1 response (Cuneo et al., 2010). Moreover, in cultured, primary human vascular smooth muscle cell (VSMC), IL-19 reduces abundance of proliferative and inflammatory gene proteins and mRNA, involving IL-1β, IL-8, and COX2 (Cuneo et al., 2010).
IL-19 expression is constitutive in monocytes, T, and B lymphocytes, and can be up regulated in these cells by LPS and G-CSF (Gallagher et al., 2000). The appearance of IL-19 mRNA in LPS-stimulated monocytes coincided with expression of IL-10 mRNA. Unlike other LPS-inducible cytokine genes such as TNF-α and IL-1β, which are expressed within 1 h post-stimulation, mRNA for the IL-19 and IL-10 genes is not detectable until 2–3 h post-stimulation. This delayed induction of IL-19 and IL-10 gene expression is consistent with a role for these cytokines as feedback inhibitors of proinflammatory cytokine production (Gallagher et al., 2000).
In our work we have detected IL-19 mRNA after 8h and 24 h post LPS stimulation of astroglial cells. These findings are in accordance with other studies mentioned above.
A study by Constantinescu and colleagues showed the expression of proinflammatory cytokines including TNF-α, IL-1β and IL-6 genes in LPS-stimulated astrocytes (Constantinescu et al., 2005). When primary astrocyte cultures derived from newborn mice are treated with LPS, the astrocytes readily release IL-1β (Blumberg et al., 2001; Hsing et al., 2007). Furthermore, TNF-α is synthesized and secreted both by astrocytes, microglia and neurons (Kim, Hong, & Ro, 2011).
Previous studies have also demonstrated that LPS stimulates IL-1β synthesis in the brain (Lau & Yu, 2001). Excessive IL-1β expression is observed in neurodegenerative diseases, the main source being two types of glial cells, namely microglia and astrocytes (Aschner, 1998).
In the current investigation, to examine inflammation induction after LPS stimulation, we have studied the expression of proinflammatory cytokines mRNA IL-1β and TNF-α in LPS-treated astroglial cells and after i.p. LPS administration in cerebral cortex.
Our results have demonstrated that LPS-treated astrocytes can express proinflammatory cytokines mRNA (IL-1β and TNF-α). Also, we have found IL-1β and TNF-α mRNA in cerebral cortex after i.p. injection of LPS.
Astrocytes can respond to many stimuli within the CNS. The usual progression of inflammation induced by cytokines first includes attenuation of the destructive and inflammatory response, followed by wound healing, and then a return to homeostasis. Regulatory cytokines released by microglia and astrocytes such as TNF-α and IL-1β can induce reactive astrogliosis. Thus, a normal response to inflammatory insults is a self-limiting process that inversely manages the initiating event. A failure to resolve the causative insult or to break the balance of pro- and anti-inflammatory agents results in prolonged tissue injury and destruction (Chen & Benveniste, 2004).
Regarding the self-limiting process of inflammation our results suggest that LPS may induce the production of IL-19 after proinflammatory cytokine expression in brain and implies that IL-19 expression is likely to contribute to down regulation of inflammation. Therefore, it is possible that IL-1β and TNF-α may function as autocrine mediators that induce IL-19 expression by astrocytes. Previous studies have demonstrated that IL-19 expression is upregulated in psoriatic lesions, in sera from asthmatic patients, and in the urine of uremic patients (Constantinescu et al., 2005) (Huang et al., 2008). Moreover, T cells in the peripheral blood and synovial fluid of rheumatoid arthritis patients also expressed elevated levels of IL-19 (Alanara et al., 2010). The role of IL-19 in neurodegenerative diseases has not yet been elucidated.
In conclusion, these results suggest that, LPS acts on astrocytes in the CNS to produce second mediators such as IL-19, IL-1β and TNF-α. These cytokines may induce different biological activities in the CNS, and modify the several neurodegenerative disease courses depending on the stage of disease activity.
The relevance of IL-19 in brain immunity remains unknown, especially with respect to the beneficial or detrimental influence on activity of astrocytes. Further investigation should be performed to disclose the molecular basis of underlying IL-19 signaling pathway in astrocytes and the exact role of IL-19 in regulation of astrocyte function in brain immunity.
This work has been performed at the level of qualitative mRNA detection by RT-PCR reactions. More research on the level of IL-19 protein and mRNA in different inflammatory conditions using sensitive methods such as western blotting, ELISA or intracellular flocytometry and Real Time PCR are necessary.
Since most neuropathological processes involve astrocytes and inflammatory cytokines, these findings which are reported for the first time have important implications for future research and therapeutic strategies.
From these data, we conclude that, astrocytes by way of cross-talk between pro- and anti-inflammatory cytokines regulate inflammatory events in the brain. It seems that, IL-19 regulation results from a complex multi-directional communication between neurons, astrocytes, and immune cells. Since IL-19 has been demonstrated to be both beneficial and detrimental, a thorough understanding of its regulation is paramount to our ability to manipulate its expression and effects in the CNS.
Acknowledgment
The study was conducted in the Neuroscience Institute of Cavaliery Otholenghy (NICO) Turin, Italy, and the National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran. The work was supported by Immunology, Asthma and Allergy Research Institute, Tehran University of Medical Sciences and Health Services, Tehran, Iran, Project Grant #88-04-40-10239.
References
- Alanara, T. , Karstila, K. , Moilanen, T. , Silvennoinen, O. , & Isomaki, P. (2010). Expression of IL-10 family cytokines in rheumatoid arthritis: elevated levels of IL-19 in the joints. Scandinavian Journal of Rheumatology, 39, 118–26 [DOI] [PubMed] [Google Scholar]
- Aloisi, F. , Ria, F. , & Adorini, L. (2000). Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunology Today, 21, 141–7 [DOI] [PubMed] [Google Scholar]
- Aschner, M. (1998). Immune and inflammatory responses in the CNS: modulation by astrocytes. Toxicology Letter, 103, 283–87 [DOI] [PubMed] [Google Scholar]
- Cuneo, A. A. , Herrick, D. , & Autieri. M. V. (2010) IL-19 Reduces VSMC Activation by Regulation of mRNA Regulatory Factor HuR and Reduction of mRNA Stability. J Mol Cell Cardiol. 49, 647–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu, U.S. , Wiesenfeld, P. W. , Collins, T. F. X. , Sprando, R. , Flynn, T. J. , & Black, T. et al. (2003). Impact of High Flaxseed Diet on Mitogen-induced Proliferation, IL-2 Production, Cell Subsets and Fatty Acid Composition of Spleen Cells from Pregnant and F1 Generation Sprague–Dawley Rats. Food and Chemical Toxicology, 41, 905–15 [DOI] [PubMed] [Google Scholar]
- Kim, D. Y. , Hong, G., U. , & Ro, J., U. (2011). Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. Journal of Neuroinflammation 8:25, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg, H. , Conklin, D. , Xu, W. F. , Grossmann, A. , Brender, T. , & Carollo, S. , et al. (2001). Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell, 104, 9–19 [DOI] [PubMed] [Google Scholar]
- Brahmachari, S. , Fung, Y. K. , & Pahan, k. (2006). Induction of Glial Fibrillary Acidic Protein Expression in Astrocytes by Nitric Oxide. Journal of Neuroscience, 26, 4930–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. H. , & Benveniste, E. N. (2004). Oncostatin M: a pleiotropic cytokine in the central nervous system, Cytokine & Growth Factor Reviews, 15, 379–91 [DOI] [PubMed] [Google Scholar]
- Constantinescu, C. S. , Tani, M. , Ransohoff, R. M. , Wysocka, M. , Hilliard, B. , & Fujioka, T. , et al. (2005). Astrocytes as antigenpresenting cells: expression of IL-12/IL-23, Journal of Neurochemistry, 95, 331–40 [DOI] [PubMed] [Google Scholar]
- Dong, Y. , & Benveniste, E. N. (2001). Immune function of astrocytes. Glia, 36, 180–90 [DOI] [PubMed] [Google Scholar]
- Gallagher, G. , Dickensheets, H. , Eskdale, J. , Izotova, L. S. , Mirochnitchenko, O. V. , & Vazquez, N. , et al. (2000). Cloning, expression and initial characterisation of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10). Genes & Immunity, 1, 442–50 [DOI] [PubMed] [Google Scholar]
- Gallagher, G. , Eskdale, J. , Jordan, W. , Peat, J. , Campbell, J. , & Boniotto, M. , et al. (2004). Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. International Immunopharmacology, 4, 615–26 [DOI] [PubMed] [Google Scholar]
- Gallagher, G. (2010). Interleukin-19: multiple roles in immune regulation and disease. Cytokine & Growth Factor Reviews, 21, 345–52 [DOI] [PubMed] [Google Scholar]
- Hsing, C. H. , Hsu. C. C. , Chen, W. Y. , Chang, L.Y. , Hwang, J. C. , & Chang, M.S. (2007). Expression of IL-19 correlates with Th2 cytokines in uraemic patients. Nephrology Dial Transplant, 22, 2230–8 [DOI] [PubMed] [Google Scholar]
- Huang, F. , Wachi, S. , Forteza, R. M. , Thai, P. , & Wu, R. (2008). Potentiation of IL-19 Expression In Airway Epithelia By IL-17A and IL-4/IL-13: Important Implications in Asthma. Journal of Allergy and Clinical Immunology, 121, 1415–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, W. J. , Eskdale, J. , Boniotto, M. , Lennon, G. P. , Peat, J. , Campbell, J. D. M. , & Gallagher, G. (2005). Human IL-19 regulates immunity through auto-induction of IL-19 and production of IL-10. European Journal of Immunology, 35, 1576–82 [DOI] [PubMed] [Google Scholar]
- Morga, E. , Amazzal, L. M. , Felten, P. , Heurtaux, T. , Moro, M. , Michelucci, A. , Gabel, S. , Grandbarbe, L. , & Heuschling, P. (2009). Jagged1 Regulates the Activation of Astrocytes Via Modulation of NF-κB and JAK/STAT/SOCS Pathways. Glia, 57, 1741–53 [DOI] [PubMed] [Google Scholar]
- Kort, J. J. , Kawamura, K. , Fugger, L. , Weissert R., & Forsthuber, T.G. (2006). Efficient presentation of myelin oligodendrocyte glycoprotein peptides but not protein by astrocytes from HLA-DR2 and HLA-DR4 transgenic mice. Journal of Neuroimmunology, 173, 23–34 [DOI] [PubMed] [Google Scholar]
- Lau, L. T. & Yu, A. C. H. (2001). Astrocytes Produced and Release Interleukin-1, Interleukin-6, Tumor Necrosis Factor Alpha and Interferon-Gamma Following Traumatic and Metabolic Injury. Journal of Neurotrauma, 18, 351–9 [DOI] [PubMed] [Google Scholar]
- Ledeboer A., Breve J. J. P., Wierinckx A., Jagt S. V.D., Bristow A. F., & Leysen J. E., et al. (2002). Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Europian Journal of Neuroscience, 16, 1175–1185 [DOI] [PubMed] [Google Scholar]
- Leng, R. X. , Pan H. F., Tao, J. H. , & Ye, D. Q. (2011). IL-19, IL-20 and IL-24: potential therapeutic targets for autoimmune diseases. Expert Opinion Therapy Targets., 15, 119–26 [DOI] [PubMed] [Google Scholar]
- Liao, Y. C. , Liang, W. G. , Chen, F. W. , Hsu, J. H. , Yang, J. J. , and Chang, M.S. (2002). IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. Journal of Immunology, 169, 4288–97 [DOI] [PubMed] [Google Scholar]
- Liao, S. C. , Cheng, Y.C. , Wang, Y. C. , Wang, C. W. , Yang, S. M. , Yu, C. K. , et al. (2004). IL-19 induced Th2 cytokines and was up-regulated in asthma patients. Journal of Immunology, 173, 6712–6718 [DOI] [PubMed] [Google Scholar]
- Minagar, A. , Shapshak, P. , Fujimura, R. , Ownby, R. , Heyes, M. and Eisdorfer, C. (2002). The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. Journal of Neurological Science, 202, 13–23 [DOI] [PubMed] [Google Scholar]
- Oral, H.B. , Kotenko, S.V. , Yilmaz, M. , Mani, O. , Zumkehr, J. , & Blaser, K. , et al. (2006). Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26, European Journal of Immunology, 36, 380–8 [DOI] [PubMed] [Google Scholar]
- Ouyang, W. , Valdez, P. A. , Rutz, S. , Crellin, N. K. , & Hymowitz, S. G. (2011). Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annual Review Immunology, 29, 71–109 [DOI] [PubMed] [Google Scholar]
- Sa, S. M. , Valdez, P. A. , Wu, J. , Jung, K. , Zhong, F. , & Hall, L. , et al. (2007). The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis, Journal of Immunology, 178, 2229–40 [DOI] [PubMed] [Google Scholar]
- Sabat, R. , Wallace E., Endesfelder S., Wolk, K. (2007). IL-19 and IL-20: two novel cytokines with importance in Inflammatory diseases. Expert Opinion Therapy Targets, 11, 601–12 [DOI] [PubMed] [Google Scholar]
- Sabat, R. (2010). IL-10 family of cytokines. Cytokine & Growth Factor Reviews, 21, 315–24 [DOI] [PubMed] [Google Scholar]
- Wolk, K. , Kunz, S. , Asadullah, K. , & Sabat, R. (2010). Cutting edge: immune cells as sources and targets of the IL-10 family members? Journal of Immunology, 168, 5397–402 [DOI] [PubMed] [Google Scholar]
- Zdanov, A. Structural analysis of cytokines comprising the IL-10 family. (2010). Cytokine & Growth Factor Reviews, 21, 325–30 [DOI] [PMC free article] [PubMed] [Google Scholar]