Abstract
Background
Pleurostylia capensis is a large tree that can reach the maximum height of 20 m long, and it have been traditionally used as cosmetic, for steam bath, ritual body wash, and as a purgative to treat symptoms of witchcraft. Using ethanol, chloroform, dichloromethane (DCM), ethyl acetate (EA), and water extracts, leaves, bark and roots of Pleurostylia capensis were investigated scientifically for their effectiveness in antimicrobial, antioxidant and anti-inflammatory activities using standard methods
Materials and Methods
The extracts were evaluated for antimicrobial activity against Gram positive (Staphylococcus aureus, Bacillus cereus, and Mycobacterium smegmatis), Gram negative (Escherichia coli, Klebsiella pneumonia, Klebsiella oxytoca, Streptococcus pyogenes, Pseudomonas aeruginosa and Salmonella typhimurium), and Candida albicans. The antioxidant activity was investigated using 2, 2-diphenlyl-1-picrylhadrazyl (DPPH), free radical scavenging assay. The anti-inflammatory activity of P. capensis extracts was evaluated against both cyclooxygenase enzymes (COX 1 and 2).
Results
The ethyl acetate extracts of P. capensis showed a strong antimicrobial activity against B. cereus, K. pneumonia, S. pyogenes, and M. smegmatis with MIC value of 0.39 and 0.78 mg/ml. While the ethanol bark extract was most active against M. smegmatis with MIC value of 0.78 mg/ml; the least potent activity was observed with dichloromethane, chloroform and water extracts, with an MIC value ranging from 1.56 mg/ml to 50.0 mg/ml. The plant extracts proved to be good antioxidant agent, whereas extracts of ethanol were the most active, with IC50 ranging from 1.00 to 1.74 µg/ml, which is lower, and in close range to Vitamin C (1.40 µg/ml).
Conclusions
Its moderation to potent inhibitory activity was observed in all extracts. Ethanol and dichloromethane extracts were among the most potent when compared to water and petroleum ether extracts. The water extracts showed to be nontoxic on the Hek cell line with an IC50 value of 204.0, and 207.3 µg/ml (roots and bark) respectively. The dichloromethane, ethyl acetate, chloroform and ethanol extracts showed to be toxic on the Hek cell, with IC50 range from 5.94 to 42.91µg/ml. The results obtained indicate the effectiveness of these plants.
Keywords: Pleurostylia capensis (P.capensis); 2, 2-diphenlyl-1-picrylhadrazyl (DPPH); Minimum inhibitory concentration (MIC); Minimum bactericidal concentration (MBC); Cyclooxygenase (COX)
Introduction
Pleurostylia capensis Turcz (Loes), can grow into a large tree to a height of about 20 m long, but it is usually a low-growing spindly shrub (Retief and Herman, 1997). It is distributed commonly in scrub, wooded ravines, along rivers and streams, and in coastal and mountain forest (Retief & Herman, 1997). Pleurostylia capensis belongs to the Celestraceae family, and is commonly known as coffee pear (English), koffiepeer (Afrikaans) and murumelela (Tshivenda) (Mabogo, 1990; Schmidt and Lotter, 2002). This species are commonly found in the Western Cape, Eastern Cape, Kwazulu-Natal, Swaziland and Limpopo Province and probably throughout tropical Africa, under the name P. African (Schmidt and Lotter, 2002). The bark of this tree is greyish-brown, and the leaves are shiny dark green to fresh green above: some are paler green below (Retief and Herman, 1997). It was previously used in wagon construction (Retief 7 Herman, 1997). In the Cape provinces of South Africa, unspecified parts are used to encourage sleep and to bring good dreams (De Jager, 1963). The bark of this plant is used as cosmetic, for steam bath, ritual body wash, and as a purgative to treat symptoms of witchcraft (Michelle and Dold, 2012). Diviners use the stem, bark and roots in powdered form in addition with other parts of semi-parasitic plants, and other ingredients of either plant or animal origin to make a magical mixture, usually blown away to affect a remote target (Mabogo,1990). In Southern Uganda, it is used in the treatment of colic pain in babies (Seqaws and Kasenene, 2007), and in the treatment of epilepsy and mental illness in East Africa (Reid et al., 2006). No previous investigation has been done on the biological activities of the extracts from this plant. This study was initiated as a preliminary screening exercise of P. capensis to determine its effects against pathogenic organisms, and to further analyse its antioxidant, anti-inflammatory and cytotoxicity activity.
Materials and Methods
The root, bark and leaves of P. capensis were collected during September 2011, at Venda in Limpopo province of South Africa. A voucher specimen (MPT0060) was prepared and identified at the University of Venda.
Preparation of extracts The bark, roots and leaves were washed with distilled water and air-dried at room temperature for two weeks before being ground in a Wiley mill grinder. Samples of the ground bark, leaves and roots were soaked in various organic solvents (water, dichloromethane (DCM), ethyl acetate (EA), chloroform and ethanol, with 50 g of sample/500 ml of solvent), for at least 24 hours, with frequent shaking. The crude extracts were then filtered, and the solvent was evaporated on the rotary evaporator under pressure at 40°C. The water extract was then frozen, after which it was placed in a freeze-dry machine for three to four days. The extracts were rotary-dried, after which they were subjected to biological assay activities.
Preliminary phyto-chemical screening
The major secondary metabolites classes such as alkaloids, flavonoids, tannins, steroids and trepenoids were screened according to the common phyto-chemical methods previously described by Trease & Evans (1978).
Antimicrobial Assay
Test microorganisms and preparation of inoculate
The micro organisms used in this study are skin pathogens; nine bacteria: Staphylococcus aureus (ATCC 25923), Bacillus cereus (ATCC 11778), Mycobacterium smegmatis (Clinical isolates), Escherichia coli (ATCC 8739), Klebsiella pneumonia (ATCC 13883), Klebsiella oxytoca, Streptococcus pyogenes, Pseudomonas aeruginosa (ATCC 9027), Salmonella typhimurium (ATCC 14028) and one fungus, Candida albicans (Med 1). Bacteria were grown in the nutrient broth medium (Merck SA (Pty) Ltd.), at 37°C for 48 hours. Sabouraud Dextrose Broth medium (Merck SA (Pty) Ltd.) was used for the culturing of Candida albicans and incubated at 37°C for 24 hours under aerobic conditions. Sub culturing was done once weekly.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The micro dilution technique using 96-well micro plates (Eloff, 1998), was used to obtain the MIC and MBC values of the crude extract against the micro organisms under study. The extract was serially diluted in the 96-well plate with 24 hour old micro organisms (5 × 106 CFU/ml), grown at 37°C; the final concentration of the extract ranged from 25.0 mg/ml to 0.39 mg/ml, and the positive control ranged from 1.25 mg/ml to 0.01 mg/ml. Microbial growth was indicated by adding 40 µl of (0.2 mg/ml) p-iodonitrotetrazolium violet (INT) (Sigma-Aldrich, South Africa) to the microplate wells and incubated at 37°C for 48 hours. The MIC was defined as the lowest concentration that inhibited the colour change of INT. The MBC was determined by adding 50 µl of the suspensions from the wells, which did not show any growth after incubation during MIC assays, to 150 µl of fresh broth. These suspensions were incubated again at 37°C for 48 hours. The MBC was determined as the lowest concentration of extract that inhibited 100% growth of micro organisms (Cohen et al., 1998)
Antioxidant Assay
The free radical scavenging activity was measured using 1, 1 diphenyl-2-picryl-hydraxyl (DPPH) assay (Chan et al., 2007), with slight modifications. The ethanol extract of P. capensis and Vitamin C (positive control) 1000 µg/ml (20 µl) was added in the first three wells of a 96well plate containing 200 µl of distilled water to make up a final concentration of 100 µg/ml and the remaining wells were filled with 110 µl of distilled water. The first wells containing the extracts were serially diluted to wells which contained 110 µl of distilled water, and later, 90 µl of methanolic solution of DPPH (90 mM), added to all the wells. The final concentrations of the extracts ranged from 100 to 0.8 µg/ml. The plates were incubated at 37°C for 30 min and the absorbance was measured at 517 nm using the ELISA plate reader. The percentage of the radical scavenging activity by P. capensis was determined by dirrect comparison with ethanol (blank). The inhibition ratio was calculated as follows: % DPPH radical-scavenging = (AC-AS)/AC × 100, where AC is absorbance of the control solution (containing only DPPH solution), and AS is the absorbance of the sample in DPPH solution. The percentage of DPPH radical-scavenging was plotted against the plant extract/compounds concentrations (µg/ml) to determine the concentration of extract/compound required to scavenge DPPH by 50% (EC50).
Anti-inflammatory Assay
The anti-inflammatory activities of nine extracts were evaluated using the enzyme-based cyclooxygenase assays; COX-1 and COX-2. Both COX-1 and COX-2 enzymes were obtained from Sigma-Aldrich (Eldeen and Van Staden, 2008). The extracts were tested at a concentration of 10 mg/ml per test solution, giving a final concentration of 250 µg/ml. Indomethicin at 5 µM for COX-1, and 200 µM for COX-2, background (the enzyme was inactivated with HCl before the addition of 14C-arachidonic acid), and solvent blanks were used as controls. For each assay, a duplicate set of samples were tested. The anti-inflammatory activity of the extracts was measured as percentage of inhibition. This was done by determining the amount of radioactivity in the solutions relative to that of the solvent blank. The formula below was used to calculate the percentage of inhibition:
% inhibition = [(Radio activity sample − Radio activity background/ Radio activity blank − Radio activity background)] × 100.
Determination of cytotoxicity
Preparation of extracts
About 2 mg of extract was dissolved in 100 µl of DMSO to a stock solution of 20 mg/ml. The sample was then placed under the sun for 15–30, min and vortexed thereafter.
Cell culture
Hek cells were maintained in a monolayer culture at 37°C, in and 5% CO2 with 10% PBS medium, 10 µg/ml of penicillin, 10 µg/ml of streptomycin, 40 µg/ml of gentamycin and 0.25 µg/ml of fungizone.
Cell proliferation assay
A microtiter plate with Hek cells was used for testing all the ethanol extracts for cytotoxicity following the method of Mosmann (1983). Cytotoxicity was measured by the XTT (sodium 3′-[1-(phenyl amino-carbonyl)-3, 4-tetrazolium]-bis-[4-methoxy-6-nitro] benzene sulfonic acid hydrate) method using a cell proliferation kit II (Roche Diagnostics GmbH). A hundred microlitres of Hek cells (1 × 105 cells/ml) was seeded onto a microtiter plate and incubated for 24 hours to allow the cells to attach to the bottom of the plate. Dilution series were made of the extracts and the various concentrations (400 to 3.1 µg/ml), were added to the micro titre plate and incubated for 48 hours. The XTT reagents were added to a final concentration of 0.3 mg/ml and the cells were incubated for 1–2 hours. The positive drug control (Actinomycin D), at a concentration range of (0.013 µg/ml to 0.001 µg/ml), was included in the assay. After incubation, the absorbance of the colour was spectrophotometrically quantified using an ELISA plate reader, which measured the optical density at 490 nm with a reference wavelength of 690 nm. The assay was carried out in triplicate. Cells' viability was calculated as followed.
% viability of cells = ((A1−A2)/A0) × 100, where A1 is the absorbance of the treated cells with XTT and extract, A2 is the absorbance with no cells with XTT and extract, A0 is the absorbance of untreated cells (DMSO) with XTT.
Statistical analysis: Statistical analysis was conveyed as means ± SD using GraphPad Prism 4.0 with a significant difference of (P < 0.05).
Results and discussion
The results of qualitative analysis showed that the ethanol, water, chloroform, dichloromethane and ethyl acetate extracts of P. capensis contains at least two classes of secondary metabolites such as alkaloids, tannins, steroids and trepanoids (Table 1). The antimicrobial effects of P. capensis extracts and Ciprofloxacin as reference standard were evaluated in vitro against three species of Gram positive, six species of Gram negative and one fungus. The antimicrobial activity considered in this study was qualitatively assessed, evaluating the presence of MIC and MBC values. The results for screening of P. capensis extracts for antimicrobials have been summarized in Tables 2 and 3. In general, most plant extracts of the different plant parts exhibited a broad spectrum of antimicrobial activity. Strong antimicrobial activity was demonstrated by ethyl acetate roots with the MIC value range from 0.39 and 0.78 mg/ml (B. cereus, K. pneumonia, S. pyogenes and M. smegmatis), and ethyl acetate bark with MIC value of 0.78 mg/ml against B. cereus. The ethanol extracts (roots and bark) showed to be active against M. smegmatis with MIC value of 0.78 mg/ml. The least activity was demonstrated by chloroform, water and DCM. It can be noted that the root ethyl acetate extract, showed strong minimum bactericidal activity against B. cereus and S. pyogenes with MBC value of 0.39 mg/ml. The leaf extracts of P. capensis were found to be inactive. It is known that many factors affect bacterial activity. This may be due to the fact that the bacterial inhibition can vary with plant extracts, the solvent used for extraction and the organism to be tested (Dogruoz and Karogoz, 2008).
Table 1.
Extraction yield and phytochemical composition of the plant extracts
| Solvents | Plant part | Yield (%) | Alkaloids | Flavonoids | Tannins | Steroids | Trepenoids |
| Ethanol | Bark | 3.96 | + | − | + | − | + |
| Roots | 4.82 | + | − | + | − | + | |
| Water | Bark | 2.23 | − | − | + | + | + |
| Roots | 5.50 | − | − | + | + | + | |
| DCM | Bark | 18.17 | − | − | + | + | − |
| Roots | 13.20 | − | − | + | + | − | |
| EA | Bark | 1.69 | − | − | + | − | + |
| Roots | 2.77 | + | − | + | − | + | |
| Chloroform | Bark | 8.30 | + | − | + | − | − |
| Roots | 6.52 | − | − | + | − | − |
(+): present; (−): Absent;* the yield was calculated as the ratio of the mass of the obtained extract/mass of the plant powder.
Table 2.
Minimum inhibition concentration (MIC) of P. capensis extracts against nine species of bacteria and C. albicans
| Plants Part | Solvents | Minimum inhibition concentration (MIC) in mg/ml | |||||||||
| B. c | E. c | K. p | S. a | K. o | S. t | S. p | P. a | M.s | C. a | ||
| Roots | Et0H | 3.13 | 6.25 | 12.50 | 1.56 | 6.25 | 6.25 | 1.56 | 6.25 | 0.78 | 3.13 |
| Water | 3.13 | 6.25 | 3.13 | 6.25 | 6.25 | 6.25 | 12.50 | 6.25 | 6.25 | 12.50 | |
| DCM | 25.00 | 6.25 | 25.00 | 3.13 | 12.50 | 12.50 | 12.50 | 6.25 | 3.13 | >25.0 | |
| EA | 0.39 | 6.25 | 0.39 | 1.56 | 12.50 | 12.50 | 0.39 | 12.50 | 0.78 | 1.56 | |
| Chloroform | 6.25 | 25.00 | 25.00 | 1.56 | 6.25 | 25.00 | 3.13 | >25.0 | 6.25 | 12.50 | |
| Bark | Et0H | 1.56 | 12.50 | 12.50 | 3.13 | 12.50 | 6.25 | 3.13 | 12.50 | 0.78 | 3.13 |
| Water | 6.25 | 6.25 | 6.25 | 6.25 | 25.00 | 12.50 | 12.50 | 6.25 | 3.13 | 25.00 | |
| DCM | 25.00 | 25.00 | 12.50 | 12.50 | 25.00 | 25.00 | 25.00 | 25.00 | 12.50 | 25.00 | |
| EA | 0.78 | 25.00 | 25.00 | 3.13 | 25.00 | 25.00 | 3.13 | 25.00 | 6.25 | 6.25 | |
| Chloroform | 6.25 | 25.00 | 25.00 | 3.13 | >25.0 | 25.00 | 3.13 | >25.0 | 3.13 | 6.25 | |
| Leaves | Et0H | 25.00 | 25.00 | >25.0 | >25.0 | 3.13 | 25.00 | >25.0 | >25.0 | 25.00 | 25.00 |
| Water | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | >25.0 | >25.0 | 25.00 | 25.00 | >25.0 | |
| DCM | 25.00 | >25.0 | 25.00 | 25.00 | >25.0 | >25.0 | >25.0 | >25.0 | >25.0 | 25.00 | |
| EA | 3.13 | >25.0 | >25.0 | 25.00 | >25.0 | >25.0 | 25.00 | >25.0 | >25.0 | 25.00 | |
| Chloroform | 6.25 | >25.0 | >25.0 | >25.0 | 25.00 | 25.00 | 12.50 | >25.0 | 25.00 | 25.00 | |
| Ciprofloxacin | 0.01 | 0.01 | 0.02 | 0.01 | 0.63 | 0.16 | 0.01 | 0.31 | 0.01 | 0.01 | |
B.c: Bacillus cereus; E.c: Escherichia coli; S.a: Staphylococcus aureus; K.o: klebsiella oxytoca; S.t: Salmonella typhimurium; S.p: Streptococcus pyogenes; P.a: Pseudomonas aeruginosa; M.s: Mycobacterium smegmaris; C.a: Candida albicans
Table 3.
Minimum bactericidal concentration (MBC) of P. capensis extracts against nine species of bacteria and C. Ibicans
| Plants Part |
Solvents | Minimum bactericidal concentration (MBC) in mg/ml | |||||||||
| B. c | E. c | K. p | S. a | K. o | S. t | S. p | P. a | M.s | C. a | ||
| Roots | EtOH | 6.25 | 12.50 | 12.50 | 6.25 | 6.25 | 12.50 | 3.13 | 12.50 | 6.25 | 6.25 |
| Water | 12.50 | 25.00 | 12.50 | 15.50 | 25.00 | 25.00 | 12.50 | 12.50 | 12.50 | 25.00 | |
| DCM | 25.00 | 25.00 | 12.50 | 3.13 | 25.00 | 25.00 | 25.00 | 25.00 | 3.13 | Nt* | |
| EA | 0.39 | 25.00 | 12.50 | 12.50 | 25.00 | 25.00 | 0.39 | 25.00 | 12.50 | 1.56 | |
| Chloroform | 6.25 | >25.0 | 25.00 | 12.50 | 12.50 | 25.00 | 12.50 | Nt* | 25.00 | 12.50 | |
| Bark | Et0H | 6.25 | 25.00 | 25.00 | 12.50 | 12.50 | 12.50 | 6.25 | 25.00 | 3.13 | 12.50 |
| Water | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 12.50 | 25.00 | 6.25 | >25.0 | |
| DCM | >25.0 | >25.0 | 25.00 | 25.00 | 25.00 | >25.0 | >25.0 | >25.0 | 25.00 | >25.0 | |
| EA | 3.13 | 25.00 | 25.00 | 25.00 | >25.0 | >25.0 | 6.25 | >25.0 | 25.00 | 6.25 | |
| Chloroform | 6.25 | >25.0 | >25.0 | 25.00 | >25.0 | >25.0 | 3.13 | Nt* | 12.50 | 6.25 | |
| Leaves | Et0H | >25.0 | 25.00 | Nt* | Nt* | Nt* | >25.0 | Nt* | Nt* | >25.0 | 25.00 |
| Water | >25.0 | >25.0 | 25.00 | >25.0 | >25.0 | Nt* | Nt* | 25.00 | >25.0 | Nt* | |
| DCM | >25.0 | >25.0 | >25.0 | >25.0 | Nt* | Nt* | Nt* | Nt* | Nt* | 25.00 | |
| EA | >25.0 | Nt* | Nt* | >25.0 | Nt* | Nt* | >25.0 | Nt* | Nt* | >25.0 | |
| Chloroform | 6.25 | Nt* | Nt* | Nt* | Nt* | >25.0 | 25.00 | Nt* | 25.00 | >25.0 | |
| Ciprofloxacin | 0.001 | 0.01 | 0.02 | 0.16 | >1.25 | 0.16 | 0.31 | 0.01 | 0.31 | 0.01 | |
B.c: Bacillus cereus; E.c: Escherichia coli; S.a: Staphylococcus aureus; K.o: klebsiella oxytoca; S.t: Salmonella typhimurium; S.p: Streptococcus pyogenes; P.a: Pseudomonas aeruginosa; M.s: Mycobacterium smegmaris; C.a: Candida albicans; *Nt= Not tested.
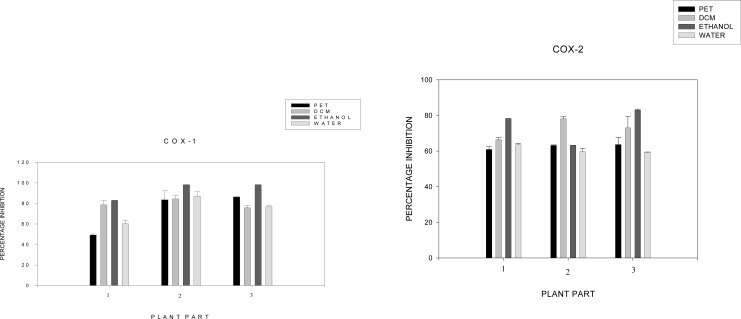
The anti-inflammatory activity results were summarized in Figure 1, which shows the inhibition of COX-1 and COX-2 enzymes by the 12 different extracts. A value of inhibition of 60% by organic solvents is considered significantly active. Moderate to potent inhibitory activity was observed in all extracts. Ethanol and dichloromethane extracts were among the most potent when compared to water and petroleum ether extracts. Ethanol (80%), bark and roots extracts resulted in the highest inhibitory activity against both COX-1 and COX-2 (Figures 1). Inhibitory effects for COX-1 were measured at 98.0% and 98.1% respectively. For COX-2, the percentage inhibitions were 78.17% and 83.07% respectively. It is important to note that water serves as the most used solvent by traditional healers, and showed more than 50%, inhibition rate against both COX-1 and COX-2 (Figures 1). The anti-oxidant activities of the extracts that were tested, compared to Vitamin C, are displayed in Table 4. The extracts revealed that they are antioxidant agents. Ethanol extracts causes strong transformation of DPPH radicals into a reduced form, with IC50 of 1.00 µg/ml and 1.74 µg/ml (roots and bark), which is in close range with Vitamin C (1.40 µg/ml). The water, ethyl acetate, dichloromethane and chloroform (bark and roots) extracts exhibited a high DPPH scavenging activity with an IC50 of 5.62 to 25.62 µg/ml. The leaf extracts of all solvents yielded the highest DPPH scavenging activity, with IC50 values ranging from 6.80 to 45.97 µg/ml. The results revealed that P. capensis extracts are good antioxidant agents. The cytotoxicity effects of the P. capensis extracts on the growth of Hek cells are shown in Table 5. The water extracts of roots and bark proved to be non-toxic in a lower concentration of 100.0 µg/ml with a cell viability of above 150%, and an IC50 value of 204.0 and 207.3 µg/ml. It was ofvery exciting to note that water extracts did not have any toxic effects on the cells, given the traditional healers normal use of water as their solvent. However, dichloromethane, ethanol, ethyl acetate and chloroform extracts showed toxic effects at a higher concentration of 25.0 to 400.0 µg/ml, with cell viability of 70 to 20%, and an IC50 range from 5.94 to 42.91 µg/ml. Table 1: Extraction yield and phytochemical composition of the plant extracts
Figure 1.
Percentage inhibition of COX1 and 2 extracts of P. capensis 1Leaf extract; 2Bark extract; 3Root extract
Table 4.
Antioxidant activity(IC 50) of P.capensis extracts please
| Solvents | Plants Part | IC 50(µg/ml) | STD |
| Ethyl acetate | Roots | 4.34 | ± 0.06 |
| Bark | 5.62 | ± 0.20 | |
| Leaves | 45.97 | ±16.38 | |
| Water | Roots | 3.53 | ± 0.11 |
| Bark | 12.42 | ± 1.11 | |
| Leaves | 30.42 | ±13.39 | |
| Ethanol | Root | 1.74 | ± 0.07 |
| Bark | 1.00 | ± 0.03 | |
| Leaves | 6.80 | ± 0.38 | |
| Dichloromethane | Roots | 14.25 | ± 0.12 |
| Bark | 59.82 | ±15.26 | |
| Leaves | 33.24 | ±22.91 | |
| Chloroform | Root | 13.34 | ± 0.46 |
| Bark | 25.62 | ± 0.62 | |
| Leaves | 6.81 | ± 4.99 | |
| Vitamin C | 1.40 | ±0.003 |
Table 5.
The IC50 (cytotoxicity activity) value of P. capensis extracts
| Solvent | Plants Part | IC50 (µg/mL) | STD |
| Ethylacetate | Roots | 5.94 | ± 0.13 |
| Bark | 7.63 | ± 0.08 | |
| Water | Roots | 204.0 | ± 2.58 |
| Bark | 207.3 | ± 1.59 | |
| Ethanol | Roots | 42.91 | ± 0.91 |
| Bark | 17.23 | ± 1.25 | |
| Dichloromethane | Roots | 8.06 | ± 0.13 |
| Bark | 14.93 | ± 0.11 | |
| Chloroform | Roots | 6.90 | ± 0.02 |
| Bark | 21.43 | ± 0.17 | |
| Actinomycin-D | 0.005458 | ± 0.00008235 |
Conclusion
The current study was initiated as a preliminary screening exercise of P. capensis in pathogenic organisms, to analyse if this plant is an effective antimicrobial agent. The roots and bark extracts of ethyl acetate showed to be a good antimicrobial agent with MIC value below 1,000 mg/ml. This plant showed a good effect on free radical DPPH solution. The present study indicates that P. capensis extracts yield good anti-inflammatory activity against both COX1 and 2, with the inhibition of both COX enzymes by 98% and 83%. The water extracts prove to be nontoxic to Hek cells, with an IC50 value of 207.3 and 204.0 µg/ml. It was also very exciting to note that water extracts did not have any toxic effects on the cells, as confirmed through the traditional healers' normal use of water as solvent. It is therefore suggested that further analysis be carried out to validate the effectiveness of this plant.
Acknowledgement
The authors would like to thank CSIR for Funding.
References
- 1.Baneerjee A, Dasgupta N, De B. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chemistry. 2005;90:727–733. [Google Scholar]
- 2.Burrell JWK, Garwood RF, Jackman LM, Oskay E, Weedon BCL. Carotenoids and related compounds. Part XIV. Stereochemistry and synthesis of geraniol, nerol, farnesol, and phytol. Journal of the Chemical Society. 1966:2144–2154. [Google Scholar]
- 3.Chan EWC, Lim YY, Chew YL. Antioxidant activity of Camellia sinesis leaves and tea from lowland plantation of Malaysia. Food Chemistry. 2007;102:1214–1222. [Google Scholar]
- 4.Cock M, Dold T. Voice from the forest: Celebrating nature and culture in Xhosaland. Jacan Media (Ltd); 2012. p. 209. [Google Scholar]
- 5.Cohen MA, Husband MD, Yoder SL, Gage JW, Roland GE. Bacterial eradication by clinafloxacin, CI-990, and ciprofloxacin employing MBC test, in vitro time kill and in vivo time kill studies. Journal of Antimicrobial Chemotheraphy. 1998;41:605–614. doi: 10.1093/jac/41.6.605. [DOI] [PubMed] [Google Scholar]
- 6.Jager De. Notes on the magical charms of the Cape Nguni tribes. Vol. 2. Fort Hare Papers; 1963. pp. 293–309. [Google Scholar]
- 7.Dogruoz N, Zeybek Z, Karogoz A. Antibacterial activity of some plants extracts. Journal of Biology. 2008;67(1):17–21. [Google Scholar]
- 8.Du Toit R, Volsteedt Y, Apostolides Z. Comparison of the antioxidant content of fruits, vegetables and teas measured as Vitamin C equivalents. Toxicology. 2001;166:63–69. doi: 10.1016/s0300-483x(01)00446-2. [DOI] [PubMed] [Google Scholar]
- 9.Eldeen MS, Van S, Taden J. In vitro pharmacological investigation of extracts from some trees used in Sudanese traditional medicine. South African Journal of Botany. 2007;73:435–440. [Google Scholar]
- 10.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plants extract for bacteria. Planta Medica. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 11.Jager AK, Huctching A, Van Staden A. Journal of Ethnopharmacology. 52. 1996:95–100. doi: 10.1016/0378-8741(96)01395-5. [DOI] [PubMed] [Google Scholar]
- 12.Loo AY, Jain K, Darah I. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chemistry. 2008;107:1151–1160. [Google Scholar]
- 13.Mabogo DEN. The ethno botany of the Vhavenda. University of Pretoria; 1990. p. 77. M.Sc. thesis. [Google Scholar]
- 14.Michelle C, Dold T. Voice from the forest: Celebrating nature and culture in Xhosaland. Jacan Media (ltd) 2012:209. [Google Scholar]
- 15.Palgrave KG. Trees of South Africa. second edition. Cape Town South Africa: Struik Publishers; 1983. pp. 516–517. [Google Scholar]
- 16.Palombo SD, Semple SJ. Antibacterial activity of traditional Australian medicinal plants. Journal of Ethnopharmacology. 2001;77:151–157. doi: 10.1016/s0378-8741(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 17.Rabe T, Van Staden J. Antibacterial activity of South African plants used for medicinal purposes. Journal of Ethnopharmacology. 1997;56:81–87. doi: 10.1016/s0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- 18.Rangkadilok N, Sitthimonchai S, Worasuttangkurn L, Mahidol C, Ruchirawat M, Satayavivad J. Evaluation of free radical scavenging and antityrosinenase activities of standardized Longan fruit extract. Food Chemistry Toxicology. 2007;44:328–336. doi: 10.1016/j.fct.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Reid KA, Maesa A, Van Staden J, Dekimpec N, Mulholland DA, Verschaevea L. Evaluation of the mutagenic and antimutagenic effects of South African plants. Journal of Ethnopharmacology. 2006;106:44–50. doi: 10.1016/j.jep.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Retief E, Herman PPJ. Plants of the Northern province of South Africa; Key and diagnostic characters. National Botanical Institute; 1997. p. 369. [Google Scholar]
- 21.Schmidt E, Lotter M. Trees and shrubs of Mpumalanga and Kruger National Park. Jacana Media; 2002. pp. 358–359. [Google Scholar]
- 22.Seqaws P, Kasenene JM. Medicinal plants diversity and uses in the Sango bay area, Southern Uganda. Journal of Ethnopharmacology. 2007;(113):521–548. doi: 10.1016/j.jep.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Zheng YT, Chan WL, Chan P, Huang H, Tam SC. Enhancement of the anti-herpetic effect of trichosanthin by acyclovir and interferon. Federation of European Biochemical Letters. 2001;496:139–142. doi: 10.1016/s0014-5793(01)02391-2. [DOI] [PubMed] [Google Scholar]