Abstract
Background
Massularia acuminata is a small tree or shrub of tropical rainforest. The leaves are used in Nigerian ethno-medicine for the treatment of microbial infections and pharmacological report suggested the leaf extract as possessing antioxidant activity. This study was therefore carried out to determine the most antioxidant and antimicrobial active fraction(s) of Massularia acuminata leaf and the constituent(s) responsible for the activities.
Matherials and Methods
The leaf of Massularia acuminata was investigated for in vitro antioxidant and antimicrobial activities, using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay and agar dilution method respectively.
Results
The ethyl acetate fraction demonstrated the best activities among the partitioned fractions tested. Bioassay guided purification of the most active ethyl acetate fraction led to isolation of a new thiophenolic glycoside, characterized as 4-(3′,3′-dihydroxy-1-mercaptopropyl)phenyl glycosylpyranoside.
Conclusion
The isolated compound from the leaf of Massularia acuminata demonstrated antioxidant and antimicrobial activities and may be responsible for the activities of leaf extract and its ethyl acetate fraction, hence this may justify its ethnomedicinal use.
Keywords: Massularia acuminata, antioxidant; antimicrobial; DPPH; agar dilution; thiophenolic glycoside
Introduction
The use of plants in medicine could be regarded as one of the landmark achievements of man's ability to intuitively tap into nature. Many plants contain curative agents, some of which have been implicated in the management of oxidative stress related degenerative diseases (Halliwell, 2007). An Antioxidant is any substance that delays or prevents oxidative damage to a target molecule (Halliwell and Gutteridge, 2007). Some of the commercially available synthetic antioxidants are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and propylgallate (PG). In recent times, the benefits of some of these synthetic antioxidants have been in doubts due to their possible carcinogenic and toxic effects. Thus, their use are now restricted by legislation in some developed and developing countries, and are gradually being replaced with plant-based antioxidants which are less toxic, readily available and affordable (Gülçin et al., 2005). Among the identified antioxidants in plants are polyphenols, a group of highly hydroxylated aromatic compounds, most of which are antimicrobials (Turkoglu et al., 2006), and it is believed that plants still possess array of new and/or novel lead compounds of antioxidant and antimicrobial properties.
Massularia acuminata (G. Don) Bullock ex Hoyle is locally known in Southwest Nigeria as “Pako Ijebu”. The leaves are used in Nigeria as fish poison and for the cure of mouth infections (Burkill, 1997). Pharmacological investigations suggest that leaf methanolic extract possessed antioxidant activity (Aladesanmi et al., 2007), the stem aqueous extract possessed antimicrobial activity especially against periodontal microbes (Taiwo et al., 1999), and the stem has also been implicated as an oral prophylactic and also reported to play an important role in gingival health (Aderinokun et al., 1999). However, the fraction(s) and constituent(s) responsible for antioxidant and antimicrobial activities in the leaf of M. acuminata have not been determined.
In this paper, we report the antioxidant and antimicrobial properties of M. acuminata leaf fractions and isolation of a new bioactive compound from the ethyl acetate leaf fraction.
Experimental
Equipments and Chemicals
The Absorbance measurement was taken on CamSpec M 107 Spectrophotometer, Gallenkamp MPD 350.BM2.5 for melting point determination, an ATR Platinum Diamond 1Refl Spectrophotometer for Infrared Spectroscopy, Bruker TOPSINI.3 Spectrometer for NMR Spectroscopy, with 1H NMR at 300 MHz and 13C NMR at 75 MHz, TMS, and a Centroid ESI-Mass Spectrometer for molecular mass determination. Column chromatography (CC) was performed on open column, with silica gel 60 (200–400 mesh, ASTM, 0.040–0.063mm, E.merck, Darmstadt, Germany) and Sephadex LH-20. Thin-Layer Chromatography (TLC) was performed on precoated silica gel (Kieselgel 60) GF254 plates (0.25mm, Merck). A 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical and L-ascorbic acid were purchased from Sigma-Aldrich Co., USA. All solvents used were of analytical grade.
Plant Material
The leaves of Massularia acuminata were collected at the Shasha Forest Reserve, Area J4, Osun State, Nigeria in October, 2010. The plant was authenticated at the Obafemi Awolowo University Herbarium, Ile-Ife, Nigeria, and Voucher number; IFE 16337 was obtained. The leaves were air dried and milled into powder prior to extraction process.
Extraction
A 1.5 kg dried and powdered leaf of M. acuminata was exhaustively extracted with 7.5 L of 80 % Ethanol by maceration, filtered and concentrated to dryness in vacuo, using a Shimadzu NE 100 rotary evaporator to afford 180 g ethanolic extract (12 % w/w).
Fractionation
The modified Kupchan method of solvent partitioning (Wagenen et al., 1993) was used for fractionation of the extract. This was done by suspending 170 g of EtOH extract in 100 ml distilled water, partitioned with gradient solvents (4 × 200 ml n-Hexane; 5 × 200 ml EtOAc; 2 × 200 ml n-BuOH) and concentrated in vacuo to afford n-Hexane (8 g), EtOAc (22 g), n-BuOH (100 g) and aqueous (35 g) fractions.
Antioxidant Activity: DPPH Radical Scavenging Assay
In this method, 1 mL DPPH solution in methanol (0.05 mg/mL) was added to 1mL sample at different concentrations (10.00 − 2.00 µg/mL for vitamin C; 100.00 − 6.25 µg/mL for extract, fractions and isolated compound) (Li et al., 2012; Sanchez-Moreno et al., 1998) . The experiment was carried out in triplicate, and the percentage inhibition of DPPH by each test sample was calculated thus:
% Inhibition of Sample = [{ABSControl − ABSSample} /ABSControl] * 100
Where ABSControl = Absorbance of negative control
ABSSample = Absorbance of positive control/extract/fraction/compound
The concentration that caused a 50% inhibition of the DPPH radical solution by each test sample was taken as the IC50 value, the result was statistically analyzed using an analysis of variance (ANOVA) with GraphPad Prism 3.0.
Antimicrobial Activity
The determination of Minimum inhibitory concentration (MIC) of extracts, fractions, column bulked fractions and isolated compounds on bacterial and fungal strains was done using agar dilution method (CLSI, 2007) on Mueller Hinton Agar (Oxoid) as described by Aladesanmi et al., (2007). Serial dilutions from stock solutions of extracts and solvent fractions (40 mg/mL), bulked column fractions (4 mg/mL), and isolated compound (2 mg/mL) were prepared. Acriflavin and Ketoconazole (BDH, UK) were used as positive controls against bacteria and fungi respectively, while negative controls used were 95 µL each of 50% aqueous methanol and Tween 80 (Sigma-Aldrich Co., USA).
Isolation and Identification of Compound
The most active ethyl acetate fraction (A, 19 g) was adsorbed unto silica gel and gradiently eluted in an open column (CC) using solvent systems from n-Hexane (100%) through EtOAc to MeOH (100 %). Eluates were bulked into thirteen sub-fractions (A1 – A13) on the basis of their TLC analysis. The most active A10 sub-fraction (4.766 g) was isocratically eluted on Sephadex LH-20 column using DCM — MeOH (8:2) and eluates were bulked into three sub-fractions (A101 – A103) on the basis of their TLC analysis. The most active A103 sub-fraction (430 mg) was further purified on silica gel CC and gradiently eluted with DCM — MeOH (8:2; 1:1) and MeOH (100 %) solvent sytems to afford Compound 1 (15 mg, DCM — MeOH 6:4).
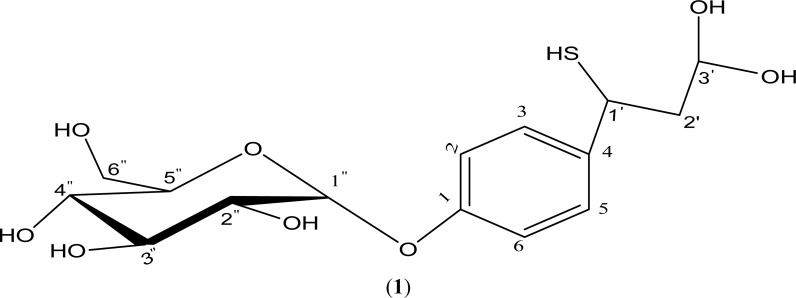
Isolated Compound (1)
4-(3′, 3′-dihydroxy-1-mercaptopropyl) phenyl glucosylpyranoside: (Brown semi-solid)
ESI-MS: Base peak [M]+ with chlorine adduct at m/z 399.4; Sodiated molecular ion [M+Na]+ m/z 385.2; molecular ion [M]+ at m/z 362 consistent with a molecular formula C15H22O8S; loss of C2H2O2 [M+2] at m/z 304.4 (C13H20O6S);
IR: (KBr), (v, cm−1): 3400–3200 (OH), 2954.88 (Aryl C – H), 2855.03 (aliphatic C – H), 1725.03 (C = O: of sugar substituted tautomeric phenone), 1269.81 (C – OH), 1071.17 (C – O), 742.82 (C – S);
1H-NMR: (CD3OD) δ - of aglycone: 2.35 (2H, bd, H-2′), 3.23–4.28 (1H, m, H-1′), 4.64 (1H, d, J = 9.0 Hz, H-3′), 6.66 (1H, dd, H-2, H-6), 7.49 (1H, dd, H-3, H-5); glucose: 3.23–4.28 (1H, m, H-2″, H-3″, H-4″, H-5″, H-6″), 5.67 (1H, d, J = 6.0, H-1″);
13C-NMR: (CD3OD) δ - of aglycone: 35.00 (C-1′), 39.78 (C-2′), 96.44 (C-3′), 115.43 (C-2, C-6), 131.95 (C-3, C-5), 142.12 (C-4), 154.67 (C-1); glucose: 62.75 (C-6″), 71.23 (C-4″), 74.93 (C-3″), 77.44 (C-2″), 78.17 (C-5″), 100.16 (C-1″);
Results
In vitro Antioxidant activity
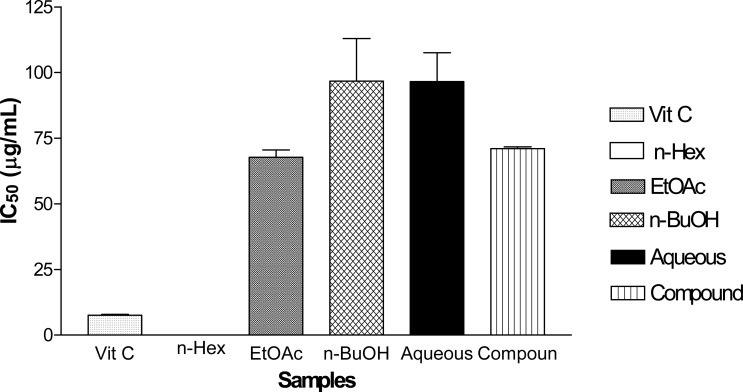
The concentration that inhibited 50 % of DPPH radical solution was taken to be the IC50 values for all tested samples. In the figure 2 below, Vitamin C, the standard drug used, demonstrated the lowest IC50 value (7.59±0.28 µg/mL) and invariably the highest antioxidant activity among all tested samples. However, ethyl acetate fraction demonstrated a significantly (p < 0.05) higher DPPH radical scavenging activity than each of the partitioned fractions tested. Its DPPH radical scavenging activity was also comparable (p < 0.05) to that of the isolated compound (1). Hence, the order of antioxidant activity: Vitamin C > Ethyl acetate fraction ≥ Isolated Compound > Aqueous fraction > n-Butanol Fraction.
Figure 2.
DPPH Free Radical Scavenging Activity of Fractions and Isolated Compound from Ethanolic extract of M. acuminata Leaf
Antimicrobial Activity
In the Table 1 below, ethyl acetate fraction exhibited the widest spectrum of antimicrobial activity (MIC range: 5 – 40 mg/mL) among the four partitioned fractions tested. Some of the susceptible pathogens were reference strain of methicillin resistant Staphylococcus aureus (Gram-positive bacteria), clinical strain of Clostridium sporogens (Gram-negative bacteria), NCTC strains of Candida albicans and C. pseudotropicalis (fungi). The isolated compound from ethyl acetate fraction demonstrated the widest spectrum of antimicrobial activity (MIC range: 0.25 – 2.00 mg/mL) among the plant samples tested. However, clinical strain of Clostridium sporogens and NCTC strain of Candida pseudotropicalis were susceptible to the isolated compound at MIC of 0.25 mg/mL. Thus, the order of antimicrobial activity: Acriflavin, ketoconazole > Isolated compound > ethyl acetate fraction > ethanolic extract > n-Butanol fraction > Aqueous fraction > n-Hexane fraction > Aq. MeOH, Tween 80.
Table 1.
Minimum Inhibitory Concentration (MIC) of M. acuminata extracts
| SAMPLE | MIC of Samples against Tested Microorganisms (mg/mL) | ||||||||
|
E. coli Clinical |
Pseudomonas aeruginosa ATCC |
Klebsiella pneumonia Clinical |
Clostridium sporogens Clinical |
MRSA Reference |
Shigella Clinical |
MRSA Clinical |
Candida albicans NCTC |
C. pseudotropicalis NCTC |
|
| n-Hexane | >40 | >40 | >40 | 40 | >40 | >40 | >40 | 40 | 20 |
| Ethyl acetate | 20 | 20 | 20 | 5 | 10 | 40 | 20 | 10 | 10 |
| n-Butanol | >40 | >40 | >40 | >40 | 40 | >40 | 40 | 40 | 10 |
| Aqueous | >40 | >40 | >40 | >40 | 40 | >40 | >40 | >40 | >40 |
| Isolated Compound | 2 | 2 | 2 | 0.25 | >4 | 2 | 1 | 2 | 0.25 |
| Acrifla-vin (mg/L) | 8 | 8 | 16 | 8 | 4 | 16 | 8 | *8 | *8 |
MIC of ketoconazole; MIC of 50% aqueous methanol and Tween 80 as negative controls: > 40 mg/mL
Discussion
The highest antioxidant activity exhibited by the ethyl acetate fraction can be attributed to the fact that it possessed the most antioxidant components or the most active structural moieties of a component which could scavenge rapidly the DPPH radical solution by reduction (Canadanovic-Brunet et al., 2005). Upon repeated column purification, the ethyl acetate fraction elaborated a thiophenolic glycoside which demonstrated a comparable (p < 0.05) DPPH radical scavenging activity as a measure of antioxidant activity. This may be due to the presence of hydroxyl (phenolic OH) and thiol groups which are free radical scavengers (Amić et al., 2003).
Compound 1 belongs to the class of polyphenols which are easily extractible with ethyl acetate and are characteristically anti-oxidative in their actions with considerable level of antimicrobial activity (Erol et al.,2009; Cowan, 1999) as was demonstrated by the ethyl acetate fraction and its constituent in this investigation. The susceptibility of clinical strains of methicillin resistant Staphylococcus aureus to compound 1 at MIC of 2 mg/mL is an indication that this compound may be useful in the treatment of some opportunistic infections and food borne diseases (Dupont et al., 2006). Candida albicans and C. pseudotropicalis which are causative agents of mouth infections (oral thrush/candidiasis) were also susceptible to compound 1 at reduced concentrations, hence, may justify the ethno-medicinal use of the leaves in the treatment of mouth infections.
However, the investigation was limited to the use of open column chromatography only. It would therefore be necessary to introduce faster and easily reproducible isolation techniques such as preparative system (PTLC), accelerated system (AGC and Flash chromatography), automated system (HPLC/UPLC), and the newer hyphenated systems (LC-MS/MS, LC-IR-NMR) which could assist in the isolation and characterization of novel leads from natural sources (Hostettmann and Marston, 2002).
Isolated Compound (1)
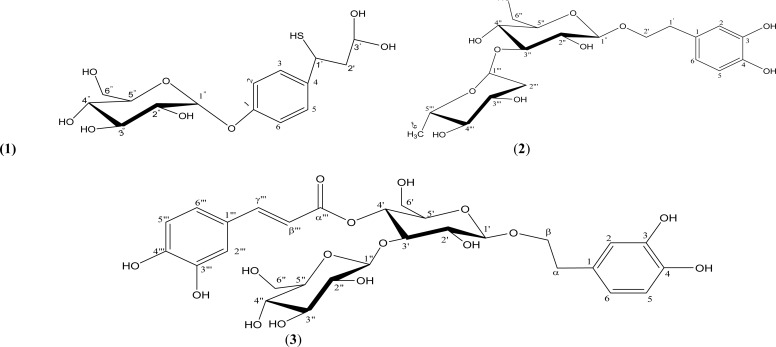
The 1H NMR spectra showed an AA′BB′ (A2B2) system (para-substitution pattern) and the assignment was further confirmed by the 13C NMR spectra (δc 131.95 for C-2′/6′ and δc 115.43 for C-3′/5′. The effect of the less electronegative sulphur in thiol group (SH) compared to nitrogen in amino group (NH2) and oxygen in hydroxyl group (OH) caused a less deshielding of C-1′ and C-2′, making them to resonate at 35.00 ppm and 39.78 ppm respectively. However, by comparison of physical data of Compound (1) with those of phenolic glycosides (Table 2) reported in literature (Arnold et al., 2002; Kanchanapoom et al., 2002) and Dictionary of Natural Products (DNP, 2012) as well as Handbook of Natural Products from Plants (Cseke et al., 2006), Compound (1) is said to be a new compound and isolated for the first time from this plant.
Table 2.
Comparison of NMR Signals of Compound (1) and Reported Phenolic glycoside
| Isolated Compound (1) | Phenolic glycoside (Kanchanapoom et al., 2002) | ||||
| Position | 1H NMR | 13C NMR | 1H NMR | 13C NMR | |
| Aromatic ring | Aromatic ring | ||||
| C-1 | - | 154.67 | - | 131.50 | |
| C-2 | 6.66 (1H, dd, J = 2.0 Hz) | 115.43 | 6.66 (1H, d, J = 2.0 Hz) | 117.10 | |
| C-3 | 7.49 (1H, dd, J = 1.5 Hz) | 131.95 | - | 144.60 | |
| C-4 | 142.12 | - | 146.10 | ||
| C-5 | 7.49 (1H, dd, J = 7.0 Hz) | 131.95 | 6.66 (1H, d, J = 8.3 Hz) | 116.30 | |
| C-6 | 6.66 (1H, dd, J = 9.0 Hz) | 115.43 | 6.51 (1H, dd, J = 8.3 Hz, 2.0 Hz) |
121.30 | |
| dihydroxyl-1-mercaptopropyl | 93.37 | α, β-Substitution | |||
| C-1′ | 3.53 (1H, m) | 35.00 | 3.90 (1H, m) | 72.20 | |
| C-2′ | 2.35 (bd) | 39.78 | 2.73 (td, J = 7.0) | 36.50 | |
| C-3′ | 4.64 (d, J = 9 Hz) | 96.44 | - | - | |
| Glucose | Glucose | ||||
| C-1″ | 5.67 (1H, d, J = 6.0 Hz) | 100.16 | 4.32 (1H, d, J = 7.8 Hz) | 104.20 | |
| C-2″ | 3.23–4.28 (m) | 77.44 | 3.37 (dd) | 76.20 | |
| C-3″ | 3.23–4.28 (m) | 74.93 | 3.78 (dd) | 81.60 | |
| C-4″ | 3.23–4.28 (m) | 71.23 | 4.96 (dd) | 70.40 | |
| C-5″ | 3.23–4.28 (m) | 78.17 | 3.66 (dd) | 76.00 | |
| C-6″ | 3.23–4.28 (m) | 62.75 | 4.12 (dd) | 62.40 | |
Conclusion
The isolated compound from the leaf of Massularia acuminata demonstrated antioxidant and antimicrobial activities and may be responsible for the activities of leaf extract and its ethyl acetate fraction, hence this may justify its ethnomedicinal use. The isolation and characterization of thiophenolic glycoside from this plant marks the first report of isolated constituent from M. acuminata and from the genus Massularia.
Figure 1.
Structure of Isolated Compound
Figure 3.
Structure of Compound (1) and Phenolic glycosides (2–3) from Literature
Acknowledgments
The authors thank Mr. T. A. Oladele of the Department of Plant Science, Faculty of Science, University of Port Harcourt, Nigeria, for plant collection and identification. Dr. Frank Schweizer of the Department of Chemistry, University of Manitoba, Winnipeg, Canada, is appreciated for making available, spectral data.
References
- 1.Aderinokun G A, Lawoyin J O, Onyeaso C O. Effect of Two Common Nigerian Chewing sticks on Gingival Health and Oral Hygiene. Odonto-Stomatologie Tropicale. 1999;87(12):1274–1281. [PubMed] [Google Scholar]
- 2.Aladesanmi A J, Iwalewa E O, Akinkunmi E O, Adebajo A C, Taiwo B J, Olorunmola F O, Lamikanra A. Antimicrobial and Antioxidant Activities of Some Nigerian Medicinal Plants. African Journal of Traditional Complementary and Alternative Medicines. 2007;4(2):173–184. doi: 10.4314/ajtcam.v4i2.31206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amić D, Davidović-Amić D, Bešlo D, Trinajstić N. Structure-Radical Scavenging Activity Relationships of Flavonoids. Croatian Chemical Acta. 2003;71(1):55–61. [Google Scholar]
- 4.Arnold U W, Zidorn C, Ellmerer E P, Stuppner H. Iridoid and Phenolic Glycosides from Wulfenia carinthiaca. Verlag der Zeitschrift für Naturforschung. 2002;57C:969–975. doi: 10.1515/znc-2002-11-1202. [DOI] [PubMed] [Google Scholar]
- 5.Burkill H M. Useful Plants of West Tropical Africa. Vol. 4. Royal Botanic Garden, Kew. UK: 1997. [Google Scholar]
- 6.Canadanovic-Brunet J M, Djilas S M, Cetkovic G S. Free-radical Scavenging Activity of Wormwood (Artemisia absinthium) Extracts. Journal of the Science of Food and Agriculture. 2005;85:265–272. [Google Scholar]
- 7.Clinical Laboratory Standard Institute (CLSI), author Document M2-A9 and M7-A7 Performance Standards for Antimicrobial Susceptibility Testing; Seventh Information Supplement. 940 West Valley Road, Suite 1400, Wayne, Pennsylvania, USA: CLSI; 2007. pp. 19087–19098. [Google Scholar]
- 8.Cowan M M. Plant products as antimicrobial agents. Clinical Microbiology Review. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cseke L J, Kirakosyan A, Kaufman P B, Warber S L, Duke J A, Brielmann H L. Natural Products from Plants. Second Edition. Boca Raton, FL 33487-2742: Taylor and Francis Group (CRC Press); 2006. [Google Scholar]
- 10.DNP, author. Dictionary of Natural Products: A Compilation of the National Institute for Occupational Safety and Health Services. USA: Department of Health and Human Services; 2012. pp. 1–210. [Google Scholar]
- 11.Dupont S, Caffin N, Bhandari B, Dykes G A. In vitro Antibacterial Activity of Australian Native Herb Extracts Against Food-related Bacteria. Food Control. 2006;17:929–932. [Google Scholar]
- 12.Erol N T, Sari F, Polat G, Velioglu Y S. Antioxidant and Antibacterial Activities of Various Extracts and Fractions of Fresh Tea Leaves and Green Tea. Tarim Bilimleri Dergisi. 2009;15(4):371–378. [Google Scholar]
- 13.Gülçin I, Alici H A, Cesur M. Determination of In vitro and Radical Scavenging Activities of Propofol. Chemica Pharmaceutica Bulletin. 2005;53:281–285. doi: 10.1248/cpb.53.281. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B. Biochemistry of Oxidative Stress. Biochemical Society Transaction. 2007;35(5):1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 15.Hostettmann K, Marston A. Twenty Years of Research into Medicinal Plants: Results and Perspectives. Phytochemistry Reviews. 2002;1:275–285. [Google Scholar]
- 16.Kanchanapoom T, Kasai R, Yamasaki K. Phenolic Glycosides from Barnettia kerrii. Phytochemistry. 2002;59:565–570. doi: 10.1016/s0031-9422(01)00476-9. [DOI] [PubMed] [Google Scholar]
- 17.Li W J, Cheng X L, Liu J, Lin R C, Wang G L, Du S S, Liu Z L. Phenolic Compounds and Antioxidant Activities of Liriope muscari. Molecules. 2012;17:1797–1808. doi: 10.3390/molecules17021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Moreno C, Larrauri J A, Saura-Calixto F A. Procedure to Measure the Antiradical Efficiency of Polyphenols. Journal of Science Food and Agriculture. 1998;76:270–276. [Google Scholar]
- 19.Taiwo O, Xu HX, Lee S F. Antibacterial Activities of Extracts from Nigerian Chewing Sticks. Phytotherapy Research. 1999;13:675–679. doi: 10.1002/(sici)1099-1573(199912)13:8<675::aid-ptr513>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Turkoglu A, Kivrak I, Mercan N, Duru M E, Gezer K, Turkoglu H. Antioxidant and Antimicrobial Activities of Morchella conica Pers. African Journal of Biotechnology. 2006;5(11):1146–1150. [Google Scholar]