Abstract
Background
Cymbopogon citratus (Poaceae) a tropical perennial herb plant that is widely cultivated to be eaten either fresh with food or dried in tea or soft drink has been reported to possess a number of medicinal and aromatic properties. This study aimed at evaluating the protective effects of C. citratus aqueous extract against liver injury induced by hydrogen peroxide (H2O2), in male rats.
Materials and Methods
Twenty-five rats were randomly divided into five different groups of five animals in each group; (1) Control. (2) Received H2O2 (0.5%) with drinking water. (3), and (4) received H2O2 and C. citratus (100 mg·kg−1 b wt), vitamin C (250 mg·kg−1 b wt) respectively. (5), was given C. citratus alone. The treatments were administered for 30 days. Blood samples were collected and serum was used for biochemical assay including liver enzymes activities, total protein, total bilirubin and malonaldehyde, glutathione in serum and liver homogenates. Liver was excised and routinely processed for histological examinations.
Results
C. citratus attenuated liver damage due to H2O2 administration as indicated by the significant reduction (p<0.05), in the elevated levels of ALT, AST, ALP, LDH, TB, and MDA in serum and liver homogenates; increase in TP and GSH levels in serum and liver homogenates; and improvement of liver histo-pathological changes. These effects of the extract were similar to that of vitamin C which used as antioxidant reference.
Conclusion
C. citratus could effectively ameliorate H2O2-induced oxidative stress and prevent liver injury in male rats.
Keywords: Cymbopogon citratus, Vitamin C, Hydrogen peroxide, Rat, Liver enzymes, Serum proteins, Histopathology
Introduction
Cymbopogon citratus, commonly known as lemon grass, is a tropical monocotyledonous hypogeal perennial herb belonging to the family Poaceae. It is commonly used in traditional Indian, Chinese, and Brazilian medicines, and its oil is used as culinary flavoring, scent, and medicine. Citronelle, which is obtained from C. citratus, acts as an antihypertensive agent by inducing the vasodilatation of vascular smooth muscles (Bastos et al., 2010; Chitra et al., 2012). C. citratus effectively treats fever and infection, headaches, digestive and nervous disorders, and rheumatic pain. It also acts as a sedative, an antispasmodic, an analgesic, and an anti-inflammatory agent (Naik et al., 2010; Figueirinha et al., 2010). In Iraq, the genus Cymbopogon was represented by 2–4 species where C. citratus was not among them (Townsend et al., 1968). However, this species was cultivated in some local gardens in Tikrit area.
Oxidative stress (OS), is induced by hydrogen peroxide (H2O2), in animal models (Ganie et al., 2011; Park et al., 2012) and produces various reactive oxygen species. These species are generated constantly in vivo and can cause oxidative damage to nucleic acids, lipids, and proteins. Furthermore, the accumulation of reactive oxygen species may lead to oxidative damage of cells (Valacchi and Davis, 2008). Several reports have demonstrated that oxidative stress has a critical role in the initiation and progression of most modern human diseases such as cardiovascular disease, cancer, diabetes, arthritis, aging diseases, and reproductive system disorders (Halliwell et al., 1992; Medina, and Moreno-Otero, 2005; Makker et al., 2009). To prevent oxidative damage, cells developed a variety of antioxidant defense mechanisms such as chain breaking antioxidants, which include the non-enzymatic antioxidant glutathione; flavonoids and phenol compounds; vitamins A, E, and C; and free radical scavenger antioxidant enzymes (Križanović et al., 2008). H2O2 is among the ROS that produce OS, and accumulating evidence has demonstrated that OS plays a critical role in the initiation and progression of a variety of liver disorders (Jones et al., 2010; Huo et al., 2011; Zamani-Moghddam et al., 2012). H2O2 generates toxic hydroxyl radicals (OHo), which are important intermediates of substances that are toxic to liver (Halliwell et al., 1992, Kehrer, 2000). Hepato-toxicity is now a commonly used method in animal model (Rats), for liver damage investigation to screen the hepato-protective activity of natural medicines. Use of natural products for liver diseases is growing because of their safety and efficacy as an alternative remedy compared with chemically synthesized drugs (Natanzi et al 2009). Histo-pathological changes in liver tissue; activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH); levels of malondialdeyde (MDA), (end-product of lipid peroxidation) and glutathione (GSH), (a chain breaking antioxidant); and other related parameters are used to assess liver toxicity and the hepato-protective activity of medicinal plants (Kumar et al., 2009; Uboh et al., 2012).
Given that the liver is involved in almost all vital biological processes, its damage has severe consequences in metabolism, immune response, detoxification, and antimicrobial defense. The protective activity of aqueous extract C. citratus against H2O2-induced liver oxidative stress damage is unknown. The present study was therefore conducted to evaluate the protective effect of C. citratus aqueous extract against H2O2induced liver injury caused by oxidative stress in male rats.
Materials and Methods
Plant Material Collection and Extraction
Lemon grass (C. citratus) leaves were collected from the Garden of Tikrit University, Iraq during the summer season (July-September). The plant were identified and authenticated by Dr. Khalil I. Al-Shemmary, Plant taxonomist, Biology Department, Faculty of Science, Tikrit University, Tikrit, Iraq. The voucher specimen (No.1298) was deposited at the herbarium of Faculty of Science, Tikrit University. The plant material was dried in the shade by exposing to air at (33 ± 2) °C and ground into a fine powder. The dry powder (10 g), was extracted with 300 mL of distilled water using a Soxhlet extractor at 40 °C. The extract was then concentrated with a rotary evaporator under reduced pressure at 40 °C. Then, the solid extract was stored in sealed, dark glass bottles under free moisture conditions and deep-frozen until used.
Animals and Experimental Protocol
25 Sprague-Dawley strain male rats weighing 200 -250 g and approximately 7 to 8 weeks old were purchased from the Faculty of Veterinary Medicine, University of Mosul, Iraq and used for the experiments. They were divided into five groups of five rats each and housed in separate cages under a controlled environment with standard conditions of temperature (25 ± 2) °C and humidity, as well as alternating 10 hr light and 14 hr dark cycle. The rats were provided with standard laboratory diet and water ad libitum. The five groups were treated for 30 days as follows. Group 1 served as control and was given only normal water and diet. Group 2 was given normal diet and 0.5% H2O2 in drinking water. Group 3 was given normal diet, 0.5% H2O2 in drinking water, and 100 mg·kg−1 body weight oral dose of C. citratus aqueous leaf extract according to Rahim et al., (2013). Group 4 was given normal diet and 0.5% H2O2 in drinking water. An oral dose of 250 mg·kg−1 body weight of vitamin C in distilled water was also given. Group 5 was given normal diet and an oral dose of 100 mg·kg−1 body weight of C. citratus aqueous leaf extract.
At the end of the experiment, the rats were starved for 24 h and then mild anesthetized by ether prior to sampling. Blood samples were collected from the retro orbital vein. Serum was separated by centrifugation at 3000 × g for 5 min and then stored at — 20 °C until use for biochemical assay. Liver was excised. The study protocol was approved by the committee for the control and supervision of experiment on animals of the Scientific Affairs, Tikrit University, Iraq.
Assay of Lipid Peroxidation
Lipid peroxidation in serum and liver homogenates was measured using the thiobarbituric acid test (TBA) (Guidet and Shah, 1989). Liver homogenate or serum samples were mixed with trichloroacetic acid and TBA and placed in boiling water for 15 min. After cooling and centrifugation, the absorbance of the red TBA malonaldehyde complex was measured at 532 nm using an ultraviolet-visible spectrophotometer (Shimadzu, Japan).
Estimation of glutathione
GSH in serum and liver homogenates was measured according to the method of Beutler (Beutler, 1975) using Ellman's reagent. The procedure is based on the reduction of the Ellman's reagent by sulfhydryl groups into 5,5′-dithiobis (2-nitrobenzoic acid), which has an intense yellow color. The solution was measured spectrophotometrically at 412 nm using a Shimadzu (Japan) spectrophotometer.
Biochemical Analysis
Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and lactate dehydrogenase activities were determined using Biomerieux diagnostic kits (France). Total protein (TP), and total bilirubin (TB) were measured using BIOLABO (SA, France), diagnostic kits.
Histopathological Studies
Following autopsy, liver tissues were immediately removed, trimmed into 2 mm thickness, and fixed with 10% buffered formaldehyde for 24 h at 4 °C. The fixed tissues were processed by dehydration and embedment in paraffin, sectioned, and rehydrated. Sections cut at 7 µm thickness were stained with hematoxylin and eosin for light microscopic examination. The histological sections were examined, and photographs were taken using a mitotic microscope with digital camera (Mercury, China).
Statistical Analysis
The data were presented as means ± standard error and compared by one way analysis of variance. Significant difference between groups was determined using Duncan's test, and probability levels of less than (p<0.05), were considered significant.
Results
Estimation of Biochemical Parameters
Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and lactate dehydrogenase enzymes activities, as well as TB and MDA levels, in serum and liver homogenates significantly (p<0.05), increased in H2O2-treated rats (group 2), compared with that in the control rats (group 1), (Tables 1 and 2). Furthermore, the TP and GSH levels in the serum and liver homogenates of H2O2-treated rats (group 2), were markedly reduced compared with that in group 1 (Tables 1 and 2). The administration of C. citratus aqueous extract (100 mg·kg−1 body weight) and vitamin C (250 mg·kg−1 body weight), (groups 3 and 4, respectively), significantly (p<0.05) decreased the levels of the parameters (Tables 1 and 2) relative to that of the H2O2-treated rats (group 2). However, TP and GSH exhibited different trends. Group 5, which was treated with lemon grass alone, showed a significant difference in AST, TB, GSH, and MDA compared with the control group (Tables 1 and 2).
Table 1.
MDA and GSH values in serum/ S (µmol.L−1), and in liver homogenates/H (µmol. g−1 wet tissue), of all groups.
| Experimental | GSH/S | GSH/H | MAD/S | MAD/H |
| Group (1) | 16.24±1–22 | 4.19±1.35 | 5.72±0.18 | 4.05±0.19 |
| Group (2) | 12.41±1.271 | 3.68±0.191 | 7.79±1.231 | 5.11±0.351 |
| Group (3) | 14.83±1.272 | 4.48±1.392 | 5.90±0.792 | 3.73±1.532 |
| Group (4) | 15.04±0.222 | 4.56±1.422 | 6.29±0.722 | 4.50±0.472 |
| Group (5) | 15.65±0.49 | 7.89±3.243 | 5.60±0.22 | 2.14±0.423 |
Table 2.
ALT, AST, ALP, LDH enzymes and TP, TB values in rat serum of all groups.
| Experimental Group |
ALT U/L |
AST U/L |
ALP U/l |
LDH U/L |
TP g/dl |
TB mg/dl |
|
| (1) | Group | 12.67±1.2 | 23.80±4.9 | 16.98±4.2 | 1832±46.6 | 5.33±0.6 | 0.76±0.07 |
| (2) | Group | 21.00±3.611 | 27.00±0.001 | 47.88±4.61 | 2752±307.41 | 3.63±0.131 | 1.45±0.411 |
| (3) | Group | 14.00±1.002 | 25.83±3.82 | 23.17±3.42 | 1372±93.82 | 4.33±0.012 | 0.87±0.072 |
| (4) | Group | 15.00±3.002 | 26.60±1.772 | 25.98±4.62 | 1466±38.32 | 4.58±0.442 | 0.89±0.082 |
| (5) | Group | 12.00±1.00 | 21.73±4.673 | 14.09±0.6 | 1715±63.6 | 4.99±0.22 | 0.41±0.033 |
Values represent the means ± SE (n=5), Significance at 1P<0.05 as compared with Control (group I), Significance at 2P<0.05 as compared with group (2), Significance at 3P<0.05 as compared with control (group 1).
Histological Studies
The results of histological study are presented in Figure 1. Histological examination results are consistent with that of the biochemical analysis. The liver of control rats (group 1) showed a normal arrangement of hepatocytes and sinusoids. The cytoplasm was not vacuolated. Areas of necrosis, infiltration by inflammatory cells, and changes in fats were not observed (Fig.1a). Group 2 rats, which were exposed to H2O2 for 30 days, exhibited histo-pathological alterations including cytoplasm vacuolization, congestion, infiltration, and degeneration of hepatocytes (necrosis) (Fig.1b) compared with the control (Fig.1a). The rats administered with C. citratus aqueous extract and vitamin C (Figs. 1c and 1d) showed significant improvement evident through a well arranged of hepatocytes with cytoplasm not vacuolated. Sinusoids well preserved, few areas of necrosis and fatty changes compared with H2O2-treated rats (Fig. 1b). Liver tissue of rats treated with C. citratus aqueous extract alone exhibited similar liver cytoarchitecture compared with the control group (Fig. 1e).
Figure 1.
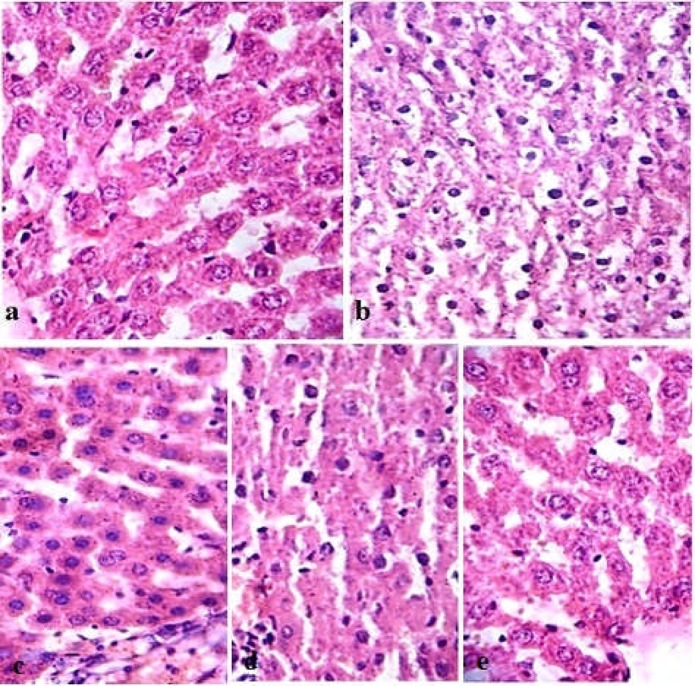
Light microscope photographs of experimental rats liver paraffin sections stained with haematoxylin and eosin.
Legend of Figure 1: (a) control, showed normal liver arrangement of hepatocytes and sinusoids. The cytoplasm was not vacuolated. Areas of necrosis, infiltration by inflammatory cells, and changes in fats were not observed. (b) Rat liver treated with H2O2 (0.5%), in drinking water, showed histo-pathological alterations including cytoplasm vacuolization, congestion, infiltration, and degeneration of hepatocytes (necrosis), in comparison with control. (c) Liver treated with aqueous extract of C. citratus (100mg.kg −1 b.wt.) concomitant with H2O2, exhibited a well arranged hepatocytes with cytoplasm not vacuolated. Sinusoids well preserved no area of necrosis, no fatty change. (d) Rat liver administered with vit. E (500mg.kg−1 b.wt.) concomitant with H2O2 showed significant improvement comparable to rat liver treated with C. citratus. (e) Aqueous extract of C. citratus administration alone exhibited similar liver cytoarchitecture compared with the control group. Magnification at × 400 for all figures.
Discussion
The hepato-protective effect of C. citratus aqueous extract was investigated in rats with oxidative stress induced by H2O2, which is a commonly used animal model (Mello Filho et al., 1984; Ganie et al., 2011; Park et al., 2012). The activities of the liver function enzymes, as well as TP, TB concentrations and histological changes are good biomarkers for liver injury and evaluating the protective effect of medicinal plants (Huo et al., 2011 Zamani-Moghddam et al., 2012; Xie et al., 2012). The present study demonstrates that C. citratus aqueous extract attenuates liver damage due to H2O2 administration as indicated by the significant reduction in the elevated levels of ALT, AST, ALP, LDH, TB, and MDA in serum and liver homogenates; increase in TP and GSH levels in serum and liver homogenates, and improvement of histological changes. These effects of C. citratus are similar to that of vitamin C used as an antioxidant reference.
Oxidative stress is one of the most important mechanisms involved in H2O2-induced toxicity and results in the increased generation of potent ROS such as the hydroxyl radical (OHo), and reduction in antioxidant enzymes (Mora et al., 2003). ROS are unstable, as well as highly reactive, and combat various biomolecules in cells such as membrane lipids, proteins, and deoxyribonucleic acids, leading to several pathological conditions and cell apoptosis (Sharma and Agarwal, 1996).
Many biologically and chemically active substances have been investigated and isolated in C. citratus. The most important among these substances are citral oil, C-glycosylflavones, orientin, isoorientin, and chlorogenic acid (Cheel et al., 2005; Akande et al., 2012). The content and free radical scavenging/antioxidant activity of these substances has been reported by several authors (Huguet et al., 1990; Budzianowski et al., 1991; Mun'im et al., 2003). The flavonoids and phenolic active compounds C. citratus can act as hepato-protective agents because of their antioxidant and scavenging properties and protect rats against H2O2-induced oxidative stress in liver. This assumption was confirmed by the observed GSH depletion and lipid peroxidation in this study. Moreover, the maximum protective effect of C. citratus aqueous extract was observed in H2O2-treated rats administered with 100 mg·kg−1 body weight of extract.
MDA content was significantly elevated in serum and liver homogenates. GSH, an important antioxidant in living cells, markedly decreased in the serum and liver homogenates of H2O2-treated rats (Table 1). Therefore, the elevation of MDA, a marker of oxidative damage, reveals the overproduction of free radicals, which is consistent with the view that H2O2 induces lipid peroxidation. The failure of the antioxidant defense mechanism is due to the overproduction of free radicals, decreased activities of the scavenging enzymes, or both. Increased MDA levels, lipid per oxidation (Zaidi and Banu, 2004; Bharrhan et al., 2010), and decreased GSH levels are associated with cell damage (Martensson and Meister, 1991). Treatment with C. citratus aqueous extract restored MDA and GSH content, which further highlights their role against H2O2-induced liver damage. Vitamin C administration showed similar trends (Table 1), to that of C. citratus aqueous extract in H2O2-treated rats. Several reports have shown the hepatoprotective effect of vitamin C against the hepatotoxicity caused by carbon tetrachloride, acetaminophen, malathion and gasoline vapor (Ademuyiwa et al., 1994; Adejuwon and Joseph, 2008; Suna et al., 2010; Uboh et al., 2012). Therefore, vitamin C possibly acts as an antioxidant by scavenging ROS, thereby reducing oxidative stress and related complications. Moreover, vitamin C restores the antioxidant abilities of vitamin E, which suggests that the major function of vitamin C is recycling tocopheroxyl radicals and glutathione (Saraswathy et al., 2010), and inhibiting lipid peroxidation and hepatocellular damage (Serbecic and Beutelspacher, 2005).
The mitochondrion is the major target in drug-induced hepatic damage (Kass et al., 2006). Mitochondrial dysfunction is generally accompanied with oxidative stress, which is a key regulator of mitochondria-mediated cell death (Boelsterli and Lim, 2007). Hence, drugs cause cell death by inducing oxidative stress in hepatic mitochondria. In the current study, oxidative stress induced by H2O2 resulted in hepatic injury as shown by the elevated levels of ALT, AST, ALP, LDH, and TB and decreased TP in serum. These parameters are the most sensitive circulating markers of hepatic injury (Sallie et al., 1991). A marked increase in hepatic oxidative stress occurred as shown by the marked increase in MDA level in serum and liver homogenates and decreased GSH level. The administration of the aqueous extract of C. citratus reduced the hepatotoxicity in H2O2-treated rats. This result was evidenced by the marked decrease in serum AST, ALT, ALP, and LDH activities and increase in serum protein level of rats treated with C. citratus relative to that of rats treated with H2O2 alone. Pei et al. (2012), also indicated the hepatoprotective effect of C. citratus against carbon tetrachloride-induced hepatic stress and toxicity. Similar results were also obtained using vitamin C concomitant with H2O2 treatment. Vitamin C protects the lipids and lipoproteins in hepatocellular membranes against oxidative damage caused by toxic free radicals. Thus, the hepatoprotective activity of vitamin C is based on the reduction of lipid peroxidation through free radical scavenging activity (Saraswathy et al., 2010).
Histopathological studies of the liver demonstrate H2O2-induced periportal inflammation, sinusoidal congestion hemorrhage, and hepatic necrosis in rat liver. These changes are consistent with those observed in the various biochemical parameters. Liver damage may be caused by the toxic effects of H2O2-mediated oxidative stress. H2O2-treated rats co-administered with C. citratus aqueous extract and vitamin C reveals significant improvement compared with H2O2-treated rats as indicated by the reversed periportal inflammation, sinusoidal congestion, hemorrhage and hepatic necrosis. The administration of C. citratus aqueous extract alone displayed similar results as that of the control, with slight amelioration in most of the studied parameters.
In conclusion, the current results demonstrate that C. citratus has a potent protective effect against H2O2-induced liver injury. C. citratus treatment significantly reduced the increase in liver enzyme activities and attenuated oxidative stress-induced pathological changes.
Acknowledgements
We would like to thank Universiti Kebangsaan Malaysia for the postdoctoral fellowship they awarded to the first author.
Abbreviations list
- (ALT)
alanine aminotransferase
- (AST)
aspartate aminotransferase
- (ALP)
alkaline phosphatase
- (LDH)
lactate dehydrogenase
- (MDA)
malondialdeyde
- (GSH)
glutathione
References
- 1.Adejuwon AA, Joseph OO. Protective effect of oral Ascorbic Acid (Vitamin C) against Acetaminophen- induced hepatic injury in rats. Afr J Biomed Res. 2008;11:183–190. [Google Scholar]
- 2.Ademuyiwa O, Adesanya O, Ajuwon OR. Vitamin C in CC14 hepatotoxicity - a preliminary report. Hum Exp Toxicol. 1994;13:107–109. doi: 10.1177/096032719401300208. [DOI] [PubMed] [Google Scholar]
- 3.Akande IS, Samuel T, Agbazue U, Olowolagba B. Omparative proximate analysis of ethanolic and water extracts of Cymbopogon citrates (lemon grass) and four tea brands. JPBMS. 2012;22:1–7. [Google Scholar]
- 4.Bastos JF, Moreira IJ, Ribeiro TP, Medeiros IA, Antoniolli AR, De Sousa DP. Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin Pharmacol Toxicol. 2010;106:331–337. doi: 10.1111/j.1742-7843.2009.00492.x. [DOI] [PubMed] [Google Scholar]
- 5.Beutler E. Glutathione. In: Beutler E, editor. Red Cell Metabolism, a Manual of Biochemical Methods. New York, NY, USA: Grune and Stratton; 1975. pp. 112–114. [Google Scholar]
- 6.Bharrhan S, Chopra K, Rishi P. Vitamin E supplementation modulates endotoxin-induced liver damage in a rat model. Am J Biomed Sci. 2010;2:51–62. [Google Scholar]
- 7.Boelsterli UA, Lim PL. Mitochondrial abnormalities - a link to idiosyncratic drug hepatotoxicity. Toxicol Appl Pharmacol. 2007;220:92–107. doi: 10.1016/j.taap.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Budzianowski J, Pakulski G, Robak J. Studies on antioxidative activity of some C-glycosylflavones. Pol J Pharmacol Pharm. 1991;43:395–401. [PubMed] [Google Scholar]
- 9.Cheel J, Theoduloz C, Rodriguez J, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.) J Agric Food Chem. 2005;53:2511–2517. doi: 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- 10.Chitra R, Sim SM, Ismil R. Effect of Cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evid Based Complement Alternat Med. 2012;53:94–175. doi: 10.1155/2012/539475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueirinha A, Cruz MT, Francisco V, Lopes MC, Batista MT. Anti-inflammatory activity of Cymbopogon citratus leaf infusion in lipopolysaccharide-stimulated dendritic cells: Contribution of the polyphenols. J Med Food. 2010;13:681–690. doi: 10.1089/jmf.2009.0115. [DOI] [PubMed] [Google Scholar]
- 12.Ganie SA, Haq E, Hamid A, Masood A, Zargar MA. Long dose exposure of hydrogen peroxide (H2O2) in albino rats and effect of Podophyllum hexandrum on oxidative stress. Eur Rev Med Pharmacol Sci. 2011;15:906–915. [PubMed] [Google Scholar]
- 13.Guidet B, Shah SV. Enhanced in vivo H2O2 generation by rat kidney in glycerol-induced renal failure. Am J Physiol. 1989;257:F440–F445. doi: 10.1152/ajprenal.1989.257.3.F440. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants and human disease; where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 15.Huguet A I, Manez S, Alcaraz MJ. Superoxide scavenging properties of flavonoids in a nonenzymic system. Z Naturforsch. 1990;C45:19–24. doi: 10.1515/znc-1990-1-205. [DOI] [PubMed] [Google Scholar]
- 16.Huo HZ, Wang B, Liang YK, Bao YY, Gu Y. Hepatoprotective and Antioxidant Effects of Licorice Extract against CCl4-Induced Oxidative Damage in Rats. Int J Mol Sci. 2011;12:6529–6543. doi: 10.3390/ijms12106529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones DP, Lemasters JJ, Han D, Boelsterli UA, Kaplowitz N. Mechanisms of pathogenesis in drug hepatotoxicity putting the stress on mitochondria. Mol Interv. 2010;10:98–111. doi: 10.1124/mi.10.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kass GE. Mitochondrial involvement in drug-induced hepatic injury. Chem - Biol Interact. 2006;163:145–159. doi: 10.1016/j.cbi.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Kehrer JP. The Haber - Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 20.Križanović D, Velimir S, Pero B, Igor S, Anamaria EK. Changes of bovine blood lipid peroxides and some antioxidants in the course of growth. Veterinarski Arhiv. 2008;78:269–278. [Google Scholar]
- 21.Kumar SS, Kumar BR, Mohan GK. Hepatoprotective effect of Trichosanthes cucumerina var. cucumerina L. on carbon tetrachloride induced liver damage in rats. J Ethanopharmacoal. 2009;123:347–350. doi: 10.1016/j.jep.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Makker K, Ashok A, Rakesh S. Oxidative stress and male infertility. Indian J Med Res. 2009;129:357–367. [PubMed] [Google Scholar]
- 23.Martensson J, Meister A. Glutathione deficiency decreases issue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Nat Acad Sci USA. 1991;88:56–60. doi: 10.1073/pnas.88.11.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medina J, Moreno-Otero R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs. 2005;65:2445–2461. doi: 10.2165/00003495-200565170-00003. [DOI] [PubMed] [Google Scholar]
- 25.Mello Filho AC, Hoffmann ME, Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984;218:273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora LO, Antunes LM, Francescato HD, Bianchi M. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2003;47:517–522. doi: 10.1016/s1043-6618(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 27.Mun'im A, Negishi O, Ozawa T. Antioxidative compounds from Crotalaria sessiliflora. Biosci Biotechnol Biochem. 2003;67:410–414. doi: 10.1271/bbb.67.410. [DOI] [PubMed] [Google Scholar]
- 28.Naik MI, Fomda BA, Jaykumar E, Bhat JA. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacteria. Asian Pac J Trop Med. 2010:535–538. [Google Scholar]
- 29.Natanzi AE, Ghahremani MH, Monsef Esphani HR, Minaei B, Nazarian H, Sabzevari O. An experimental model for study of the hepatoprotective activity of Nasturtium officinale (Watercress) against acetaminophen toxicity using in situ rat liver system. Eur j sci Res. 2009;38:556–564. [Google Scholar]
- 30.Park KJ, Kim YJ, Kim J, Kim SM, Lee SY, Bae JW, Hawang KK, Kim DW, Cho MC. Protective Effects of Peroxiredoxin on Hydrogen Peroxide Induced Oxidative Stress and Apoptosis in Cardiomyocytes. Korean Circ J. 2012;42:23–32. doi: 10.4070/kcj.2012.42.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei HK, Mokhtar RA, Mohammad I. Antioxidant potential of Cymbopogon citratus extract: alleviation of carbon tetrachloride-induced hepatic oxidative stress and toxicity. Hum Exp Toxicol. 2012;31:81–91. doi: 10.1177/0960327111407226. [DOI] [PubMed] [Google Scholar]
- 32.Uboh FE, Ebongi PE, Akpan HD, Usoh IF. Hepatoprotective effect of vitamins C and E against gasoline vaporinduced liver injury in male rats. Turk J Biol. 2012;36:217–223. [Google Scholar]
- 33.Rahim SM, Taha EM, Mubark ZM, Aziz SS, Simon KD, Mazlan AG. Protective effect of Cymbopogon citratus on hydrogen peroxide-induced oxidative stress in the reproductive system of male rats. Syst Biol Reprod Med. 2013 doi: 10.3109/19396368.2013.827268. [DOI] [PubMed] [Google Scholar]
- 34.Sallie R, Tredger JM, Willam R. Drugs and the Liver. Biopharm Drug Dispos. 1991;12:251–259. doi: 10.1002/bdd.2510120403. [DOI] [PubMed] [Google Scholar]
- 35.Saraswathy GR, Maheswari E, Santhrani T. Effect of Vitamin C Supplementation on Phenytoin Induced Hepatotoxicity. Global J Pharmacol. 2010;4:127–135. [Google Scholar]
- 36.Serbecic N, Beutelspacher SC. Antioxidative vitamins prevent lipid peroxidation and apoptosis in corneal endothelial cells. Cell Tissue Res. 2005;320:465–475. doi: 10.1007/s00441-004-1030-3. [DOI] [PubMed] [Google Scholar]
- 37.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 38.Suna K, Gokce Uzun F, Durak D, Demir F, Kalender Y. Malathion-induced hepatotoxicity in rats: The effects of vitamins C and E. Food Chem Toxicol. 2010;48:633–638. doi: 10.1016/j.fct.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 39.Valacchi G, Davis P. Oxidants in biology, a question of Balance. 1st edition. New York: Springer; 2008. [Google Scholar]
- 40.Xie Q, Guo FF, Zhou W. Protective effects of cassia seed ethanol extract against carbon tetrachloride-induced liver injury in mice. Acta Biochim Pol. 2012;59:265–270. [PubMed] [Google Scholar]
- 41.Zaidi SMKR, Banu N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta. 2004;340:229–233. doi: 10.1016/j.cccn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Zamani-Moghddam E, Azami K, Minaei-Zangi B, Mousavie SZ, Sabzevari O. Protective activity of Fumaria villantii extract and monomethyl fumarate on acetaminophen induced hepatotoxicity in mice. Int J pharmac. 2012;8:177–184. [Google Scholar]


