Abstract
Background
The genus Asphodeline (Liliaceae) is represented in Turkey by 20 taxa, which are traditionally used for medicinal purposes in Anatolia.
Materials and Methods
In this study, we tested the phytochemical content and antioxidant effect of different solvent extracts obtained from different anatomical parts of Asphodeline anatolica. The different extracts of each plant parts were tested for antioxidant activity using different chemical assays. The total antioxidant components were also calculated.
Results
Generally, acetone extracts produced the seed and root exhibited significantly higher antioxidant activity with high antioxidant components. Total phenolic content of extracts were significantly correlated with antioxidant potentials (except for, metal chelating activity).
Conclusion
On the basis of the results obtained, A. anatolica extracts should be regarded as a valuable source of natural antioxidants for food and therapeutic applications.
Keywords: Asphodeline, Antioxidant activity, Free radical scavenging, Phenolics, Solvent extracts, Turkey
Introduction
Free radicals, especially reactive oxygen species (ROS), are highly unstable molecules; react with various organic substrates such as lipids, proteins, carbohydrates and DNA. The excessive production of free radicals is called “oxidative stress” and can result in serious diseases including cancer, atherosclerosis, rheumatoid arthritis and neurodegenerative diseases (Arouma, 2010). Defense against oxidative stress is therefore very important in preventing the development of diseases mentioned. Antioxidants are vital substances which possess the ability to protect the body from the damage caused by these radicals (Silva et al., 2005). Hence, it has been suggested that there is an inverse relationship between dietary intake of antioxidants and the incidence of the diseases related to oxidative stress (Rice-Evans et al., 1997; Lu and Foo, 2000). Although synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG) are commonly used for food processing, their toxic properties and unwanted side effects limit their widespread use (Lindenschmidt et al., 1986; Kehrer and DiGiovanni, 1990). For this reason, there has been a particular interest in the potential health benefits of natural antioxidants in medicinal and aromatic plants. In fact, many functional phytochemicals such as phenolics, flavonoids and carotenoids possessed powerful antioxidant activities (Wang et al., 2013)
The genus Asphodeline belongs to the Liliaceae family and is present in south-west of Asia, Middle-Eastern countries and Mediterranean region. The genus comprises of 14 species worldwide. In Turkey, this genus represented by 20 taxa and 11 of them are endemic to Turkey (Mathews and Tuzlaci, 1984; Tuzlaci, 1987). The high number of endemics shows that Turkey is one of the gene centre of this genus. The Asphodeline species are known as çiriş plants and are abundant especially in the mountains and steppes of inner Anatolia region of Turkey. Besides their attractive flowers, many of the Asphodeline genus have significant applications in Anatolia traditional medicine. For example, A. damascena and A. cilicica are used for treatment of earaches. Likewise, A.globifera is used as a medicament for alleviating haemorrhoids symptoms, A. lutea and A. taurica are consumed in salads (Tuzlaci, 1985). Owing to their potential use in different purposes, several studies focused on secondary metabolites especially antraquinones, sesquiterpene and naphthalene components of Asphodeline species (Ulubelen et al., 1988; Ulubelen et al., 1989; Todorova et al., 2010). However, no scientific studies are reported on the antioxidant properties of different solvent extracts obtained from different parts of Asphodeline anatolica, which is endemic to Turkey.. The aim of the present study was to evaluate on total antioxidant components, antioxidant potentials, free radical scavenging activities, reducing power activities and metal chelating abilities of extracts from parts of A. anatolica in order to understand the usefulness of this plant as a foodstuff as well as in medicine in food and pharmaceutical industries. This study creates a scientific basis for ethnopharmacological use of Asphodeline species in the Anatolian traditional medicine.
Materials and Methods
Plant material and Preparation of Extracts
The herbal parts of A. anatolica was collected from Sarkikaraagac-Yenisarbademli road, 38°03′07″ N, 31°17′51″E, 1144 m, Isparta-Turkey when the end of flowering season (July 2012). Taxonomic identification of the plant material was confirmed by the senior taxonomist Dr. Murad Aydin Sanda, from the Department of Biology, Selcuk University. The voucher specimen was deposited at the KNYA Herbarium of Department of Biology, Selcuk University, Konya-Turkey (Voucher No: GZ 1001). The plant materials (stem, root, seed and leaf) were dried at the room temperature. The dried parts were ground to a fine powder using a laboratory mill. For each of the powdered parts (10 g) were separately extracted with acetone and methanol in a Soxhlet apparatus for 6–8 h. The extracts concentrated under vacuum at 40 °C by using a rotary evaporator. To obtain water extracts, the powdered samples were boiled with 250 mL of distilled water for 30 min. The aqueous extracts were filtered and lyophilized (−80°C, 48 h). Extracts were stored at + 4°C in dark until use.
Determination of total bioactive components
Total phenolic content
The total phenolic content was determined by employing the methods given in the literature (Slinkard and Singleton, 1977) with slight modification. Sample solution (0.25 mL) was mixed with diluted Folin-Ciocalteu reagent (1 mL, ratio of 1:9) and shaken vigorously. After 3 min, Na2CO3 solution (0.75 mL, 1%) was added and the sample absorbance was read at 760 nm after 2 hrs incubation at room temperature. The total phenolic content was expressed as equivalents of gallic acid (mgGAEs/g).
Total flavonoid content
The total flavonoid content was determined using the Dowd method as adapted by Berk et al. (2011). Briefly, sample solution (1 mL) was mixed with the same volume of aluminium trichloride (2%) in methanol. Similarly, a blank was prepared by adding sample solution (1 mL) to methanol (1 mL) without AlCl3. The sample and blank absorbances were read at 415 nm after a 10 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The total flavonoid content was expressed as equivalents of rutin (mgREs/g).
Total saponins content
The total saponins content was determined by the vanillin-sulfuric acid method (Aktumsek et al., 2013). Sample solution (0.25 mL) was mixed with vanillin (0.25 mL, 8%) and sulfuric acid (2 mL, 72%). The mixture was incubated for 10 min at 60 °C. Then the mixture was cooled for another 15 min, followed by the sample absorbance measurement at 538 nm. The total saponin content was expressed as equivalents of Quillaja (mgQEs/g).
Total condensed tannin content
The total condensed tannin content was determined by the vanillin method (Bekir et al., 2013) with slight modification. Sample solution (0.5 mL) was mixed with vanillin reagent (1.5 mL, 1% in 7 M H2SO4) in an ice bath and then mixed well. Similarly, a blank was prepared by adding sample solution (0.5 mL) to 7 M H2SO4 (1.5 mL). The sample and blank absorbances were read at 500 nm after a 15 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The total condensed tannin content was expressed as equivalents of (+)-catechin (mgCEs/g).
Total flavanol content
The total flavanol content was determined by employing the methods given in the literature (Quettier-Deleu et al., 2005) with slight modification. Sample solution (0.25 mL) was added to 5 ml of 0.1% DMACA (p-dimethylaminocinnamaldehyde) in methanolic:HCl ( ratio of 3:1) reagent. The sample absorbance was read at 640 nm after 10 min incubation at room temperature. The total flavanol content was expressed as equivalents of (+)-catechin (mgCEs/g).
Radical scavenging activity
Free radical scavenging activity (DPPH)
The effect of the samples on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical was estimated according to Sarikurkcu (2011). Sample solution (1 mL) was added to a 4 ml of a 0.004% methanol solution of DPPH. The sample absorbance was read at 517 nm after a 30 min incubation at room temperature in dark. The DPPH radical scavenging activity was expressed as equivalents of trolox (mgTEs/g).
ABTS (2,2 Azino-bis (3-ethylbenzothiazloine-6-sulfonic acid)) radical cation scavenging activity
The scavenging activity aganist ABTS cation radical was measured according to the method of Re et al. (1999) with slight modification. Briefly, ABTS.+ radical cation was produced directly by reacting 7 mM ABTS solution with 2.45 mM potassium persulfate and allowing the mixture to stand for 12–16 in dark at the room temperature. Prior to beginning the assay, ABTS solution was diluted with methanol to an absorbance of 0.700±0.02 at 734 nm. Sample solution (1 mL) was added to ABTS solution (2 mL) and mixed. The sample absorbance was read at 734 nm after a 30 min incubation at room temperature. The ABTS radical cation scavenging activity was expressed as equivalents of trolox (mgTEs/g).
Reducing power
Cupric ion reducing (CUPRAC) method
The cupric ion reducing activity (CUPRAC) was determined according to the method of Apak et al. (2006). Sample solution (0.5 mL) was added to premixed reaction mixture containing CuCl2 (1 mL, 10 mM), neocuproine (1 mL, 7.5 mM in ethanol) and NH4Ac buffer (1 mL, 1 M, pH 7.0). Similarly, a blank was prepared by adding sample solution (0.5 mL) to premixed reaction mixture (3 mL) without CuCl2. Then, the sample and blank absorbances were read at 450 nm after a 30 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. CUPRAC activity was expressed as equivalents of trolox (mgTEs/g).
Ferric reducing antioxidant power (FRAP) method
The FRAP assay was carried out as described by Aktumsek et al. (2013) with slight modification. Sample solution (0.1 mL) was added to premixed FRAP reagent (2 mL) containing acetate buffer (0.3 M, pH 3.6), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) (10 mM) in 40 mM HCl and ferric chloride (20 mM) in a ratio of 10:1:1 (v/v/v). Then, the sample absorbance was read at 593 nm after a 30 min incubation at room temperature. FRAP activity was expressed as equivalents of trolox (mgTEs/g)
Total antioxidant activity
Phosphomolybdenum method
The total antioxidant activity of the samples was evaluated by phosphomolybdenum method according to Berk et al. (2011) with slight modification. Sample solution (0.3 mL) was combined with 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The sample absorbance was read at 695 nm after a 90 min incubation at 95 °C. The total antioxidant capacity was expressed as equivalents of trolox (mgTEs/g)
β-carotene-linoleic acid method
In this assay antioxidant activity is determined by measuring the inhibition of the volatile organic compounds and the conjugated diene hydroperoxides arising from linoleic acid oxidation (Sarikurkcu et al., 2012) with slight modification. A stock solution of β-carotene-linoleic acid mixture was prepared as following: 0.5 mg β-carotene was dissolved in chloroform (1 mL, HPLC grade). 25 µL linoleic acid and 200 mg Tween 40 was added. Chloroform was completely evaporated using a vacuum evaporator. Then 100 mL of oxygenated distilled water was added with vigorous shaking; 1.5 mL of this reaction mixture was dispersed to test tubes and sample solution (0.50 mL, 1 mg/mL) were added and the emulsion system was incubated for up to 2 h at 50 °C. The same procedure was repeated with the standards (BHA, BHT and trolox) and a blank. After this incubation period, the sample absorbance was read at 490 nm. Measurement of absorbance was continued until the color of β-carotene disappeared. The bleaching rate (R) of β-carotene was calculated according to Eq. (1).
Where, ln=natural log, a=absorbance at time 0, b=absorbance at time t (30, 60, 90, 120 min). The antioxidant activity (AA) was calculated in terms of percent inhibition relative to the control using Eq. (2).
Metal chelating activity on ferrous ions
The metal chelating activity on ferrous ions was determined by the method described by Aktumsek et al. (2013). Briefly, sample solution (2 mL) was added to FeCl2 solution (0.05 mL, 2 mM). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL). Similarly, a blank was prepared by adding sample solution (2 mL) to FeCl2 solution (0.05 mL, 2 mM) and water (0.2 mL) without ferrozine. Then, the sample and blank absorbances were read at 562 nm after 10 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The metal chelating activity was expressed as equivalents of EDTA (mgEDTAs/g).
Results and Discussion
Evaluation of antioxidant properties of plants cannot be performed accurately by any single method due to complex nature of phytochemicals (Du et al., 2009). Accordingly, we different antioxidant capacity assays, including free radical scavenging (DPPH and ABTS), reducing power (FRAP and CUPRAC), metal chelating, phosphomolybdenum and β-carotene/linoleic acid test system were used to analyze the antioxidant capacity of the anatomical parts of A. anatolica.
Antioxidant components
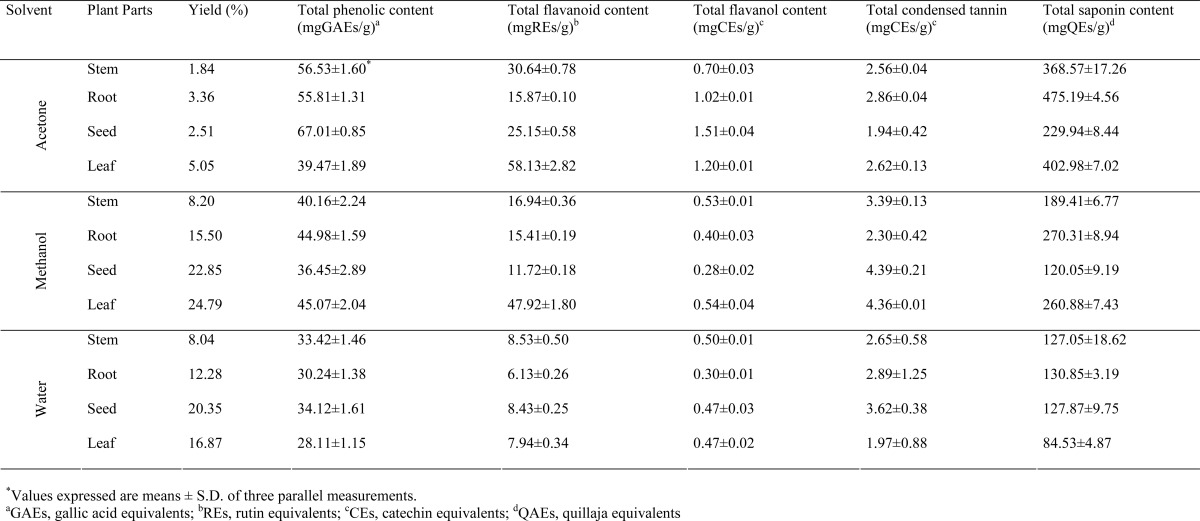
Plant phenolics, flavonoids, tannin and saponins represent major groups of plant constituents that work predominantly as powerful antioxidants or scavenger of free radicals. In the present study, solvents with different polarities (acetone, methanol and water) were used to extract antioxidants from A. anatolica. It is expected that using different solvents, there would be various amounts of phenolics and non-phenolics such as saponins (Sun and Ho, 2005). The total antioxidant components namely phenolic, flavonoid, flavanol, tannin and saponin contents in the various extract of different parts of A. anatolica were presented in Table 1. Our results revealed that the acetone extract of seed had higher total phenolic content (67.01 mgGAEs/g) followed by acetone extracts of stem (56.53 mgGAE/g) and root (55.81 mgGAE/g). As regards the flavonoid content, the results obtained from evaluation of total flavonoid content indicated great variations. In acetone extracts, the content of flavonoid compounds as rutin equivalents were notably higher than methanolic and water extracts studied. Acetone extract of leaves contains the highest level flavonoid concentrations (58.13 mgRE/g). The lowest flavonoid concentration was measured in water extracts studied. From our results, it was apparent that the total phenolic and flavonoid content were dependent on extraction solvents and their polarity. Total flavanol content of the extracts was examined using a colorimetric DMACA method. Similar to phenolic and flavonoids, the results of quantitative analysis revealed that higher level of flavanol was observed in acetone extracts. The flavanol content of acetone extracts ranged from 0.70 to 1.51 mgCEs/g, whereas that of water extracts ranged from 0.30 to 0.5 mgCEs/g. It was in agreement with previous studies, which also found that acetone extracts obtained plant species contained the highest amount of phenolic compounds (Zhao et al., 2006). Condensed tannin content was detected using the vanillin-HCl assays and these results were evaluated as catechin equivalents (mgCEs/g). Table 1 shows that the methanolic extract of seed contain large amounts of tannin (4.39 mgCEs/g), which was very similar to the value of methanolic extract obtained from leaves (4.36 mgCEs/g). As to total saponin content, root extracts showed the highest saponin content in different anatomical parts tested in this study, followed by leaves, stem and seed, respectively. Moreover, the total saponin content decreased in the following order: acetone>methanol>water. According to our study, acetone is the best solvent for the extraction of antioxidant components from A. anatolica.
Table 1.
Total antioxidant components of different solvent extracts obtained from different parts of A. anatolica
 |
Free radical scavenging activities
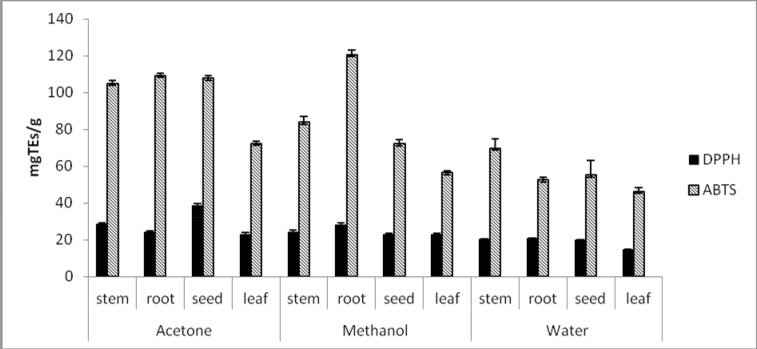
The free radical quenching abilities of different parts of A. anatolica were tested by DPPH and ABTS assays. The activities were evaluated as trolox equivalents (mgTEs/g) (Figure 1). The DPPH is stable free radical, it can be readily undergo scavenging by an antioxidant that loses this absorption on accepting an electron or a free radical species (Wang et al., 2013). In DPPH assay, seed and root extracts had stronger scavenging activity. As expected, acetone extracts exhibited superior scavenging activity than other solvent extracts. Among all the organs tested, acetone extract of seed exhibited the highest scavenging activity with the highest TE value of 38.55 mg/g, followed by stem, root and leaves, respectively. The lowest DPPH scavenging activity was observed in water extract of leaf with very low TE value of 14.74 mg/g. The results for superior DPPH radical scavenging activity of acetone extracts could be explained by presence of greater concentration of phenolics in these extracts.
Figure 1.
ABTS and DPPH radical scavenging activity of different solvent extracts obtained from different parts of A. anatolica (Values expressed are means ± S.D. of three parallel measurements).
ABTS is one of the radicals generally used for testing the preliminary radical quenching activity of plant extracts. The ABTS radical scavenging activity assay is presented as an excellent tool for determining the hydrogen donating and chain-breaking capacity of plant extracts (Leong and Shiu, 2002). On ABTS assay, TE values were as follows: 52.55 mgTEs/g to 120.55 mgTEs/g for extracts of root, 55.21 mgTEs/g to 107.74 mgTEs/g for extracts of seed, 69.6 mgTEs/g to 104.78 mgTEs/g for extracts of stem and 46.34 mgTEs/g to 72.36 mgTEs/g for extracts of leaf (Figure 1). In compared extracts tested, acetone extracts obtained from A. anatolica parts had higher quenching capacity than methanolic and water extracts. Interestingly, the methanolic extract of root had the grater ABTS radical scavenging capacity than acetone extract (120.55 mgTEs/g and 109.12 mgTEs/g extract, respectively). These results were in agreement with Wang et al. (2013) and Khan et al. (2012) who found a strong correlation between DPPH and ABTS assay. Likewise, many literatures reported show a strong relationship between the total phenolic content and the free radical scavenging activity of plant extracts (Shabir et al., 2011; GonÇalves et al., 2013; Othman et al., 2007).
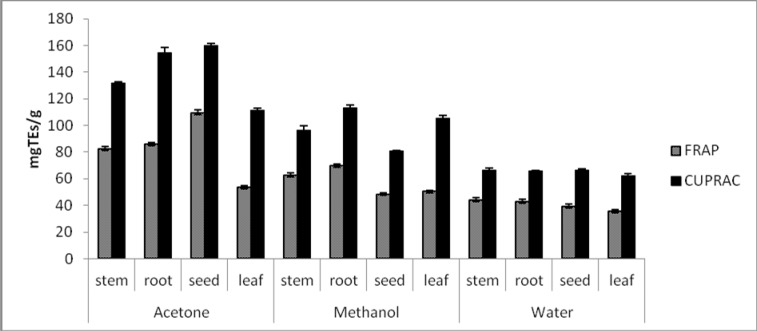
Reducing Power Activity by FRAP and CUPRAC assays
Fe3+ or Cu2+ reduction are often used to measure electron donation activity which is an important mechanism of antioxidants. Therefore, in order to assess the electron-donating powers of A. anatolica extracts, theirs ability to reduce Fe(III) and Cu(II) were investigated. The reducing activities of extracts studied are presented in Figure 2. The results are expressed as trolox equivalents (mgTEs/g extract). High values of TEs are indicative of high reducing activity. FRAP assay is based on the reduction of Fe (III)/TPTZ complex to the ferrous form (Fe(II)/TPTZ) in the presence of antioxidants. Generally, the highest FRAP activity was obtained for the acetone extracts and the lowest for water extracts. The CUPRAC assay is based on the reduction of Cu(II) to Cu(I) by antioxidants present in the plant extracts using copper(II)-neocuproine reagent as the chromogenic oxidant. The Cu(I)-neocuproine complex has an absorption maximum at 450 nm (Apak et al., 2006). The cupric reducing ability of the extracts tested was in order of acetone>methanol>water; the same trend shown by FRAP assay. For all the anatomical parts, the acetone extract of seed had the best of ferric and cupric reduction potentials. The antioxidant mechanism for ferric and cupric reducing power of acetone extracts might be due to the high level of phenolic compounds that act as electron donors. A similar correlation between the reducing power and total phenolic content of various solvent extracts were reported by GonÇalves et al. (2013) and Nithiyanantham et al. (2013).
Figure 2.
Ferric and cupric reducing powers of different solvents extracts obtained from different parts of A. anatolica (Values expressed are means ± S.D. of three parallel measurements).
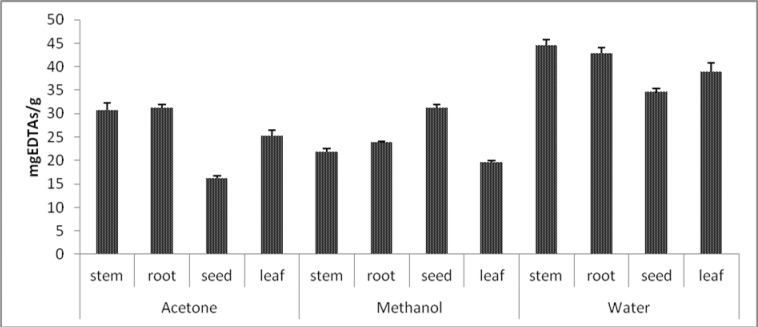
Figure 3.
Metal chelating activities of different solvent extracts obtained from different parts of A. anatolica (Values expressed are means ± S.D. of three parallel measurements).
Total antioxidant capacity by Phosphomolybdenum and β-carotene/linoleic acid bleaching assays
Phosphomolybdenum assay was described by Prieto et al. (1999) as cheap, easy and rapid for the evaluation of total antioxidant capacity of plant extracts. In the presence of antioxidant, Mo(VI) is reduced to Mo(V) and forms green phsosphate-Mo(V) complex which has its maximum absorption at 695 nm. Higher activity of 513.56, 469.89 and 438.32 mg TEs/g extract was observed acetone extract of stem, methanolic extract of leaf and acetone extract of seed, respectively (Table 2). The higher activity in acetone extracts were may be due to high contents of antioxidant components, especially phenolics. Our results are supported by other investigations (Prasad et al., 2009; Jayaprakasha et al., 2008). Lower activity was noted for water extracts compared to acetone and methanolic extracts (expect for seed).
Table 2.
Total antioxidant activity of different solvent extracts obtained from different parts of A. anatolica
| Plant Parts | Phosphomolybdenum assay (mgTEs/g)a |
β-Karoten/Linoleic acid system (%) |
|
| Acetone | stem | 513.56±2.34* | 82.71±2.38 |
| root | 418.85±6.74 | 83.34±1.51 | |
| seed | 438.32±2.87 | 85.23±1.60 | |
| seed | 397.60±13.60 | 90.46±1.12 | |
| Methanol | stem | 310.27±19.34 | 72.27±0.69 |
| root | 381.97±20.04 | 80.71±2.25 | |
| seed | 237.69±1.09 | 80.33±2.69 | |
| leaf | 469.89±35.52 | 89.42±1.06 | |
| Water | stem | 275.16±25.66 | 77.48±0.89 |
| root | 290.79±19.63 | 55.56±0.68 | |
| seed | 258.34±13.85 | 60.70±0.01 | |
| leaf | 275.45±26.18 | 44.37±3.88 | |
|
Standard Antioxidants |
BHA | nt | 86.36±1.15 |
| BHT | nt | 97.07±1.79 | |
| Trolox | nt | 89.34±0.94 | |
Values expressed are means ± S.D. of three parallel measurements.
TEs: Trolox equivalents; nt:no tested.
The total antioxidant capacity was also evaluated by using β-carotene/linoleic acid bleaching assay. The assay evaluated by the activity to neutralize the linoleate-free radical and other free radicals formed in the system which attack the highly unsaturated β-carotene models (Barros et al., 2009) and these results are depicted in Table 2. As expected, acetone extracts exhibited significantly stronger antioxidant activities (82.71–90.46%) than methanolic (72.27–89.42%) and water extracts (44.37–77.48%). When compared with the inhibition values of positive controls, including BHA (86.36%), BHT (97.07%) and trolox (89.34%), the acetone extract of leaf (90.46%) had higher value than BHA and trolox, but lower than BHT. Therefore, the acetone extracts can be considered as inhibitors safer than synthetic antioxidants for lipid oxidation in the food industry. In water extracts, the maximum activity was shown by stem, which was 77.48%. As far as our literature survey could as certain, there is no study on inhibition against linoleic acid oxidation of Asphodeline. Therefore, this study is the first in this area.
Ferrous ion-chelating ability
Iron is known as the most important lipid oxidation pro-oxidant due to its high reactivity. In this direction, the ability of substances or plant extracts to chelate iron can be important mechanism for antioxidant property (Manian et al., 2008). We therefore assessed the ferrous ion chelating capacity of extracts by measuring Fe+2-ferrozine test system. The results are expressed as EDTA an equivalents, which is known as an excellent chelator and summarized in Figure 3. From the figure, it is clear that chelating powers of water extracts were higher as compared to the other two extracts. Water extract of stem (44.62 mgEDTAs/g extract) had the strongest metal chelating activity, while acetone extract of seed (16.13 mgEDTAs/g extract) showed lowest activity among the extracts tested. In this case, no correlation was found between iron chelating capacities and phenolic content for A. anatolica extracts. This may indicate the presence of non-phenolic antioxidants, such as ascorbic acid and citric acid responsible for metal chelation (Lee et al., 2004).
Conclusion
In conclusion, our investigation on different parts of A. anatolica endemic to Turkey, indicate, that acetone extracts are rich sources of antioxidants, with significant high level of phenolic and flavonoid content. All anatomical parts studied possess antioxidant potentials; however, the extracts from seed and root exhibited good antioxidant activity in test systems used compared to stem and leaf. Therefore, the present study suggests a basis for the possible use of A.anatolica as alternative natural antioxidants for nutraceutical, functional food and pharmacological applications. Our study is the first report of in vitro antioxidant activity of A.anatolica. The present study forms a scientific basis for the use of Asphodeline species in traditional medicine. Further studies are needed to identify the antioxidant components and to investigate the antioxidant efficacy of A.anatolica in vivo.
Acknowledgements
This study was supported financially as a project. The authors thank Selcuk University Scientific Research Foundation (BAP) for providing financial support for this study.
References
- 1.Aktumsek A, Zengin G, Guler GO, Cakmak YS, Duran A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem Toxicol. 2013;55:290–296. doi: 10.1016/j.fct.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Apak R, Guclu K, Ozyurek M, Karademir SE, Ercag E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr. 2006;57:292–304. doi: 10.1080/09637480600798132. [DOI] [PubMed] [Google Scholar]
- 3.Arouma OI. Free radicals, oxidative stress, and antioxidants in human health and diseases. J Am Oil Chem Soc. 2010;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barros L, Heleno SA, Carvalho AM, Ferreira IOCFR. Systematic evaluation of the antioxidant potential of different parts of Foeniculum vulgare Mill. From Portugal Food Chem Toxicol. 2009;47:2458–2464. doi: 10.1016/j.fct.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Bekir J, Mars M, Souchard JP, Bouajila J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem Toxicol. 2013;55:470–475. doi: 10.1016/j.fct.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Berk S, Tepe B, Arslan S, Sarikurkcu C. Screening of the antioxidant, antimicrobial and DNA damage protection potentials of the aqueous extract of Asplenium ceterach DC. Afr J Biotechnol. 2011;10:8902–8908. [Google Scholar]
- 7.Du GR, Li MJ, Ma FW, Liang D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009;113:557–562. [Google Scholar]
- 8.GonÇalves S, Gomes D, Costa P, Romano A. The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Ind Crop Prod. 2013;43:465–471. [Google Scholar]
- 9.Jayaprakasha GK, Girennavar B, Patil B S. Radical scavenging activities of Rio Red grapefruits and Sour orange fruit extracts in different in vitro model systems. Biores Technol. 2008;99:4484–4494. doi: 10.1016/j.biortech.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 10.Kehrer JP, DiGiovanni J. Comparison of lung injury induced in 4 strains of mice by butylated hydroxytoluene. Toxicol Lett. 1990;52:55–61. doi: 10.1016/0378-4274(90)90165-i. [DOI] [PubMed] [Google Scholar]
- 11.Khan RA, Khan MR, Sahreen S, Ahmed M. Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Chem Cent J. 2012;6:1–11. doi: 10.1186/1752-153X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Renita M, Fioritto RJ, StMartin SK, Schwartz SJ, Vodovotz Y. Isoflavone characterization and antioxidant actitivty of Ohio soybeans. J Agric Food Chem. 2004;5(9):2647–2651. doi: 10.1021/jf035426m. [DOI] [PubMed] [Google Scholar]
- 13.Leong LP, Shiu G. An investigated of antioxidant capacity of fruits. An investigated of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. [Google Scholar]
- 14.Lindenschmidt ROC, Tryka AF, Goad ME, Witschi HP. The effects of dietary butylated hydroxytoluene on liver and colon tumor development in mice. Toxicol. 1986;38:151–160. doi: 10.1016/0300-483x(86)90116-2. [DOI] [PubMed] [Google Scholar]
- 15.Lu YR, Foo LY. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000;68:81–85. [Google Scholar]
- 16.Manian R, Anusuya N, Siddhuraju P, Manian S. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camelia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 2008;107:1000–1007. [Google Scholar]
- 17.Mathews VA, Tuzlaci E. Asphodeline Reichb. In: Davis PH, editor. Flora of Turkey and East Aegean Islands. Vol. 8. Edinburgh University Press; 1984. pp. 88–97. [Google Scholar]
- 18.Nithiyanantham S, Siddhuraju P, Francis G. A promosing approach to enhance the total phenolic content and antioxidant activity of raw and processed Jatropha curcas L. kernel meal extracts Ind Crop Prod. 2013;43:261–269. [Google Scholar]
- 19.Othman A, Ismail A, Abdul Ghani N, Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100:1523–1530. [Google Scholar]
- 20.Prasad KN, Yang B, Dong X, Jiang G, Zhang H, Xie H, Jiang Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov Food Sci Emerg. 2009;10:627–632. [Google Scholar]
- 21.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphor molybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 22.Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M, Cazin J-C, Bailleul F, Trotin F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol. 2005;72:35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 23.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 24.Rice-Evans C, Sampson J, Bramley PM, Hollway DE. Why do we expect carotenoids to be antioxidants in vivo? Free Radic Res. 1997;26:381–398. doi: 10.3109/10715769709097818. [DOI] [PubMed] [Google Scholar]
- 25.Sarikurkcu C. Antioxidant activities of solvent extracts from endemic Cyclamen mirabile Hildebr. tubers and leaves. Afr J Biotechnol. 2011;10:831–839. [Google Scholar]
- 26.Sarikurkcu C, Eryigit F, Cengiz M, Tepe B, Cakir A, Mete E. Screening of the antioxidant activity of the essential oil and methanol extract of Mentha pulegium L. from Turkey. Spectrosc Lett. 2012;45:352–358. [Google Scholar]
- 27.Shabir G, Anwar F, Sultana B, Khalid ZM, Afzal M, Khan QM, Ashrafuzzaman M. Antioxidant and antimicrobial attributes and phenolics of different solvent extracts from Leaves, flowers and bark of Gold Mohar [(Delonix regia (Bojer ex Hook.) Raf)] Molecules. 2011;16:7302–7319. doi: 10.3390/molecules16097302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva BA, Ferreres F, Malva JO, Dias AOCP. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005;90:157–167. [Google Scholar]
- 29.Slinkard K, Singleton VL. Total phenol analyses: Automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- 30.Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90:743–749. [Google Scholar]
- 31.Todorova G, Lazarova I, Mikhova B, Kostova I. Anthraquinone, naphthalene and napthoquinone components of Asphodeline lutea. Chem Nat Compd+ 2010;46:322–323. [Google Scholar]
- 32.Tuzlaci E. Çiriş Plants of Turkey. J Pharm Univ Mar. 1985;1:69–89. [Google Scholar]
- 33.Tuzlaci E. Revision of the genus Asphodeline (Liliaceae). A new infrageneric classification. Candollea. 1987;42:559–576. [Google Scholar]
- 34.Ulubelen A, Tuzlaci E. Sesquiterpene lactones, flavonoids and anthraquinones from Asphodeline globifera and Asphodeline damascena. Phytochemistry. 1985;24:2923–2924. [Google Scholar]
- 35.Ulubelen A, Terem B, Tuzlaci E. Antraquinones and sesquiterpene lactones from Asphodeline anatolica. Fitoterapia. 1988;2:159. [Google Scholar]
- 36.Ulubelen A, Tuzlaci E, Atilan N. Oxepine derivates and anthraquinones from Asphodeline tenuior and A. taurica. Phytochemistry. 1989;28:649–650. [Google Scholar]
- 37.Wang L, Wang Z, Li X. Preliminary phytochemical and biological activities study of solvent extracts from a cold-field fruit Malus baccata (Linn.) Borkh. Ind Crop Prod. 2013;47:20–28. [Google Scholar]
- 38.Zhao HF, Dong JJ, Lu J, Chen J, Li Y, Shan LJ, Lin Y, Fan W, Gu GX. Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.) J Agr Food Chem. 2006;54:7277–7286. doi: 10.1021/jf061087w. [DOI] [PubMed] [Google Scholar]