Abstract
Background
The main functions of Radix Glycyrrhizae include regulating middle warmer, moistening lung, relieving toxicity, harmonizing property of drugs which is a traditional Chinese medicine widely used in clinical settings. The objective of the paper is to isolate and identify the constituents in ethanol extract of Radix Glycyrrhizae, and to study their anticancer activity.
Materials and Methods
Column chromatography, ODS column chromatography, preparative thin layer chromatography and NMR spectroscopy techniques were used to isolate compounds from ethanol extract of Radix Glycyrrhizae; optical microscopy and flow cytometry were used to determine the anticancer effect of Radix Glycyrrhizae extract.
Results
Four compounds were isolated from the ethanol extract of Radix Glycyrrhizae, namely oleanolic acid, isoliquiritin, glycyrrhetinic acid and licochalcone A. Optical microscopic observation showed that the growth of gastric cancer cell line SGC-7901 was inhibited in the experimental groups, and apoptotic morphological changes were observed in adherent cells; flow cytometry with PI staining showed that Radix Glycyrrhizae extract could induce SGC-7901 cell apoptosis within a concentration range of 0.5–1.5 mg/mL, compared with the control group, the apoptosis was positively correlated with the drug concentration, which exhibited an apparent dose-dependence.
Conclusion
We conclude Ethanol extract of Radix Glycyrrhizae has an anti-proliferative activity on SGC-7901 cells.
Keywords: Radix Glycyrrhizae, oleanolic acid, isoliquiritin, glycyrrhetinic acid, licochalcone A, SGC-7901 cell
Introduction
Radix Glycyrrhizae is the root and rhizome of Glycyrrhiza uralensis Fisch., G. inflata Bat. or G. glabra L. of family Leguminosae. Its main functions include regulating middle warmer, moistening lung, relieving toxicity, harmonizing property of drugs, etc. which is a traditional Chinese medicine widely used in clinical settings (Suzuk et al., 2004; Torkin et al., 2005). The main active fraction of Radix Glycyrrhizae is flavonoids, domestic and foreign scholars have conducted a number of studies on them (Surget et al., 2011; Paizanis et al., 2007). In this paper, the ethanol extract of Radix Glycyrrhizae is studied, and its inhibitory effect on SGC-7901 cells is explored.
Materials
Instruments and reagents
DRX-500 NMR spectrometer (Bruker, USA); rotary evaporator (Shanghai Yarong Biochemical Instrument Factory); CO-150 CO2 incubator (NBS, USA); flow cytometer (BD Biosciences); RPMI 1640 medium, purchased from Gibco, USA; propidium iodide (PI), purchased from Sigma, USA.
Cells and drugs
Radix Glycyrrhizae, purchased from the pharmacy, which was identified as Glycyrrhiza uralensis Fisch. of family Leguminosae by Pro. Diao YP from Dalian Medical University, stored in herbarium. Radix Glycyrrhizae extract, self-prepared. SGC-7901 cell lines, purchased from China Medical University.
Methods
Extraction and isolation of compounds from Radix Glycyrrhizae
5 kg of Radix Glycyrrhizae was taken, ground into a coarse powder, and then extracted by heat reflux three times with 95% ethanol, each extraction lasted 2 h. The extracts were combined, and solvent was removed to give 1.6 kg of ethanol extract. The ethanol extract was added with appropriate amount of water, and extracted with ethyl acetate, then solvent was removed under reduced pressure to give 125 g of ethyl acetate extraction fraction. The extract was then isolated by column chromatography, and gradient-eluted with dichloromethane and methanol as solvents, identical fractions were combined, and four fractions were obtained. The first and second fractions were subjected to polyamide chromatography, ODS column chromatography and preparative thin layer chromatography to obtain four compounds.
Cell culture
Gastric cancer SGC-7901 cell lines were cultured in RPMI 1640 medium containing 10% fetal bovine serum, and routinely subcultured in a 37°C, 5% CO2 incubator, cells in logarithmic growth phase were taken and set aside.
Optical microscopic observation
Logarithmic growth phase SGC-7901 cells were taken, and treated with different concentrations of Radix Glycyrrhizae extracts (final concentrations of 0.5, 1.0 and 1.5 mg/ml) for 24 h, control group was added with an equal volume of PBS. Cell morphology was observed under an optical microscope.
Detection of cell cycle change
The SGC-7901 cells were seeded in culture flasks at 5×104 cells/mL, and incubated at 37°C under 5% CO2 for 24 h, then the medium was replaced and the flasks were added with Radix Glycyrrhizae extracts with final concentrations of 0.5, 1.0 and 1.5 mg/ml, and incubated for 48, 72 h. After digestion with 0.25% trypsin, the cells were collected, centrifuged for 5 min, washed twice with PBS, added with 75% ethanol, and allowed to stand overnight at 4°C. After removal of ethanol by centrifugation, the cells were washed twice with PBS, PI stained, and incubated at 37°C under dark conditions for 30 min, followed by determination of cell cycle distribution by flow cytometry.
Results
Structural identification of compounds
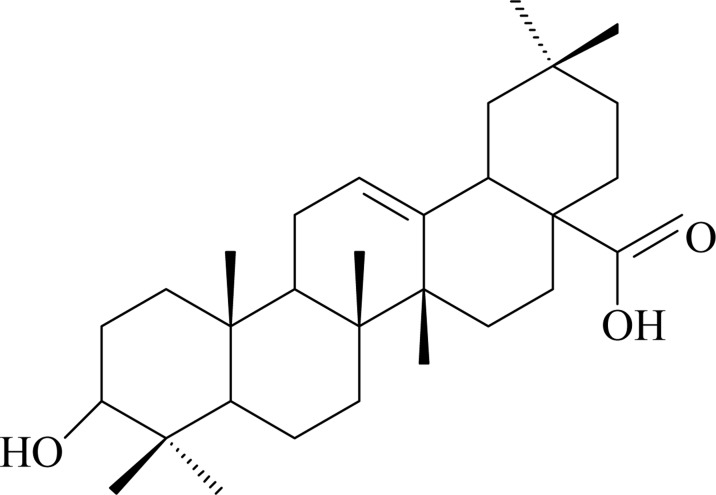
Compound 1: white powder. 1H-NMR (300MHz, CDCl3) δ: 0.79 (3H, s, H-23), 0.78 (3H, s, H-24), 0.92 (3H, s, H-25), 0.91 (3H, s, H-26), 0.95 (3H, s, H-29), 0.98 (3H, s, H-30), 1.15 (3H, s, H -27), 5.24 (1H, t, J = 2.4 Hz, H-12), 3.24 (1H, dd, J = 4.4, 11.6 Hz, H-3α), 2.85 (1H, dd, H-18); 13C-NMR (300 MHz, CDCl3) δ: 38.6 (C-1), 27.3 (C-2), 79.2 (C-3), 38.6 (C-4), 55.4 (C-5), 18.5 (C-6), 32.8 (C-7), 39.3 (C-8), 47.4 (C-9), 37.3 (C-10), 23.6 (C-11), 122.7(C-12), 143.8 (C-13), 41.6 (C-14), 27. 5 (C-15), 23.7 (C-16), 46.4 (C-17), 41.2 (C-18), 45.7 (C-19), 30.5 (C-20), 33.6 (C-21), 32.6 (C-22), 28.3 (C-23), 15.7 (C-24), 15.4 (C-25), 17.3 (C-26), 25.7 (C-27), 183.4 (C-28), 33.2 (C-29), 22.8 (C-30). The above data were consistent with the reported literature (Ding et al., 2010), so the compound was identified as oleanolic acid.
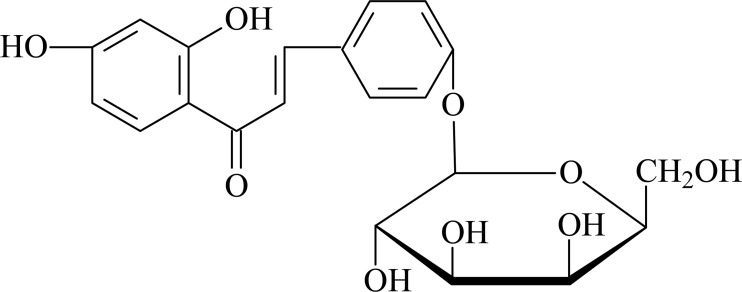
Compound 2: yellow crystals, mp: 148∼150□, easily soluble in methanol and ethyl acetate. 1H-NMR (300MHz, CDCl3) δ: 6.33 (1H, d, J=2.3Hz), 6.54 (1H, dd, J=9.0Hz, 2.3Hz), 8.03 (1H, d, J=9.0Hz), 7.45 (1H, d, J=18Hz), 7.76 (2H, d, J=18Hz), 7.69 (2H, d, J=8.6Hz), 7.43 (2H, d, J=8.6Hz); 13C-NMR (300MHz, CDCl3) δ: 145.4 (C-1), 120.5 (C-2), 193.3 (C-3), 114.7 (C-4), 167.6 (C-5), 104.5 (C-6), 166.5 (C-7), 109.4 (C-8), 133.4 (C-9), 130.6 (C-10), 131.7 (C-11), 118.5 (C-12), 161.8 (C-13), 118.7 (C-14), 131.6 (C-15), 102.4 (C-16), 78.3 (C-17), 78.4 (C-18), 75.4 (C-19), 71.6 (C-20), 62.4 (C-21). The above data were consistent with the reported literature (Wang et al., 2004), so the compound was identified as isoliquiritin
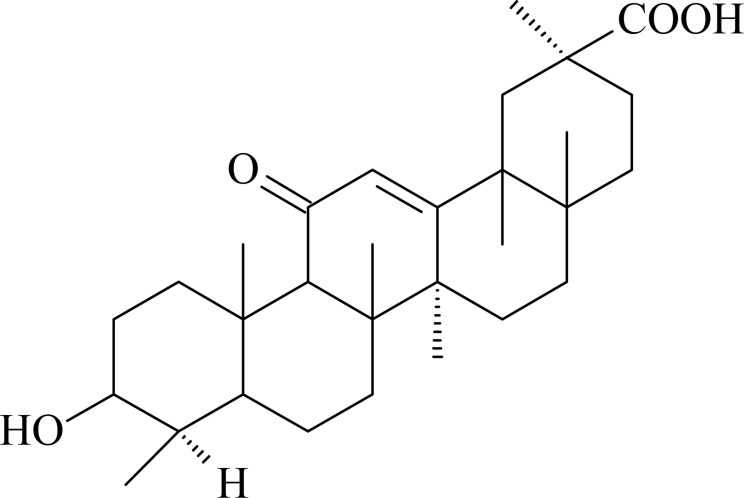
Compound 3: white powder, 1H-NMR (300MHz, CDCl3) δ: 12.23(1H, s, 29-COOH), 3.42 (1H, dd, H-3), 3.15 (1H, dd, H-5), 2.76 (2H, d, H-2), 2.32 (1H, m, H-9), 0.7-1.5 (21H, 7×CH3); 13C-NMR (300 MHz, CDCl3) δ: 38.3 (C-1), 26.6 (C-2), 76.1 (C-3), 7.2 (C-4), 54.5 (C-5), 6.5 (C-6), 30.2 (C-7), 42.6 (C-8), 48.5 (C-9), 36.8 (C-10), 177.8 (C-11), 127.5 (C-12), 169.3 (C-13), 44.3 (C-14), 25.5 (C-15), 28.5 (C-16), 32.6 (C-17), 61.6 (C-18), 39.3 (C-19), 31.3(C-20), 38.5 (C-21), 43.4 (C-22), 27.4 (C-23), 17.6 (C-24), 16.5 (C-25), 16.3 (C-26), 18.6 (C-27), 28.3 (C-28), 199.3 (C-29), 23.5 (C-30). The above data were consistent with the reported literature (Chim, 2001), so the compound was identified as glycyrrhetinic acid.
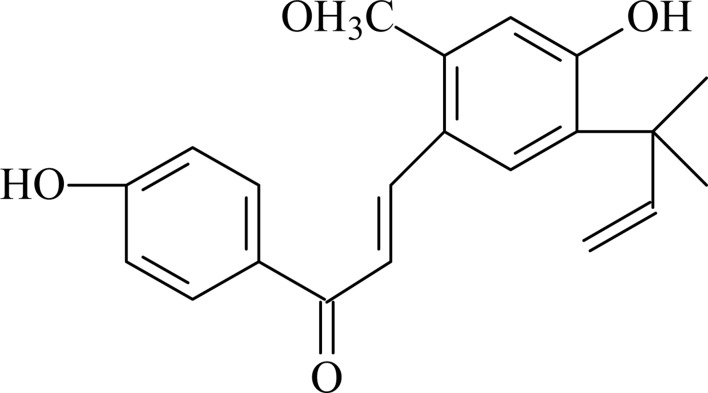
Compound 4: orange crystals, 1H-NMR ( 300MHz, CDCl3) δ: 7.32 (1H, d, A-H), 7.89 (1H, d, B-H), 6.76 (2H, d, 3c, 5c-H), 7.86 (2H, d, 2c, 6c-H), 6.34 (1H, s, 3-H), 7. 42 (1H, s, 6-H), 3. 69 (3H, s, -OCH3), 1.52[6H, s, Ar-C(CH3)2], 6.18 (1H, dd, -CH=), 5.43 (1H, dd, cis-HC=CH2), 5.33 (1H, dd, trans-CH-CH2); 13C-NMR (300MHz, CDCl3) δ: 115.5 (C-1), 158.6 (C-2), 99.7 (C-3), 161.5 (C-4), 126.3 (C-5), 127.5 (C-6), 117.3 (C-A), 138.4 (C-B), 129.2 (C-1c), 130.3 (C-2c), 115.6 (C-3c), 159.5 (C-4c), 115.6 (C-5c), 130.6 (C-6c), 39.7 (C-1d), 147.3 (C-2d), 110.6 (C-3d), 55.3 (−OCH3), 187.6 (C=O), 26.3 (C-4d), 26.4 (C-5d). By comparison, the data of compound 4 was basically consistent with the literature (Zou, 1993), so it was identified as licochalcone A.
Observation of cell morphology
Optical microscopic observation showed that the growth of gastric cancer cell line SGC-7901 was inhibited in the experimental groups, and apoptotic morphological changes were observed in adherent cells, that is, cell shape gradually changed from spindle to round, and transparency and adhesion of cells were reduced; cell growth in the control group was normal.
Change in cell cycle
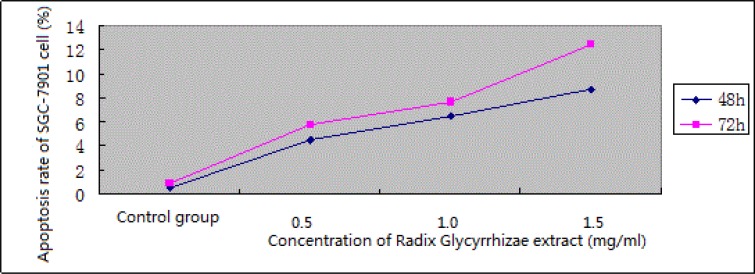
Flow cytometry with PI staining showed that Radix Glycyrrhizae extract could induce SGC-7901 cell apoptosis within 0.5–1.5 mg/mL, compared with the control group, the apoptosis was positively correlated with the drug concentration, which exhibited an apparent dose-dependence. The results in Tab. 1 show that after 72 h of action, the number of G0/G1 phase cells increased apparently in various Radix Glycyrrhizae extract groups compared with the control group, indicating that different concentrations of Radix Glycyrrhizae extracts can effectively induce SGC-7901 cell cycle arrest in the G0/G1 phase in a dose-dependent manner.
Table 1.
Effect of Radix Glycyrrhizae extract on cell cycle of SGC-7901 cells (72 h)
| Group | Concentration (mg/mL) |
G0/G1 phase | S phase | G2/M phase |
| Control group | 47.43±6.54 | 19.45±4.97 | 33.13±5.75 | |
| Radix Glycyrrhizae extract |
0.5 | 54.35±4.65 | 18.47±5.73 | 27.18±4.34 |
| 1.0 | 63.57±5.85* | 17.54±6.64 | 18.89±4.48 | |
| 1.5 | 71.24±7.37* | 16.54±5.73 | 12.22±3.23 |
Note: comparison with the blank control group,
P<0.05
Discussion
Radix Glycyrrhizae flavonoids are a class of constituents with relatively strong physiological activity contained in Radix Glycyrrhizae. There have been relatively many studies on the pharmacological activity of Radix Glycyrrhizae flavonoids, which are mainly focused on anti-depressant and anti-cancer aspects. Fan Zi-zhou et al. established chronic stress depression model by administering nine different stimuli to rats, and continuously intervened the established model using different doses of Radix Glycyrrhizae flavonoids, whose anti-depressant effects were determined by open field test, forced swimming test and tail suspension test. Their results suggested that the total flavonoid fraction of Radix Glycyrrhizae has a good antidepressant pharmacological activity on chronic unpredictable stress-induced depressive behaviors in rats, as well as a protective effect on stress-induced hippocampal neurogenesis impairment at larger doses (Li et al., 2010; Wang et al., 2004; Fan et al., 2012). Radix Glycyrrhizae flavonoids can induce typical apoptotic changes in tumor cells, including reduced nucleus volume, nuclear chromatin condensation into clumps and formation of apoptotic bodies. Electron microscopy and agarose gel electrophoresis found that Radix Glycyrrhizae flavonoids can cause relatively obvious apoptotic morphological changes and DNA fragmentation in tumor cells (Ji et al., 2005; Zhao et al., 2005; Ji et al., 2004; Ji et al., 2002).
In this paper, four compounds, namely oleanolic acid, isoliquiritin, glycyrrhetinic acid and licochalcone A, were isolated from the ethanol extract of Radix Glycyrrhizae by column chromatography, ODS column chromatography, preparative thin layer chromatography and NMR spectroscopy techniques.
In the experiment on the growth inhibitory effect of Radix Glycyrrhizae extract on gastric cancer cell line SGC-7901, optical microscopic observation showed that the growth of gastric cancer cell line SGC-7901 was inhibited in the experimental groups, and apoptotic morphological changes were observed in adherent cells, that is, cell shape gradually changed from spindle to round, and transparency and adhesion of cells were reduced; cell growth in the control group was normal. Flow cytometry with PI staining showed that Radix Glycyrrhizae extract could induce SGC-7901 cell apoptosis within 0.5–1.5 mg/mL, compared with the control group, the apoptosis was positively correlated with the drug concentration, which exhibited an apparent dose-dependence. The results in Tab. 1 show that after 72 h of action, the number of G0/G1 phase cells increased apparently in various Radix Glycyrrhizae extract groups compared with the control group, indicating that different concentrations of Radix Glycyrrhizae extracts can effectively induce SGC-7901 cell cycle arrest in the G0/G1 phase in a dose-dependent manner.
Figure 1.
Structure of oleanolic acid
Figure 2.
Structure of isoliquiritin
Figure 3.
Structure of glycyrrhetinic acid
Figure 4.
Structure of licochalcone A
Figure 5.
Effect of Radix Glycyrrhizae extract on apoptosis of gastric cancer SGC-7901 cells
References
- 1.Chim J. Synthesis and antitumor activity of Glycyrrhetic acid derivatives. Applied Chemistry. 2001;18:869–872. [Google Scholar]
- 2.Ding XJ, Chen Z, Li X, Li XR, Xu QM, Yang SL. Study on chemical constituents of Pulsatilla chinensis (Bge.) Regel. Chinese Traditional and Herbal Drugs. 2010;41(12):1952–1956. [Google Scholar]
- 3.Fan ZZ, Zhao WH, Guo J, Cheng RF, Zhao JY, Yang WD, Wang YH, Li W, Peng XD. Antidepressant activities of flavonoids from Glycyrrhiza uralensis and its neurogenesis protective effect in rats. Acta Pharmaceutica Sinica. 2012;47(12):1612–1617. [PubMed] [Google Scholar]
- 4.Ji YB, Gao SY, Kong Q. Influence of sea pyrimidine on apoptosis of SGC-7901 human stomach cancer cells. Journal of Harbin Institute of Technology. 2002;34(1):91–94. [Google Scholar]
- 5.Ji YB, Gao SY. Effects of Haimiding on the functioning of red cell membrane of FC and H22 tumor-bearing mice. Chinese Journal of Pharmaceuticals. 2004;39(12):913–916. doi: 10.3748/wjg.v11.i6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji YB, Jiang W, Shang M, Wang HL. Effect of Glycyrrhiza flavonoids on DNA and RNA in tumor cell of S180 and H22 tumor-bearing mice. Chinese Traditional and Herbal Drugs. 2005;36(10):1518–1520. [Google Scholar]
- 7.Li W, Li SP, Lin L, Bai H, Wang YH, Kato H, Asada Y, Zhang QB, Koike K. Bioassay-guided isolation and quantification of the alpha-glucosidase inhibitory compound, glycyrrhisoflavone, from Glycyrrhiza uralensis. Natural Product Communications. 2010;5:1049–1053. [PubMed] [Google Scholar]
- 8.Paizanis E, Kelaï S, Renoir T, Hamon M, Lanfumey L. Life-long hippocampal neurogenesis: environmental, pharmacological and neurochemical modulations. Neurochemical Research. 2007;32:1762–1771. doi: 10.1007/s11064-007-9330-0. [DOI] [PubMed] [Google Scholar]
- 9.Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Molecular Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuk R, Kohno H, Murakami A, Koshimizu K, Ohigashi H, Yano M, Tokuda H, Nishino H, Tanaka T. Citrus nobiletin inhibits azoxymethane-induced large bowel carcinogenesis in rats. Biological factors. 2004;22(1–4):111–114. doi: 10.1002/biof.552210121. [DOI] [PubMed] [Google Scholar]
- 11.Torkin R, Lavoie JF, Kaplan DR, Yeger H. Induction of Caspase-dependent, p53 mediated apoptosis by apigenin in human neuroblastoma. Molecular Cancer Therapy. 2005;4(1):01–11. [PubMed] [Google Scholar]
- 12.Wang YH, Bai H, Dou DQ, Pei YP, Chen YJ, Li W, Xiao CYN, Xing SR, Er JTB. Study on the flavonoids constituents of cultivated licorice. Northwest Pharmaceutical Journal. 2004;19(6):252–253. [Google Scholar]
- 13.Zhao SY, Wang NP, Zhong ZG, Nong ZX. Experimental study on induction of hepatoma cell apoptosis by Glycyrrhiza flavonoids. Journal of Guangxi Medical University. 2005;22(2):235–237. [Google Scholar]
- 14.Zou K. Study on the chemical constituents of Glycyrrhiza inflata. Natural Product Research and Development. 1993;5(4):1–4. [Google Scholar]