Abstract
Background
Alkaloids of Sophora alopecuroides have good biological activity, and are widely used in clinical settings, which not only have pharmacological activities of anti-cancer, cancer suppression, as well as the inhibition, and killing of various microorganisms; but also possess extensive pharmacological effects on immune system, nervous system and cardiovascular system. The objective of this paper was to extract and isolate total alkaloids of Sophora alopecuroides (TASA), and to study their anti-tumor effects in H22 tumor-bearing mice.
Materials and Methods
TASA were extracted and isolated using thin-layer chromatography, and column chromatography; and the isolated compounds were analyzed using nuclear magnetic resonance. The inhibitory effects of TASA on tumor in H22-bearing mice were determined by MTT assay.
Results
Three compounds were isolated from Sophora alopecuroides L., which were matrine, oxymatrine and sophoridine, respectively. Meanwhile, mouse H22 sarcoma model was established and different doses of TASA apparently inhibited solid H22-tumor in mice; it inhibited the thymus, and spleen to some extent; the degree of inhibition was more obvious for the spleen.
Conclusion
TASA has an anti-tumor effect in H22 tumor-bearing mice.
Keywords: Sophora alopecuroides L., H22 tumor-bearing mice, matrine, oxymatrine and sophoridine
Introduction
Chinese medicine Ku Dou Zi (Sophora alopecuroides L.) is a plant in the genus Sophora, of family Fabaceae; also named Ku Shen Cao, Ku Dou Gen, Ku Gan Cao, Xi Dou Gen, Ku Dou Cao, Ou Ku Shen, etc. It is bitter in taste, cold in nature, and has the heat clearing, detoxifying, and pathogenic wind dispelling; dampness removing, analgesic, insecticidal, anti-bacterial as well as anti-inflammatory effects (Yang et al., 1998; Ren., 2000). It is mainly distributed in China's Northwest desert region, North China, and Central Asian region; it grows in the desolate salt marshes, or saline-alkali lands, which is characterized by tolerance to high salinity, alkalinity and drought.
In recent years, studies on Sophora alopecuroides L. have gradually increased. Alkaloids of Sophora alopecuroides have good biological activity, and are widely used in clinical settings, which not only have pharmacological activities of anticancer, cancer suppression, as well as the inhibition, and killing of various microorganisms, but also possess extensive pharmacological effects on immune system, nervous system and cardiovascular system. Studies have also found that TASA have a broad-spectrum anti-bacterial activity, and can induce apoptosis of hepatoma SMMC-7721, cells (Ye et al., 2009; Zhang et al., 2005). In this study, the alkaloid chemical constituents in the seed of Sophora alopecuroides L. were studied; three compounds were isolated from the seed, which were matrine, oxymatrine and sophoridine. Meanwhile, mouse H22, sarcoma model was established to observe the inhibitory effects of TASA on growth of H22-tumor in mice, and to investigate their anti-tumor effects and mechanisms.
Materials and Methods
Instruments and medicinal materials
VORIAN-600, nuclear magnetic resonance spectrometer (with TMS as internal standard); Shimadzu UV-2401 UV-visible spectrophotometer (Shimadzu Corporation, Japan); silica gel for thin-layer chromatography, silica gel for column chromatography, and alumina for column chromatography, Qingdao Haiyang Chemical Co., Ltd.; all other reagents were of analytical grade. Sophora alopecuroides L. crude drug was purchased from Shenyang medicine market, which was identified as Sophora alopecuroides L. of genus Sophora, family Fabaceae by Prof. Qiguo Wu from Yunnan Univercity.
Isolation and identification of chemical constituents of alkaloids from Sophora alopecuroides
Extraction and isolation
5kg, of Sophora alopecuroides L. coarse powder (10–20 mesh), was weighed, added with a 4-fold volume of 5% NaOH solution, and soaked overnight, the alkaline liquid was discarded, and the residue was further extracted with 5% sulfuric acid solution, the extract was then passed through a cat-ion exchange resin; eluent was 95%, ethanol containing 3% ammonia, the eluents were combined, ethanol was recovered, after activated carbon adsorption, 312g of total alkaloids were obtained, which were then isolated by a neutral alumina column, gradient eluted with petroleum ether-acetone to give various elution fractions. Each fraction was combined, and recrystallized to obtain three compounds.
Structural identification
Compound 1: white columnar crystals (petroleum ether), easily soluble in methanol, chloroform, acetone, and slightly soluble in petroleum ether. C15H24N2O, m.p 81–83°C, data were attributed as follows: 1H-NMR (300 MHz, DMSO-d6) δ: 2.08 (1H, m, 2Ha), 2.82 (1H, m, 2He), 1.95 (1H, m, 3Ha), 1.45 (1H, m, 3He), 1.46-1.36 (m, 4Ha), 1.95 (m, 4He), 1.78-1.55 (m, 6H), 1.78-1.55 (m, 6H), 1.78-1.55 (m, 7H), 1.47-1.36 (m, 8H), 1.82 (m, 9Ha), 1.43-1.34 (m, 9He), 2.11 (m, 10Ha), 2.82 (m, 10He), 3.83 (dt, J=9.6, 6.0Hz), 1.52 (m, 12Ha), 1.97 (m, 12He), 1.76-1.54 (m, 13H), 2.22 (m, 14Ha), 2.45 (m, 14He), 3.08 (t, J=12.6Hz, 17Ha), 4.42 (dd, J=12.6, 4.2Hz, 17He); 13C-NMR (75 MHz, DMSO-d6) δ: 57.4 (C-2), 21.3 (C-3), 27.3 (C-4), 35.4 (C-5), 63.9 (C-6), 41.2 (C-7), 26.7 (C-8), 20.9 (C-9), 57.3 (C-10), 53.3 (C-11), 27.8 (C-12), 19.1 (C-13), 32.8 (C-14), 169.5 (C-15), 43.3 (C-17). Based on the above spectroscopic data and by contrasting the data in the literature (Song et al., 2008), the compound was identified as matrine. The structure is shown in Fig. 1.
Figure 1.
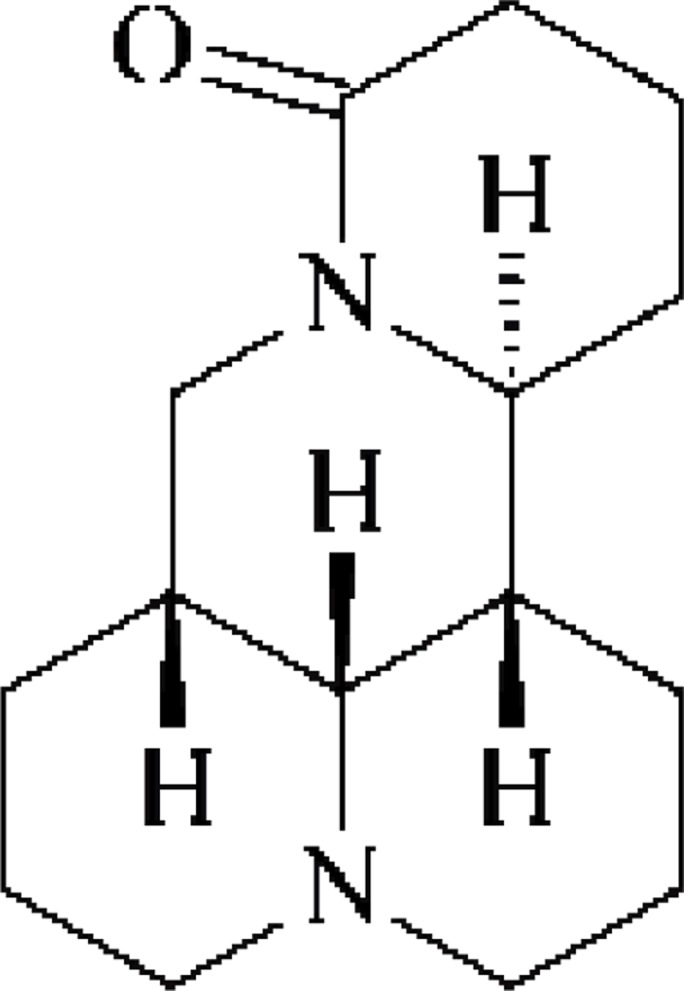
Matrine
Compound 2: white cubic crystals (petroleum ether), easily soluble in chloroform, methanol, and insoluble in petroleum ether. C15H24N2O, experimental value of m.p 106–107°C, literature value of m.p 105–107°C. Data were attributed as follows: 1H-NMR (300 MHz, DMSO-d6) δ: 2.15 (m, 2Ha), 2.84 (m, 2He), 2.05 (m, 3Ha), 1.84-1.42 (m, 3He), 1.84-1.42 (m, 3He), 1.92 (m, 4He), 2.03 (m, 5H), 1.84-1.40 (m, 6H), 1.84-1.40 (m, 7H), 1.84-1.40 (m, 8H), 1.07 (m, 9Ha), 2.19 (m, 10Ha), 2.83 (m, 10He), 3.46-3.25 (m, 11H), 1.84-1.41 (m, 12Ha), 1.92 (m, 12He), 1.85-1.40 (m, 13H), 2.39 (m, 14H), 3.47-3.25 (m, 17H); 13C-NMR (75 MHz, DMSO-d6) δ: 55.8 (C-2), 23.7 (C-3), 28.3 (C-4), 30.4 (C-5), 63.4 (C-6), 41.1 (C-7), 21.7 (C-8), 21.9 (C-9), 50.3 (C-10), 55.6 (C-11), 30.4( C-12), 19.1 (C-13), 32.5 (C-14), 169.6 (C-15), 47.3 (C-17). Based on the above spectroscopic data and by contrasting the data in the literature (Li et al., 2004), the compound was identified as sophoridine. The structure is shown in Figure 2.
Figure 2.
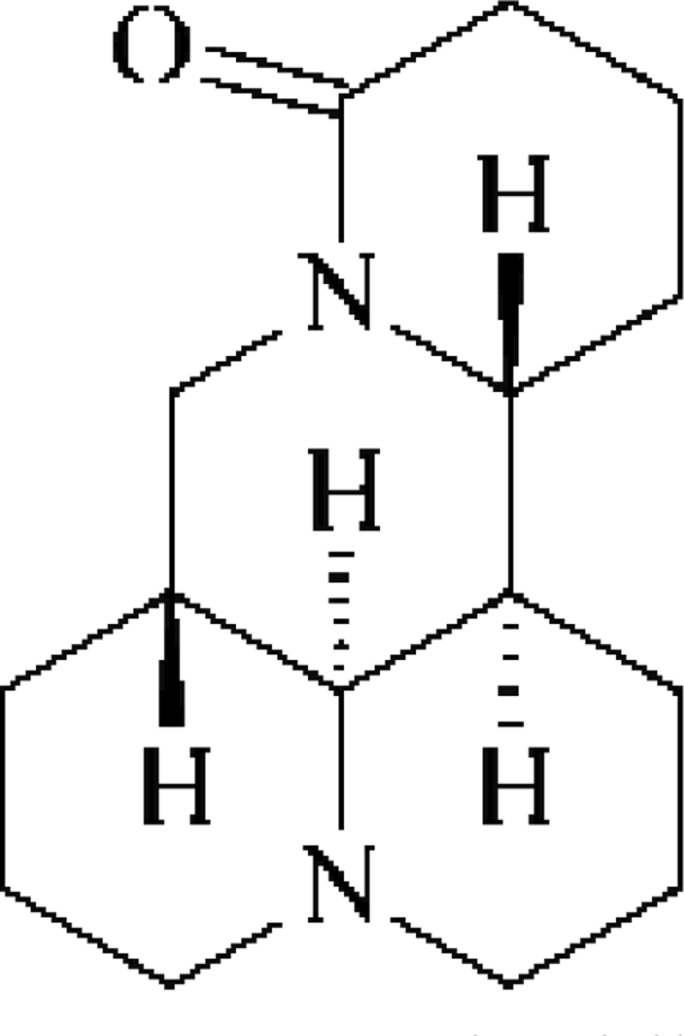
Sophoridine
Compound 3: white prismatic crystals (chloroform-ether); easily soluble in chloroform, methanol, slightly soluble in acetone, and insoluble in petroleum ether. C15H24N2O2, experimental value of m.p 207–208°C, literature value of m.p 205–207°C. Data were attributed as follows: 1H-NMR (300 MHz, DMSO-d6) δ: 3.11 (m, 2H), 2.72 (m, 3Ha), 1.87-1.53 (m), 1.86-1.53 (m, 4H), 1.86-1.53 (m, 5H), 3.10 (m, 6H), 1.86-1.54 (m, 7H), 1.86-1.54 (m, 8H), 2.06 (m, 8He), 2.71 (m, 9H), 3.12 (m, 10H), 5.13 (dt, J=10.2, 5.4Hz, 11H), 1.27 (m, 12Ha), 2.24 (m, 12He), 1.86-1.53 (m, 13H), 2.25 (m, 14Ha), 2.48 (m, 14He), 4.21 (t, J=12.6Hz, 17Ha), 4.42 (dd, J=12.6, 5.4Hz, 17He); 13C-NMR (75 MHz, DMSO-d6) δ: 69.4 (C-2), 17.3 (C-3), 26.4 (C-4), 34.4 (C-5), 67.5 (C-6), 42.2 (C-7), 24.7 (C-8), 17.9 (C-9), 69.3 (C-10), 53.1 (C-11), 28.8 (C-12), 18.8 (C-13), 32.9 (C-14), 170.2 (C-15), 41.3 (C-17). Based on the above spectroscopic data and by contrasting the data in the literature (Li et al., 2004), the compound was identified as oxymatrine. The structure is shown in Figure 3.
Figure 3.
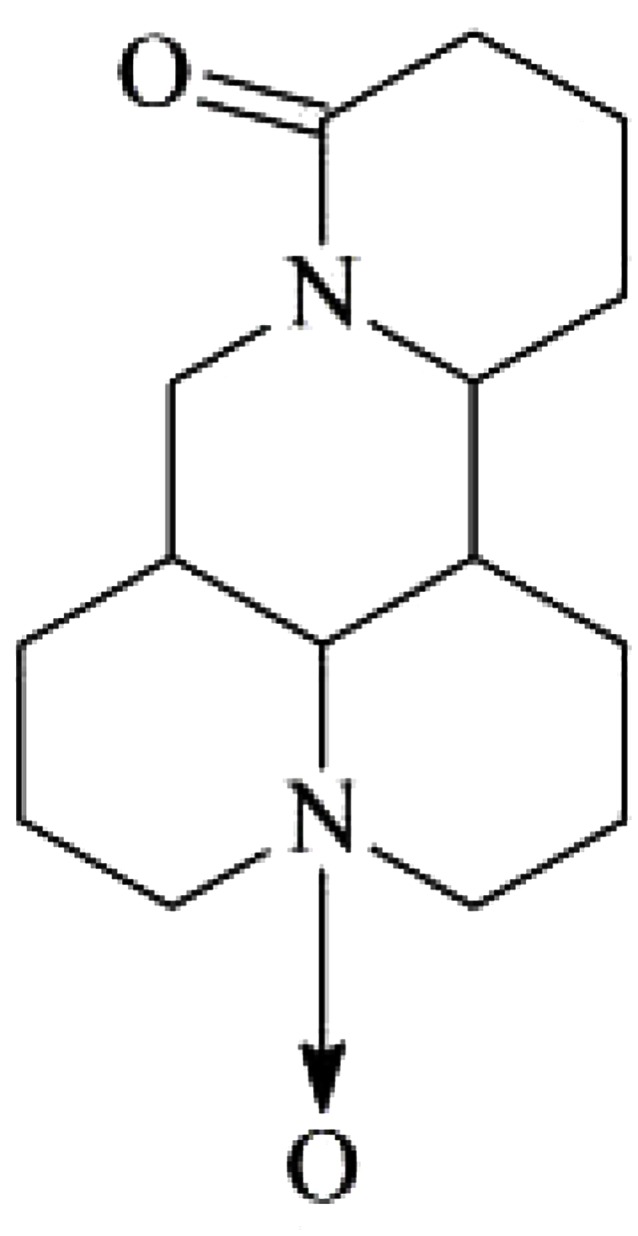
Oxymatrine
Study on the inhibitory effect of TASA on H22 hepatoma in mice
Materials and instruments
TASA (self-prepared), 5-fluorouracil injection (Shanghai Xudong Haipu Pharmaceutical Co., Ltd. batch number: 0500326). Kunming mice, body weight (20±2.0)g, half male and half female (purchased from China Medical University); mouse H22 hepatoma cell lines (provided by China Medical University).
Experimental methods
Mice were randomized into five different groups (n=10/group), namely the normal control group; 5-FU group [15 mg/(kg·d)]; and TASA low-, medium-, and high-dose groups (50, 100, and 200 mg/kg). All experimental procedures were approved by the Animal Research Ethics Committee.
Model establishment and administration
Tumor-bearing mice which have been inoculated with H22 hepatoma ascites tumor for 12 days with well grown tumors were selected, and sacrificed by cervical dislocation; ascitic fluid was drawn, diluted to a hepatoma cell suspension with a concentration of 5×107cells/mL, with normal saline, and inoculated subcutaneously into the right anterior armpit of mice under sterile conditions. 24hrs after the inoculation, sterile intraperitoneal administration began in each group; each mouse was administered 0.2mL of drug once daily for seven consecutive days. 24hrs after the last administration, mice were weighed, and then sacrificed by cervical dislocation, also the tumor mass; spleen and thymus were removed, and weighed. And the tumor growth inhibition rate calculated.
Tumor inhibition rate = [(average tumor weight of the control group -average tumor weight of the treatment group) / average tumor weight of the control group] × 100%.
Statistical analysis
Data of each group were expressed as x±s, statistical analysis was performed by t-test and variance test.
Experimental results
Anti-tumor effect of TASA in tumor-bearing mice
The results are shown in Tab. 1. Different dose groups of TASA all apparently inhibited solid H22 tumors. Tumor weights were reduced in each group compared with the negative control group, and the differences were significant.
Table 1.
Inhibitory effect of TASA on solid H22 tumor in mice (x±s, n=10)
| Group | Dose (mg/kg) | Tumor weight (g) | Tumor inhibition rate (%) |
| Control group | 1.524±0.36 | ||
| 5-FU group | 15 | 0.726±0.28 | 52.4** |
| 50 | 1.217±0.37 | 20.1* | |
| TASA group | 100 | 0.874±0.22 | 42.7* |
| 200 | 0.719±0.28 | 52.8** |
Note: Comparison with the control group,
P<0.05,
P<0.01.
Effect of TASA on immune organs in tumor-bearing mice
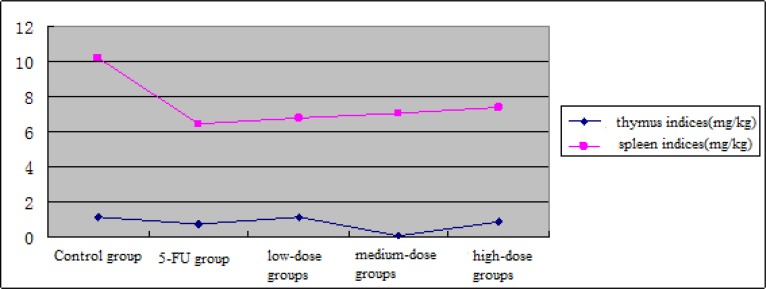
Compared with the control group, thymus index had no significant change while spleen index significantly decreased (P<0.01), in the mice of low-dose TASA group; thymus and spleen indices of mice in the high-dose TASA group were both lower than the control group, the decrease of spleen index was significantly different (P<0.01), indicating that TASA had some inhibitory effect on thymus and spleen of mice, the degree of inhibition was more obvious for spleen.
Discussion
With increase of pressures in life, and the deterioration of living environment, malignant tumor has become an important factor threatening human health. With the deepening of people's awareness about cancer, the diagnosis and treatment of cancer have been changed from the traditional “find and destroy (mainly by taking surgical, radio-therapeutic, and chemotherapeutic means)”, into “targeting and control”; the exploration of tumor preventive and control mechanisms of action; and the search of highly-targeted low-toxicity anti-neoplastic drugs have become the focus of researches in cancer prevention and control (Zhang et al., 2004; Jiang et al., 2000; Yu et al., 2004).
Chinese herbal medicines are characterized by slow action, multi-channeled, and multi-targeted, etc., which can play a role in treating both symptoms, and root causes in either inhibition or killing of tumor cells, or in the promotion of postoperative adjustment of cancer patients. In this experiment, based on the structural characteristics of TASA, acidic conditions were used for extraction, gradient elution was performed with petroleum ether-acetone, and three chemical constituents i.e. matrine, oxymatrine; and sophoridine were isolated through the identification of each fraction.
In this study, the inhibitory effect of TASA on in vitro growth of H22 cells was observed by MTT assay, it was found that different dose groups of TASA could all apparently inhibit the solid H22 tumor in mice, and the inhibitory effect of TASA increased gradually with the increase in drug concentration, presenting a clear dose-effect relationship, the experimental results were consistent with the reported literature (Huang et al., 2002; Hong et al., 2008; Huang et al., 2001).
This study used a mouse H22-tumor model to observe the in vivo, anti-tumor effect of TASA. The study found that TASA had an apparent inhibitory effect on growth of solid H22-tumor in mice, the tumor inhibition rate reached over 50%, demonstrating the potential clinical application value of TASA as an antineoplastic drug.
Figure 4.
Effect of TASA on immune organs in tumor-bearing mice
References
- 1.Hong G, Liu P X, Shen X. Inhibition of Sophora alopecuoride Alkaloid Derivative SPRIDA on H22 Liver Cancer of Mice. Herald of Medicine. 2008;27(4):369–371. [Google Scholar]
- 2.Huang X M, Li B. Review on the Pharmacology Study of Alkaloids from Sophora alopecuroides L. Chinese Pharmaceutical Affairs. 2002;16(3):175–178. [Google Scholar]
- 3.Huang X M, Li B, Shen L Z. Inhibitory Effects of Four Alkaloids of Sophora alopecuroides on TNF α production by Murine Peritoneal Macrophages. Pharmacology and Clinics of Chinese Materia Medica. 2001;17(3):12–14. [Google Scholar]
- 4.Jiang Y X, Yu J Q, Peng J Z. The Inhibitory Effects of Oxymartrine on Central Nervous System in Mice. Journal of Ningxia Medical University. 2000;22(3):157–158. 162. [Google Scholar]
- 5.Li K, Wang Y L, Zhu H Q. Determination of Three Kinds of Alkaloids in Kudouzi by HPLC. Northwest Pharmaceutical Journal. 2004;19(2):53–54. [Google Scholar]
- 6.Ren D. Development and Clinical Application of compounded Sophora alopecuroides Oral Ulcer Composite film. Chinese Traditional Patent Medicine. 2000;22(8):590–591. [Google Scholar]
- 7.Song Y Q, Wei Y H, Liu W J. Simultaneous Determination of Sophoridine, Matrine and Sophocarpine in Sophora alopecuroides Cream by HPLC. China pharmacy. 2008;19(24):1878–1879. [Google Scholar]
- 8.Yang J X, Yu Z F. Research Progress of Sophora alopecuroides L. Tianjin Pharmacy. 1998;10(1):43–45. [Google Scholar]
- 9.Ye G, Ma C H, Huang X Y, Li Z X, Huang C G. Components of Sophora alopecuroides Seeds. Chemistry of Natural Compounds. 2009;45(4):545–546. [Google Scholar]
- 10.Yu Y Q, Ding P L, Chen D F. Determination of Quinolizidine Alkaloids in Sophora Medicinal Plants by Capillary Eectrophoresis. Analytica Chimica Acta. 2004;523:15–20. [Google Scholar]
- 11.Zhang W M, Pu. P, Zhang D G. Studies on Extraction of Alkaloids in Seeds of Sophora alopecuraides L. Chinese Agricultural Science Bulletin. 2005;(21):7–9. [Google Scholar]
- 12.Zhang M J, Huang J. Recent Research Progress of Anti-tumor Mechanism of Matrine. China Journal of Chinese Materia Medica. 2004;27(2):115–118. [PubMed] [Google Scholar]