Abstract
Background
A Chinese herb Corydalis yanhusuo W.T. Wang that showed anticancer and anti-angiogenesis effects in our previous studies was presented for further studies. In the present study, we studied the anticancer proliferation and adhesion effects of five alkaloids which were isolated from Corydalis yanhusuo.
Materials and Methods
MTT dose response curves, cell migration assay, cell invasion assay, as well as three types of cell adhesive assay were performed on MDA-MB-231 human breast cancer cells. The mechanism of the compounds on inhibiting heterotypic cell adhesion were further explored by determining the expression of epidermal growth factor receptor (EGFR), Intercellular adhesion molecule 1 (ICAM-1), αv-integrin, β1-integrin and β5-integrin by western blotting assay.
Results
In five tested alkaloids, only protopine exhibited anti-adhesive and anti-invasion effects in MDA-MB-231 cells, which contributed to the anti-metastasis effect of Corydalis yanhusuo. The results showed that after treatment with protopine for 90 min, the expression of EGFR, ICAM-1, αv-integrin, β1-integrin and β5-integrin were remarkably reduced.
Conclusion
The present results suggest that protopine seems to inhibit the heterotypic cell adhesion between MDA-MB-231 cells, and human umbilical vein endothelial cells by changing the expression of adhesive factors.
Keywords: Corydalis yanhusuo, Protopine, Adhesion, Breast cancer, Integrins
Introduction
Corydalis yanhusuo W.T. Wang is a wildly used herb in China for invigorating the circulation of blood. We previously investigated the cancer chemo-preventive activities of Corydalis yanhusuo extracts on human breast cancer cells. Corydalis yanhusuo was shown to exert anti-proliferative effects on MDA-MB-231 cells and anti-angiogenesis effects on human umbilical vein endothelial cells in vitro (Gao et al., 2008; Gao et al., 2009b). However, the bioactive substance responsible for the anticancer activity of Corydalis yanhusuo remains unclear. The present study aimed to evaluate the anticancer proliferative effects of main bioactive substances isolated from Corydalis yanhusuo, namely protopine, dehydrocorydaline (DHC), dl-tetrahydropalmatine (THP), berberine and palmatine (Gao et al., 2012), as a result, only protopine exhibited anti-adhesive and anti-invasion effects in MDA-MB-231 cells, which contributed to the anti-metastasis effect of C. yanhusuo.
Protopine, a benzylisoquinoline alkaloid with a molecular weight of 353.37 g/mol, is wildly present in many plants, such as the Papaveraceae family (Cahlikova et al., 2012; Chen et al., 2009; Kulp et al., 2011; Lee et al., 2011; Lu et al., 2012), Elaeocarpaceae (Munoz et al., 2011), and Fumariaceae family (Rathi et al., 2008; Wang et al., 2012). Protopine exhibits anti-thrombotic and anti-inflammatory activities (Bae et al., 2012; Saeed et al., 1997; Teng et al., 1991), antispasmodic and relaxant activity (Hiller et al., 1998; Huang et al., 1991), antifungal effect (Orhana et al., 2007), hepato-protective activity (Janbaz et al., 1998; Rathi et al., 2008; Wei and Liu, 1997), neuro-protective activity (Xiao et al., 2007), anticholinesterase and antiamnesic effects (Kim et al., 1999), anti-cancer effect (Chen et al., 2012), antidepressant-like effect (Xu et al., 2006) and analgesic effect. The pharmacological activities of protopine might be associated with its ability on inhibiting K+ (ATP) channel subunits or Ca2+-channel (Jiang et al., 2004; Ko et al., 1992), activating GABAA receptor (Haberlein et al., 1996), antioxidant (Xiao et al., 2008), inhibiting NF-κB activity and depressing phosphorylation of MAPK (Bae et al., 2012). Otherwise, promoting tubulin polymerization (Chen et al., 2012), inhibiting serotonin transporter and noradrenaline transporter might be also involved in protopine's activities.
The most critical biological process in cancer cell metastasis is cell adhesion (Hirohashi and Kanai, 2003). Cell adhesion is a cortical step. Cell adhesion is the attachment of a cell, either to another cell or to an underlying substrate such as the ECM, via cell adhesion molecules. Nevertheless, the suppression effect of Corydalis yanhusuo and protopine on cell adhesion was never reported. It is necessary to confirm this effect with more direct and accurate method. In this paper, we designed 3 types of cell models, namely homotypic cell adhesion, heterotypic cell adhesion, and heterophilic adhesion, to simulate the effect of Corydalis yanhusuo and protopine on three types of cell adhesion.
To the best of our knowledge, a large group known as cell adhesion molecules is involved in cell adhesive process, such as integrins and ICAM-1. In order to further clarify the mechanism of the anti-adhesive effect of Corydalis yanhusuo and protopine, we detected the expression of ICAM-1, integrins αv, β1, and β5 in MDA-MB-231 cells by protopine and C. yanhusuo extract treatment.
Materials and Methods
Reagents and materials
RPMI 1640 medium, DMEM medium, F12K medium, FBS, PBS, streptomycin and penicillin (PS), endothelial cell growth supplement (ECGS), and 0.25% (w/v) trypsin /1 mM EDTA were purchased from Invitrogen (Carlsbad, CA, USA). Antibodies against epidermal growth factor receptor (EGFR), β1-integrin, β5-integrin and αV-integrin were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibody against ICAM-1 was obtained from SANTA CRUZ Biotechnology Inc. TPA, heparin and DMSO were purchased from Sigma (St Louis, MO, USA). Matrigel™ basement membrane matrix was purchased from BD Biosciences (Bedford, MA, USA). Corydaline, DHC, dl-THP, protopine, corybulbine, berberine, bicuculline, boldine, fumaric acid, palmatine, tetrahydroberberine, and stigmasterol were purchased from International Laboratory (San Bruno, CA, USA) or ChromaDex (Irvine, CA, USA). The crude drug of C. yanhusuo was purchased from Huadong Medicine Group Co., Ltd (Hangzhou, Zhejiang, China).
Sample Preparation
The rhizome of C. yanhusuo was cut into small pieces, ground into a fine powder, and extracted by 95% alcohol for five times. After retrieving the alcohol, the extract was freeze dried, producing a powdery form of the extract. The yield of crude extract of C. yanhusuo is 1.85% (w/w). HPLC showed the contents of main compounds (Gao et al., 2008; Gao et al., 2009b). The stock solution of the extract (100, 30 and 10 mg/ml) was prepared by DMSO.
The berberine, corydaline, DHC, palmatine, corybulbine, bicuculline, boldine, fumaric acid and stigmasterol were dissolved in DMSO to give stock solutions of 20 mg/ml. Protopine and dl-THP were dissolved in DMSO at 5 mg/ml. DMSO in a concentration of 0.5% was used as a vehicle control for HL-60 cells and 1% for other 4 kinds of cells.
Cell lines and culture
Human hepatoma cell line HepG2, human promyelocytic leukemia cell line HL-60, human breast carcinoma cell lines MCF7 and MDA-MB-231, HUVE-12 (Human Umbilical Vein Endothelial Cell-12) and human normal diploid fibroblasts cell line Hs68 were all purchased from American Type Culture Collection (ATCC). Hs68 cells were cultured in DMEM supplemented with 10% (v/v) FBS and 1% PS. HL-60 cells were cultured in RPMI medium supplemented with 20% (v/v) FBS and 1% PS. HepG2, MDA-MB-231 and MCF7 cells were cultured in RPMI medium supplemented with 10% (v/v) FBS and 1% PS. E12 cells were maintained in F12K medium supplemented with 100 µ/ml heparin, 30 .g/ml ECGS, 10% heat-inactivated FBS, and 1% PS. The tissue culture flasks and plates used for culturing HUVECs were pre-coated with 0.1% gelatin. All cells were cultured in a humidified atmosphere of 95% air/5% CO2 at 37 °C.
MTT assay
A modified MTT assay was used to examine the cell proliferation as described (Gao et al., 2009a). Briefly, different kinds of cells (2×104 cells/well density, 50–70% density), were seeded in 96-well plates. Drugs were added to the cells at variable concentrations or solvent control. At about 48 h after treatment, 15 µl MTT dye solution were added to each well and incubated for additional 4 h. Subsequently, 100µl/well DMSO was added to dissolve formazan crystals. Absorbance at 570 nm was measured using a Multilabel counter (Perkin Elmer, 1420 Multilabel Counter Victor3, Wellesley, USA).
Three-dimensional cell migration assay
A three-dimensional cell migration assay was performed with the Transwell system, which allows cells to migrate through an 8 µm pore size polycarbonate membrane. MDA-MB-231 cells were trypsinized, washed, and re-suspended in serum-free RPMI 1640 (2.5×105 cells/ml). 500 µl RPMI 1640 medium (containing 1% FBS and different concentrations of protopine, berberine and DHC), was added to the 24-well plate (the lower chamber of the Transwell), and a 200 µl cell suspension (containing corresponding concentrations of compounds) was added to the upper chamber. After incubation for 12 h at 37 °C, the non-migrating cells were carefully removed from the upper surface of the insert with a wet cotton swab. The migrated cells were fixed overnight in 3.7% formaldehyde at 4°C and stained with Hoechst 33258 in PBS (1:1000) for 15 min. The filters were then rinsed thoroughly in PBS and mounted on glass slides. To quantify cell motility, cells that had migrated to the bottom surface of the filter were counted. Three evenly-spaced fields of cells were counted in each well at 100× magnification using an Axiovert 200 fluorescent inverted microscope (Carl Zeiss, HK) and an Axi°Cam HRC CCD camera (Carl Zeiss, HK). The images were counted with Metamorph Imaging Series software (Molecular Devices, Tokyo, Japan). All assays were performed in triplicate.
Cell invasion assay
Cell invasion assay was carried out using the same method as three-dimensional cell migration assay with a slight modification in that, both sides of the insert were pre-coated with Matrigel. Briefly, different amounts of Matrigel (40 µg for the upper side and 32 µg for the lower side), were coated on the chambers for 6 h at 37 °C. MDA-MB-231 cells were suspended in serum-free RPMI 1640 (2.5×105 cells/ml). 500 µl RPMI 1640 medium (containing 1% FBS and different concentrations of protopine, berberine and DHC) was added to the lower chamber, and a 200 µl cell suspension (containing corresponding concentrations of compounds) was added to the upper chamber. The plate was incubated for 12 h at 37 °C in the presence of 5% CO2.
Heterotypic cell adhesion
For the heterotypic cell adhesion model, HUVECs and MDA-MB-231 cells were used to study the adhesive ability between two different kinds of cell types. Briefly, HUVECs (1.5×104 each well) were grown to confluence on fibronectin-coated wells of 96-well plates. The plates were blocked with HBSS containing 1% BSA (HBSS–BSA) for 30 min before the adhesion assay. BSA-coated wells serve as a negative control. The MDA-MB-231 cells were trypsinized and suspended in HBSS–BSA, and then labeled with 8 µg calcein-AM by 30 min incubation at 37 °C followed by washing with HBSS–BSA. The labeled MDA-MB-231 cells were then suspended in HBSS–BSA to a final density of 4.0×105 cells/ml and different dosages of drugs were added. Cell suspension 100 µl was incubated with HUVECs at 37 °C as designed, and quantified by fluorescence measurements immediately. Cultures were carefully washed three times with PBS to remove non-adherent cells and quantified again by fluorescence measurements using excitation of 485 nm and emission of 530 nm using the Multilabel counter. The results are expressed as OD value (after washed with PBS)/ OD value (before washed).
Homotypic cell adhesion
Briefly, MDA-MB-231 cells (2×104 each well) were grown to confluence on 96-well plates. The plates were blocked with HBSS–BSA for 30 min before the adhesion assay. MDA-MB-231 cell was trypsinized and suspended in HBSS–BSA, and then labeled with 8 µg calcein-AM by 30 min incubation at 37 °C followed by washing with HBSS-BSA. The labeled MDA-MB-231 cells were then suspended in HBSS-BSA to a final density of 4.0×105 cells/ml and different dosages of drugs were added. Cell suspension 100 µl was incubated with the substrate MDA-MB-231 cells at 37 °C as designed, and quantified by fluorescence measurements immediately. Cultures were carefully washed three times with PBS to remove non-adherent cells and quantified again by fluorescence measurements using excitation of 485 nm and emission of 530 nm using Multilabel counter. The results are expressed as OD value (after washed with PBS)/ OD value (before washed).
Heterophilic adhesion
Heterophilic adhesion model is used to test the adhesive ability of cancer cells and ECM. 96-well plates were incubated with 50 µl Matrigel (1:20 in cool PBS buffer), at 37 °C overnight, then blocked with 100 µl 1% HBSS-BSA for 30 min at 37 °C. MDA-MB-231 cell was trypsinized and suspended in HBSS-BSA, and then labeled with 8 µg calcein-AM by 30 min incubation at 37 °C followed by washing with HBSS-BSA. The labeled MDA-MB-231 cells were then suspended in HBSS-BSA to a final density of 4.0×105 cells/ml and different dosages of drugs were added. Cell suspension 100 µl was incubated with the substrate MDA-MB-231 cells at 37 °C as designed. OD values were measured before and after PBS wash.
Western blotting
SDS-PAGE and western blotting were performed to evaluate the protein expression levels of ICAM-1, EGFR, β1-integrin, β5-integrin and αV-integrin. Briefly, cells were treated as designated in blank RPMI 1640 for 90 min. Cell pellets were lysed in RIPA lysis buffer (Santa Cruz, CA) with 1% PMSF. After treatment on ice for 30 min, cell lysates were clarified by centrifugation at 11,419 ×g for 15 min at 4 °C to remove cell debris, and the protein content was measured using a BCA protein assay kit (PIERCE, Rockford, IL). Aliquots of the lysates were subjected to 10% SDS-PAGE (with 6% stacking gel) and transferred to a PVDF membrane (Bio-Rad, Hercules, CA). The membrane was probed with primary antibody (antibody dilutions: 1:1000) followed by second antibody and visualized using an ECL advanced western blotting detection kit (Amersham, UK) according to the manufacturer's protocol. Densitometric measurements of band intensity in the Western blots were performed using Quantity One Software (provided by Bio-Rad, Hercules, CA).
Statistical Analysis
Data were expressed as mean±S.D. Statistical significances between vehicle group and drug-treatment group were determined by one-way analysis of variance. The IC50 was calculated by SPSS software. A value of P< 0.05 was considered statistically significant.
Results
Corydalis yanhusuo extract and its pure compounds inhibited cancer cell proliferation
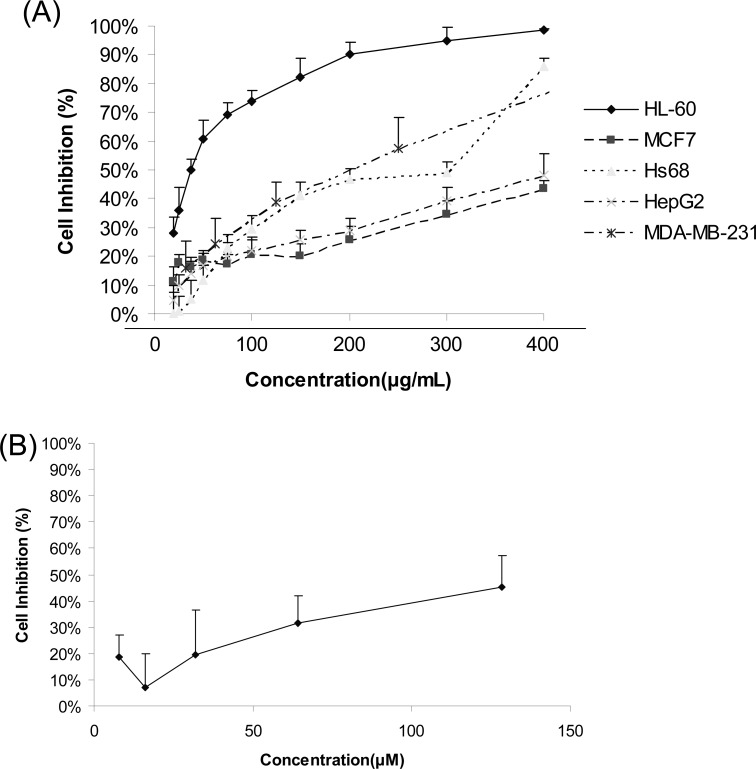
C. yanhusuo 95% ethanol extract displayed a remarkably inhibit effect in HL-60 cells; the IC50 is 46 µg/ml. However, it just showed a slightly inhibition effect in three other cell lines (Figure 1A). Berberine could strongly depress the growth of HL-60 cells with IC50 of 25 µM. Protopine have slight inhibitory effect in HL-60 cells (Figure 1B). The IC50 of those compounds were shown in Table 1. Other compounds purified from C. yanhusuo, such as boldine, protopine and palmatine only slightly inhibit the proliferation of HL-60 cells.
Figure 1.
Cell proliferation assay (MTT assay). (A) The anti-proliferation effect of Corydalis yanhusuo extract on 4 human cancer cell lines and 1 normal human cell line. (B) Effect of protopine to the cell viability of HL-60 cancer cells for 48 h treatment. The result was measured by MTT assay from three independent experiments (n=12). Each value represents the mean ± S.D. of the results.
Table 1.
Effects of Corydalis yanhusuo extracts and pure compounds on cell viability of HL-60, MCF7, HepG2, MDA-MB-231 and Hs68 cells
| Compounds | IC50 (µg•ml−1 or µM)* | ||||
| HL-60 | MCF7 | HepG2 | MDA-MB-231 | Hs68 | |
| C. yanhusuo extract | 46 | 992 | 552 | 224 | 198 |
| Corydaline | - | - | - | - | - |
| Dehydrocorydaline | - | - | - | 191 | - |
| dl-THP | - | - | - | - | - |
| Protopine | 162 | - | - | - | - |
| Corybulbine | - | - | - | - | - |
| Berberine | 25 | - | - | 127 | - |
| Bicuculline | - | - | - | - | - |
| Boldine | 214 | - | 403 | 197 | 153 |
| Fumaric acid | - | - | - | - | - |
| Palmatine | 264 | - | - | 531 | - |
| tetrahydroberberine | - | - | - | - | - |
| Stigmasterol | - | - | - | - | - |
The result was measured by MTT assay from three independent experiments.
Extract (µg•ml−1) and pure compounds (µM).
The IC50 value was calculated by the Probit regression analysis of SPSS software.
- No significant antiproliferation effect on the cells.
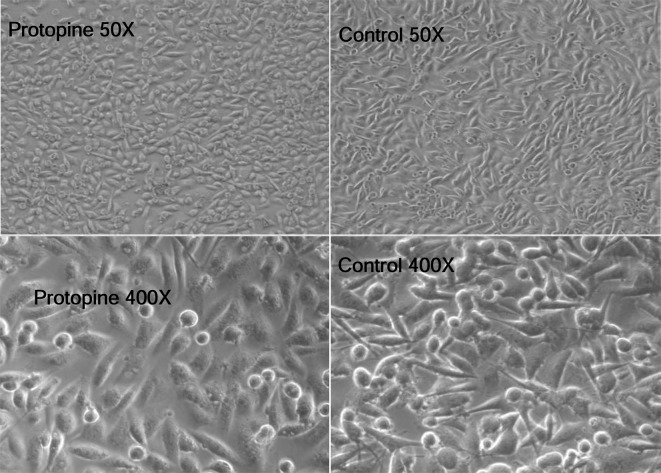
The morphologic changes of MDA-MB-231 cells after protopine treatment
The MDA-MB-231 cells were exposed to different agents including C. yanhusuo and protopine for 24 h. Observations were made on the morphologic changes of the cells. It was found that protopine could markedly affect the morphology of MDA-MB-231 cells. The morphologic changes of MDA-MB-231 cells were observed under the light microscope and were shown in Figure 2 (50× and 400× magnification). The MDA-MB-231 cells change to round shape rather than an irregular shape in the control group.
Figure 2.
The morphologic changes of MDA-MB-231 cells after exposed to 100 µM protopine for 24 h. Images were observed under the light microscope (50× and 400× magnification).
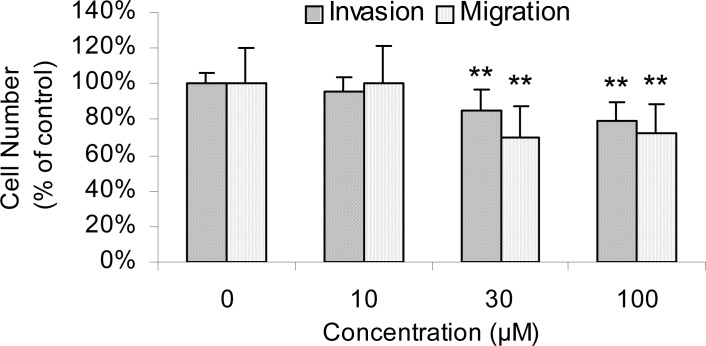
Effect of protopine on MDA-MB-231 cell invasion
We also detected the anti-migration and anti-invasion abilities of protopine in MDA-MB-231 cells. As indicated in Figure 3, protopine produced a significant, dose-dependent inhibition of MDA-MB-231 cell migration, as well as cell invasion on Matrigel (Figure. 3). The ability of cells to invade was reduced to 95.2%, 85.4%, and 79.1% after treatment with protopine at concentrations of 10, 30, and 100 µM, respectively. The result from 3D-migration assay indicated that the anti-invasion effect of protopine maybe related with its suppression in cell motility. Otherwise, DHC and berberine couldn't change the cell invasive ability (Data not shown).
Figure 3.
Effects of protopine on cell invasive and migration abilities in MDA-MB-231 cells. Each value represents the mean ± S.D. of the results from three independent experiments (n=9). **p < 0.01 vs. the vehicle control (0.1% DMSO).
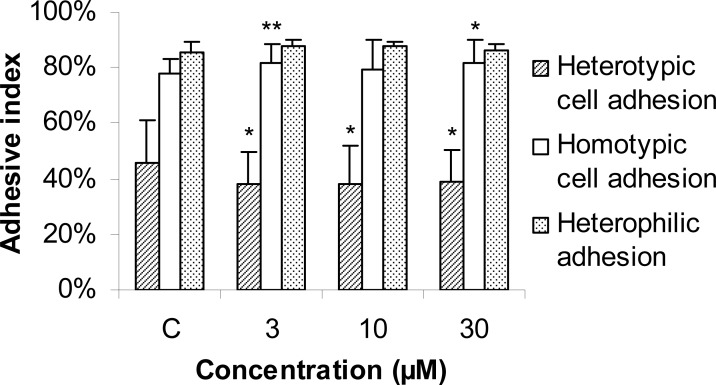
The effect of protopine in cell adhesion
There was no clear evidence of effects of C. yanhusuo in the adhesive ability of MDA-MB-231 cells in previous studies. In present study, C. yanhusuo couldn't remarkably inhibit the cell adhesive ability of MDA-MB-231 cells at the concentrations ranging from 0.1 – 30 µg/ml after being treated for 90 min (data not show). However, protopine could suppress the adhesive ability between MDA-MB-231 & HUVEC after 90 min treatment (Figure 4). In contrast, the homotypic cell adhesive ability of MDA-MB-231 cells was markedly increased by protopine, which could be helpful to maintain the adhesion stabilization of primary cancer cell, as well as decrease the detachment of cancer cells from the primary tumor.
Figure 4.
The cell adhesion index of MDA-MB-231 cells after treatment with protopine for 90min. Each value represents the mean ± S.D. of the results from three independent experiments (n=12). **p < 0.01, *p < 0.05 vs. the vehicle control (0.1% DMSO).
The effect of Corydalis yanhusuo and protopine on protein expression
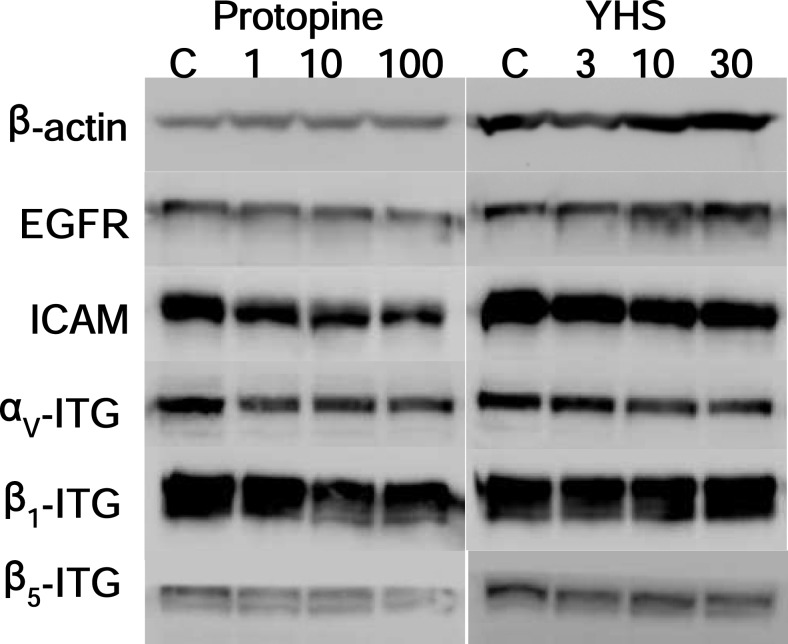
Since integrins are the major cell surface adhesion molecules expressed by all cell types, we examined whether incubation with C. yanhusuo extract and protopine could reduce integrins expression. The results shows that after the cells were treated with protopine for 90 min, the expression of EGFR, ICAM-1, αV-integrin, β1-integrin and β5-integrin (Figure 5) were reduced. On the other hand, the expression of ICAM-1, αV-integrin and β5-integrin (Figure 5) was reduced after the treatment of C. yanhusuo extract for 90 min.
Figure 5.
The effects of the protopine and Corydalis yanhusuo extract on ICAM-1, EGFR and integrins expression. Malignant human breast carcinoma cells were incubated with the protopine (1, 10, 100 µM) and Corydalis yanhusuo extract (3, 10, 30 µg/ml) for 90 min.
Discussion
One of the most important properties of cells is their ability to adhere to ECM proteins or other cells. Cell adhesion is crucial physiological event in the formation and maintenance of coherent multi-cellular structures, as well as in cancer tissues. There are two steps in metastasis in relation to cell adhesive ability, namely, the detachment of cells from the primary tumor and the lodging in blood vessels of cell cluster in distant organs (Oppenheimer, 2006). Analysis of cell-ECM and/or cell-cell adhesion, therefore, is of important value to experimental biologists as well as clinical investigators. Recently, several research groups have tried to identify cell adhesion suppressors which could inhibit cancer metastasis by blocking the lodging in blood vessels in the distant organs of disseminated cancer cells or cell clusters (Grimaldi et al., 2006).
There are three types of adhesion, including 1) homotypic cell adhesion, 2) heterotypic cell adhesion and heterophilic adhesion between cell-cell where physical bonds are formed between adjacent cells, and 3) cell-ECM adherence where cells bind to adhesive proteins in the ECM, should affect cancer cell metastasis. Over the past several decades, many different cell-adhesion assays have been developed. Here, three types of static adhesion assay models were used for characterization of the anti-adhesive properties of protopine, namely the adhesive ability between the epithelial cells or fibroblasts and cancer cells, as well as the adhesion of many types of cancer cells to the ECM.
Cell adhesion is not only one event but also a complex initial cell-surface interactions and adhesion stabilization. Present results indicated that protopine could induce morphological changes of MDA-MB-231 breast cancer cells. After 24 h, all cells treated by protopine became profoundly rounded, which maybe related with the microtubule-stabilizing activity of protopine (Chen et al., 2012). In addition, protopine also inhibits heterotypic cell adhesion, induces homotypic cell adhesion, but couldn't change the heterophilic cell adhesion ability. These observations suggested that the protopine could change the cell adhesive ability of breast cancer cells, including inhibiting the detachment of aggressive tumour cells from primary tumor and the attachment of cancer cells with blood vassals.
In the cell adhesion process, several groups of cells expressing different CAM are mixed together and interacted with each other to mediate cell adhesion. CAMs are multi-functional proteins involved in a number of regulatory processes, including cell growth, differentiation and proliferation, migration, and regeneration. Integrins are important mediators of the malignant phenotype during oncogenic transformation. In fact, cells alter their adhesive profile to assume a more migratory phenotype by increasing the affinity of integrins for their ligand.
Whereas the expression of some integrins, such as α6β4 and αvβ3, is increased during tumorigenesis, the reduced expression of the α1, α6, β1 or β4 integrin subunit is associated with the formation of neoplasms in breast epithelial tissue (Hood and Cheresh, 2002). Another report showed that β1-integrins are closely related with the cell sticking and adhesion of HT-29 colon carcinoma cells with ECM-coated surfaces under laminar flow conditions (Haier et al., 1999). Therefore, we detected the expression of β1-integrin, β5-integrin and αV-integrin with western blotting analysis to exhibit the mechanism of protopine on cell adhesion. We found that protopine caused down-regulation of β1-integrin, β5-integrin and αV-integrin (Figure 5).
Another important adhesive factor involved in heterotypic cell adhesive effect is ICAM-1. Our result indicated that protopine may have an inhibitory effect on ICAM-1 expression. In addition, MDA-MB-231 cells is a EGFR over expressed breast cancer cell, it was reported that over-expression and activation of integrin β1 induce EGFR tyrosine kinase inhibitors resistance via c-MET signaling pathway (Ju and Zhou, 2013). In the present study, it is reasonable to conceive that protopine inhibition of EGFR is attributable in part to integrin β1 inhibition.
In conclusion, we showed for the first time that protopine specifically inhibited heterotypic cell adhesion. Although the mechanisms of the beneficial effect of protopine against cancer metastasis remain an aspect to be elucidated, it was suggested in part that protopine affects the EGFR, ICAM-1, β1-integrin, β5-integrin and αV-integrin expression in MDA-MB-231. The findings of the present study may shed light on the pharmacological basis for the clinical application of protopine for preventing cancer metastasis.
Acknowledgements
The reported work was supported by China National Natural Science Foundation (No. 81102852, 81228024), the Zhejiang Provincial Key Laboratory Project (No. 2012E10002) and the Innovation Group Project of Zhejiang Chinese Medical University.
List of abbreviations
- DHC
Dehydrocorydaline
- THP
dl-Tetrahydropalmatine
- GABAA receptor
Gamma-aminobutyric acid type A receptor
- ATP
Adenosine triphosphate
- NF-κB
Nclear factor κB
- MAPK
Mitogen-activated protein kinase
- ECM
Extracellular matrix
- ICAM-1
Intercellular adhesion molecule 1
- CAM
Cell adhesion molecules
- EDTA
Ethylenediaminetetraacetic acid
- FBS
Fetal bovine serum
- PBS
Phosphate buffered saline
- DMSO
Dimethyl sulfoxide
- HPLC
High performance liquid chromatography
- MTT
3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- HUVEC
Human Umbilical Vein Endothelial Cells
- BSA
Bovine serum albumin
- HBSS
Hank's balanced salt solution
- OD value
Optical density
- EGFR
Epidermal growth factor receptor
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PVDF
Polyvinylidene difluorideF
- ECL
Enhanced chemiluminescence
- IC50
growth inhibition concentration
- C-MET
MET or MNNG HOS Transforming gene
References
- 1.Bae DS, Kim YH, Pan CH, Nho CW, Samdan J, Yansan J, Lee JK. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages. BMB reports. 2012;45:108–113. doi: 10.5483/BMBRep.2012.45.2.108. [DOI] [PubMed] [Google Scholar]
- 2.Cahlikova L, Kucera R, Host'alkova A, Klimes J, Opletal L. Identification of pavinane alkaloids in the genera Argemone and Eschscholzia by GC-MS. Natural product communications. 2012;7:1279–1281. [PubMed] [Google Scholar]
- 3.Chen CH, Liao CH, Chang YL, Guh JH, Pan SL, Teng CM. Protopine, a novel microtubule-stabilizing agent, causes mitotic arrest and apoptotic cell death in human hormone-refractory prostate cancer cell lines. Cancer letters. 2012;315:1–11. doi: 10.1016/j.canlet.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 4.Chen YZ, Liu GZ, Shen Y, Chen B, Zeng JG. Analysis of alkaloids in Macleaya cordata (Willd.) R. Br. using high-performance liquid chromatography with diode array detection electrospray ionization mass spectrometry. Journal of chromatography A. 2009;1216:2104–2110. doi: 10.1016/j.chroma.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 5.Gao JL, Chen ZF, Shi JM, Wang YT, Lv GY. Content Determination of 5 Kinds of Alkaloids in Corydalis yanhusuo and Stability Study. China Pharmacy. 2012;23:1386–1388. [Google Scholar]
- 6.Gao JL, He TOC, Li YB, Wang YT. A traditional Chinese medicine formulation consisting of Rhizoma Corydalis and Rhizoma Curcumae exerts synergistic anti-tumor activity. Oncology reports. 2009a;22:1077–1083. doi: 10.3892/or_00000539. [DOI] [PubMed] [Google Scholar]
- 7.Gao JL, Shi JM, He K, Zhang QW, Li SP, Lee SM, Wang YT. Yanhusuo extract inhibits metastasis of breast cancer cells by modulating mitogen-activated protein kinase signaling pathways. Oncology reports. 2008;20:819–824. [PubMed] [Google Scholar]
- 8.Gao JL, Shi JM, Lee SM, Zhang QW, Wang YT. Angiogenic pathway inhibition of Corydalis yanhusuo and berberine in human umbilical vein endothelial cells. Oncology research. 2009b;17:519–526. doi: 10.3727/096504009789745575. [DOI] [PubMed] [Google Scholar]
- 9.Grimaldi C, Pisanti S, Laezza C, Malfitano AM, Santoro A, Vitale M, Caruso MG, Notarnicola M, Iacuzzo I, Portella G, Di Marzo V, Bifulco M. Anandamide inhibits adhesion and migration of breast cancer cells. Experimental cell research. 2006;312:363–373. doi: 10.1016/j.yexcr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Haberlein H, Tschiersch KP, Boonen G, Hiller KO. Chelidonium majus L.: components with in vitro affinity for the GABAA receptor. Positive cooperation of alkaloids. Planta medica. 1996;62:227–231. doi: 10.1055/s-2006-957865. [DOI] [PubMed] [Google Scholar]
- 11.Haier J, Nasralla MY, Nicolson GL. Beta1-integrin-mediated dynamic adhesion of colon carcinoma cells to extracellular matrix under laminar flow. Clinical & experimental metastasis. 1999;17:377–387. doi: 10.1023/a:1006658414040. [DOI] [PubMed] [Google Scholar]
- 12.Hiller KO, Ghorbani M, Schilcher H. Antispasmodic and relaxant activity of chelidonine, protopine, coptisine, and Chelidonium majus extracts on isolated guinea-pig ileum. Planta medica. 1998;64:758–760. doi: 10.1055/s-2006-957576. [DOI] [PubMed] [Google Scholar]
- 13.Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer science. 2003;94:575–581. doi: 10.1111/j.1349-7006.2003.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nature reviews. Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 15.Huang YH, Zhang ZZ, Jiang JX. Relaxant effects of protopine on smooth muscles. Zhongguo yao li xue bao = Acta pharmacologica Sinica. 1991;12:16–19. [PubMed] [Google Scholar]
- 16.Janbaz KH, Saeed SA, Gilani AH. An assessment of the potential of protopine to inhibit microsomal drug metabolising enzymes and prevent chemical-induced hepatotoxicity in rodents. Pharmacological research : the official journal of the Italian Pharmacological Society. 1998;38:215–219. doi: 10.1006/phrs.1998.0353. [DOI] [PubMed] [Google Scholar]
- 17.Jiang B, Cao K, Wang R. Inhibitory effect of protopine on K(ATP) channel subunits expressed in HEK-293 cells. European journal of pharmacology. 2004;506:93–100. doi: 10.1016/j.ejphar.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Ju L, Zhou C. Association of integrin beta1 and c-MET in mediating EGFR TKI gefitinib resistance in non-small cell lung cancer. Cancer cell international. 2013;13:15. doi: 10.1186/1475-2867-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SR, Hwang SY, Jang YP, Park MJ, Markelonis GJ, Oh TH, Kim YOC. Protopine from Corydalis ternata has anticholinesterase and antiamnesic activities. Planta medica. 1999;65:218–221. doi: 10.1055/s-1999-13983. [DOI] [PubMed] [Google Scholar]
- 20.Ko FN, Wu TS, Lu ST, Wu YOC, Huang TF, Teng CM. Ca(2+)-channel blockade in rat thoracic aorta by protopine isolated from Corydalis tubers. Japanese journal of pharmacology. 1992;58:1–9. doi: 10.1254/jjp.58.1. [DOI] [PubMed] [Google Scholar]
- 21.Kulp M, Bragina O, Kogerman P, Kaljurand M. Capillary electrophoresis with LED-induced native fluorescence detection for determination of isoquinoline alkaloids and their cytotoxicity in extracts of Chelidonium majus L. Journal of chromatography A. 2011;1218:5298–5304. doi: 10.1016/j.chroma.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Lee DU, Park JH, Wessjohann L, Schmidt J. Alkaloids from Papaver coreanum. Natural product communications. 2011;6:1593–1594. [PubMed] [Google Scholar]
- 23.Lu Z, Sun W, Duan X, Yang Z, Liu Y, Tu P. Chemical constituents from Corydalis yanhusuo. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2012;37:235–237. [PubMed] [Google Scholar]
- 24.Munoz O, Christen P, Cretton S, Backhouse N, Torres V, Correa O, Costa E, Miranda H, Delporte C. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. The Journal of pharmacy and pharmacology. 2011;63:849–859. doi: 10.1111/j.2042-7158.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 25.Oppenheimer SB. Cellular basis of cancer metastasis: A review of fundamentals and new advances. Acta histochemica. 2006;108:327–334. doi: 10.1016/j.acthis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Orhana I, Ozcelik B, Karaoglu T, Sener B. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species. Zeitschrift fur Naturforschung. Journal of biosciences. 2007;62:19–26. doi: 10.1515/znc-2007-1-204. [DOI] [PubMed] [Google Scholar]
- 27.Rathi A, Srivastava AK, Shirwaikar A, Singh Rawat AK, Mehrotra S. Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2008;15:470–477. doi: 10.1016/j.phymed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Saeed SA, Gilani AH, Majoo RU, Shah BH. Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacological research : the official journal of the Italian Pharmacological Society. 1997;36:1–7. doi: 10.1006/phrs.1997.0195. [DOI] [PubMed] [Google Scholar]
- 29.Teng CM, Ko FN, Wang JP, Lin CN, Wu TS, Chen COC, Huang TF. Antihaemostatic and antithrombotic effect of some antiplatelet agents isolated from Chinese herbs. The Journal of pharmacy and pharmacology. 1991;43:667–669. doi: 10.1111/j.2042-7158.1991.tb03561.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Dong H, Shu X, Zheng Z, Yang B, Huang L. Large-scale separation of alkaloids from Corydalis bungeana Turcz. by pH-zone-refining counter-current chromatography. Molecules (Basel, Switzerland) 2012;17:14968–14974. doi: 10.3390/molecules171214968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei HL, Liu GT. Protective action of corynoline, acetylcorynoline and protopine against experimental liver injury in mice. Yao xue xue bao = Acta pharmaceutica Sinica. 1997;32:331–336. [PubMed] [Google Scholar]
- 32.Xiao X, Liu J, Hu J, Li T, Zhang Y. Protective effect of protopine on the focal cerebral ischaemic injury in rats. Basic & clinical pharmacology & toxicology. 2007;101:85–89. doi: 10.1111/j.1742-7843.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- 33.Xiao X, Liu J, Hu J, Zhu X, Yang H, Wang C, Zhang Y. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca(2+) antagonism and antioxidant mechanisms. European journal of pharmacology. 2008;591:21–27. doi: 10.1016/j.ejphar.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 34.Xu L-F, Chu W-J, Qing X-Y, Li S, Wang X-S, Qing G-W, Fei J, Guo L-H. Protopine inhibits serotonin transporter and noradrenaline transporter and has the antidepressant-like effect in mice models. Neuropharmacology. 2006;50:934–940. doi: 10.1016/j.neuropharm.2006.01.003. [DOI] [PubMed] [Google Scholar]