Abstract
Objective:
To minimize complement-mediated damage in acute relapses of neuromyelitis optica (NMO) by adding treatment with a complement inhibitor, purified C1-esterase inhibitor, to the current standard of care (high-dose glucocorticoids).
Method:
We conducted an open-label phase 1b safety and proof-of-concept trial in 10 patients with NMO–immunoglobulin G seropositive NMO or NMO spectrum disease (NMOSD) who presented with acute transverse myelitis and/or optic neuritis. In addition to treating with 1 g of daily IV methylprednisolone, we infused 2,000 units of C1-esterase inhibitor daily for 3 days, beginning on day 1 of hospitalization. The primary outcome measure was safety, and the secondary efficacy measure was change in Expanded Disability Status Scale (EDSS) scores.
Results:
Ten patients with NMO/NMOSD were enrolled, 7 of whom presented with acute transverse myelitis and 3 with acute optic neuritis. C1-esterase inhibitor proved to be safe in all 10 patients, with no serious adverse events recorded. There were no thromboembolic events or related lab abnormalities in any of the subjects. EDSS scores dropped from a median of 4.5 on admission to 4.0 on discharge and then down to 2.5 on 30-day follow-up. All but 1 patient returned to preattack EDSS or better and only 2 patients required escalation to plasmapheresis.
Conclusions:
C1-esterase inhibitor is a safe add-on therapy for patients with NMO/NMOSD presenting with acute transverse myelitis and optic neuritis. Preliminary evidence suggests a promising benefit with C1-esterase inhibitor in reducing neurologic damage and improving outcomes. A placebo-controlled trial is necessary to confirm these findings.
Classification of evidence:
This study provides Class IV evidence that for patients with NMO with acute transverse myelitis or optic neuritis, C1-esterase inhibitor is safe and improves disability.
Neuromyelitis optica (NMO) is a recurrent neuroinflammatory disease primarily targeting the optic nerves and spinal cord leading to blindness and paralysis.1 The role of complement in the immunopathogenesis of an acute NMO relapse as an effector of damage in the spinal cord and optic nerve was first proposed based on the demonstration of perivascular complement product deposition (C9neo) in affected tissue.2 Antibody-mediated complement fixation generally involves the classical complement pathway initiated by C1 binding to immunoglobulin (Ig) G, triggering activation of C1r and C1s components of C1, which leads to subsequent downstream activation of the membrane attack complex causing cell lysis and death.
C1-esterase inhibitor is the only known blocker of C1. It works by preventing C1r and C1s from becoming activated and also by inactivating them once triggered by antibody binding. In addition, C1-esterase inhibitor blocks downstream components of the mannan-binding lectin, fibrinolytic, clotting, and kinin pathways.3 A purified human C1-esterase inhibitor has been tested in conditions of harmful complement activation, including sepsis and myocardial infarction, with promising results.4,5
The standard of care for management of acute relapses of NMO is high-dose corticosteroids, but most patients are left with residual disability following each attack despite early and aggressive immunosuppression.6 In this study, we added C1-esterase inhibitor to try to mitigate the complement-mediated tissue injury. This phase 1 open-label trial was designed to test the safety and efficacy of using purified human C1-esterase inhibitor in preventing CNS damage during acute NMO relapses.
METHODS
This was a single-center, phase I, Class IV, open-label study of C1-esterase inhibitor in NMO patients with acute relapses. Patients with NMO or NMO spectrum disorder aged 18–65 years who presented to the Johns Hopkins Hospital between February 2013 and June 2013 with new neurologic symptoms were eligible for enrollment if they demonstrated a new contrast-enhancing lesion on MRI of the spinal cord, brainstem, or optic nerves. On days 1, 2, and 3 of admission, patients received 1,000 mg of IV methylprednisolone plus 2,000 units of IV purified human C1-esterase inhibitor. The dose of C1-esterase inhibitor was chosen based on successes in myocardial infarction and sepsis.4,5 On days 4 and 5, patients received only 1,000 mg of IV methylprednisolone. Plasmapheresis was initiated after day 5 in patients who showed no improvement with corticosteroids and C1-esterase inhibitor; 2 additional doses of 1,000 units of C1-esterase inhibitor were infused after the 2nd and 5th exchanges. All patients except for one were enrolled within 10 days of symptom onset.
The primary endpoint was safety and the secondary endpoint was efficacy on reducing disability. NMO-IgG was tested at Mayo Clinic Labs, Quest, or LabCorp. Hospitalized patients were monitored daily for adverse events and examined on admission, discharge, and 30-day follow-up using the standardized Expanded Disability Status Scale (EDSS). Statistical analysis on EDSS scores was performed using 2-tailed Wilcoxon matched-pairs signed rank test, with significant p values defined as < 0.05.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Johns Hopkins Institutional Review Board and informed consent was obtained prior to enrollment. The identifier for the trial on the ClinicalTrials.gov Web site is NCT01759602.
RESULTS
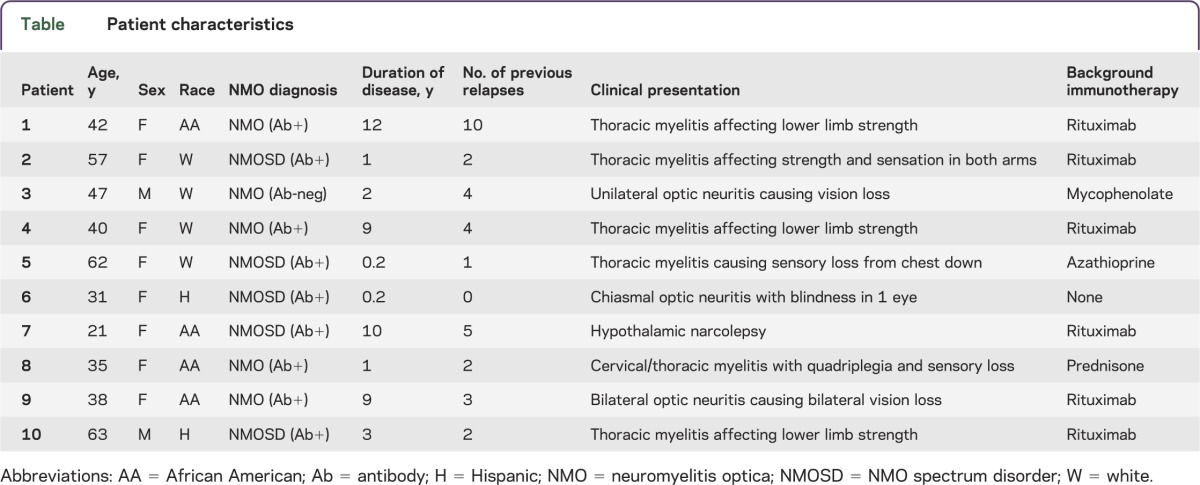
Ten patients were screened for eligibility, enrolled, given the intervention, and followed up 30 days later for examination to complete the study (figure 1). Five of the 10 patients met the Wingerchuk 2006 clinical criteria for NMO,7 4 of whom were positive for the NMO-IgG. The other 5 subjects carried a diagnosis of NMO spectrum disorder, as they were seropositive for the NMO-IgG in the context of transverse myelitis or optic neuritis but did not meet all clinical criteria.8 Seven of the 10 presented with acute transverse myelitis and 3 presented with acute optic neuritis (table).
Figure 1. Flow diagram of single-center trial.
Ten patients were screened, gave consent, and were enrolled in the trial. All 10 patients received the full study intervention and completed the trial at 30-day follow-up.
Table.
Patient characteristics
There were no serious adverse events (SAEs) in this study. Based on previous studies of C1-esterase inhibitor in other conditions,9 the SAE of greatest concern was thromboembolism manifesting as deep vein thrombosis (DVT), acute myocardial or cerebral infarction, or pulmonary embolism, especially in immobilized hospitalized patients. None of these occurred in this 10-patient NMO study population. Hospitalized patients received routine prophylaxis for DVT with subcutaneous heparin and routine hematologic and metabolic screening and were monitored daily for significant changes in vital signs and worsening neurologic examination. No abnormalities were reported among the 10 patients during or after the study except for a mild elevation in alanine aminotransferase liver function test to a peak of 111 mg/dL in 1 patient; this lab abnormality resolved by the discharge date. Adverse effects of C1-esterase inhibitor were logged daily. One patient reported a mild headache following an initial infusion; with fluids, the headache resolved within 4 hours.
Although this study was not powered for efficacy and there was no control arm, we looked for a signal of efficacy by measuring the impact of C1-esterase inhibitor on disability following an acute relapse. The EDSS score was calculated for each patient at baseline before the relapse based on previous clinic notes, at the peak of relapse, upon discharge from the hospital, and again 30 days later on follow-up in the outpatient clinic. In 7 patients, the EDSS score at the peak of relapse was higher than their baseline, due to a combination of inflammation and tissue damage from the new MRI-confirmed NMO lesion (figure 2). Following a 5-day course of steroids plus a 3-day course of C1-esterase inhibitor, EDSS scores declined back to baseline or better in 9 patients. The median baseline EDSS score was 2.25, which increased to a median of 4.5 at the peak of the attack. The median EDSS score on discharge was 4.0, which was 5 days after admission for 8 of the 10 patients and 19 days after admission for 2 patients. At 30-day follow-up, the median EDSS score was 2.5, which was not statistically different from baseline (figure 3).
Figure 2. Individual EDSS scores.
EDSS scores were recorded for each patient at baseline in clinic, at peak of relapse in the hospital, upon discharge, and at 30-day follow-up in clinic. In 7 patients, the EDSS score increased from baseline to the acute relapse, and in 8 patients the EDSS score declined back to baseline or better by 30-day follow-up. Patient 7 required plasmapheresis; patients 8 and 10 showed improvement in neurologic examination with corticosteroids plus C1-esterase inhibitor not captured by the EDSS.
Figure 3. Box plot of EDSS scores.
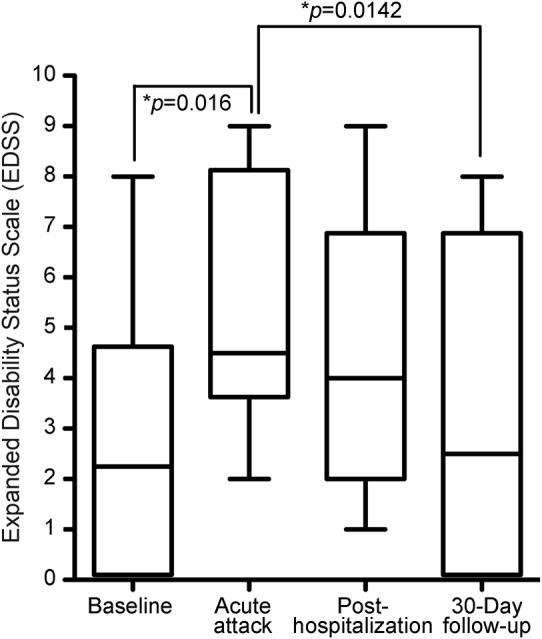
The median EDSS score at baseline was 2.25, which increased to 4.5 at peak of relapse (p = 0.016). The median EDSS score at discharge was 4.0 and at 30-day follow-up was 2.5. There was a statistical difference in the EDSS scores at peak of relapse compared to 30-day follow-up (p = 0.0142) but not between baseline and 30-day follow-up (p = 1.000). Differences were calculated using Wilcoxon matched-pairs signed rank test for paired nonparametric comparisons. Error bars represent the minimum and maximum data points.
DISCUSSION
This single-center, phase 1, open-label study of C1-esterase inhibitor add-on therapy in 10 patients with acute NMO relapses met its objective to test the safety of C1-esterase inhibitor in this patient population. There were no SAEs in the course of this trial, including no serious thromboembolic events and no significant metabolic or hematologic abnormalities as a result of using a high dose (2,000 units daily for 3 days) of C1-esterase inhibitor. Although not designed for efficacy, this trial demonstrated that neurologic disability returned to baseline in 9 out of the 10 patients on 30-day follow-up, suggesting C1-esterase inhibition may prevent complement-mediated damage in the spinal cord and optic nerve during an NMO relapse. There are obvious limitations to making efficacy conclusions based on this study alone. This phase 1 trial was not controlled with a group that received standard of care only or placebo, and the open-label design is subject to performance and detection biases. A blinded, randomized, placebo-controlled prospective trial is needed to determine the accurate impact of C1-esterase inhibition on neurologic disability.
Patients with NMO do not lack endogenous C1-esterase inhibitor and are likely to have normal (16–33 mg/dL) or higher circulating levels during a relapse. Lack of C1-esterase inhibition leads to low circulating complement C4 levels,10 but NMO relapses do not generally result in low C4 levels, measured systemically, as in sepsis or acute attacks of hereditary angioedema (a C1 inhibitor deficiency disorder).11 Rather, complement activation is taking place in relatively limited lesions within the spinal cord and/or optic nerve in NMO. Thus, the presumed primary mechanism of action of C1-esterase inhibition in acute NMO relapses is the blocking of the damage caused by localized classical and mannan-binding lectin complement activation. There may be other immunosuppressive effects mediated by C1-esterase inhibition on T-cell proliferation and cytokine production12 or other effects of C1-esterase inhibition on the kallikrein and bradykinin pathways.13 Of interest, a recent phase 2 open-label study of complement C5 inhibition in NMO showed that it was effective in preventing relapses, suggesting that chronic complement inhibition is immunosuppressive on other autoimmune elements of NMO, not just complement activity.14
Although there are no placebo-controlled trials to confirm the efficacy of glucocorticoids, they are widely considered the standard of care. Because of the high risk of permanent tissue damage from a single relapse, future prospective efficacy trials in acute NMO should be designed as randomized, placebo-controlled add-on studies of glucocorticoids plus C1-esterase inhibitor compared to glucocorticoids alone. In addition to measures of disability, a good outcome measure in this patient population would be the number of patients who require escalated immunosuppression with plasmapheresis, which results in increased length of hospital stay, increased cost of care, and the potential for additional morbidity from this intervention. A trial in which fewer patients treated with glucocorticoids plus experimental drug require plasmapheresis for an acute NMO relapse compared to the control group that received glucocorticoids plus placebo would suggest that the experimental drug is beneficial and would likely result in better long-term outcomes.
GLOSSARY
- DVT
deep vein thrombosis
- EDSS
Expanded Disability Status Scale
- Ig
immunoglobulin
- NMO
neuromyelitis optica
- SAE
serious adverse event
AUTHOR CONTRIBUTIONS
Ms. Mealy: study concept and design, acquisition of data, analysis and interpretation, critical revision of manuscript. Dr. Levy: study concept and design, acquisition of data, analysis and interpretation, critical revision of manuscript, study supervision.
STUDY FUNDING
This study was funded by ViroPharma, Inc., manufacturer of C1-esterase inhibitor (Cinryze). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and the preparation or approval of the manuscript.
DISCLOSURE
M. Mealy received honoraria from Novartis Pharmaceuticals. Dr. Levy receives research support from ApoPharma Inc., NIH, Guthy Jackson Charitable Foundation, Viropharma, Acorda, Sanofi, NeuralStem, and Genentech; is on the scientific advisory board and has received travel funding from Asterias; and serves as a consultant for Chugai Pharmaceuticals, GlaxoSmithKline, and Medimmune. Go to Neurology.org/nn for full disclosures.
REFERENCES
- 1.Oh J, Levy M. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol Res Int 2012;2012:460825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 2002;125(pt 7):1450–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caliezi C, Wuillemin WA, Zeerleder S, Redondo M, Eisele B, Hack CE. C1-Esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol Rev 2000;52:91–112 [PubMed] [Google Scholar]
- 4.Igonin AA, Protsenko DN, Galstyan GM, et al. C1-esterase inhibitor infusion increases survival rates for patients with sepsis. Crit Care Med 2012;40:770–777 [DOI] [PubMed] [Google Scholar]
- 5.Fattouch K, Bianco G, Speziale G, et al. Beneficial effects of C1 esterase inhibitor in ST-elevation myocardial infarction in patients who underwent surgical reperfusion: a randomised double-blind study. Eur J Cardiothora Surg 2007;32:326–332 [DOI] [PubMed] [Google Scholar]
- 6.Mutch K, M3ethley A, Moore P, Jacob A. Life on hold: the experience of living with neuromyelitis optica. Disabil Rehabil 2013 [DOI] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489 [DOI] [PubMed] [Google Scholar]
- 8.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815 [DOI] [PubMed] [Google Scholar]
- 9.Kalaria S, Craig T. Assessment of hereditary angioedema treatment risks. Allergy Asthma Proc 2013;34:519–522 [DOI] [PubMed] [Google Scholar]
- 10.Markovic SN, Inwards DJ, Frigas EA, Phyliky RP. Acquired C1 esterase inhibitor deficiency. Ann Intern Med 2000;132:144–150 [DOI] [PubMed] [Google Scholar]
- 11.Veszeli N, Fust G, Csuka D, et al. A systematic analysis of the complement pathways in patients with neuromyelitis optica indicates alteration but no activation during remission. Mol Immunol 2014;57:200–209 [DOI] [PubMed] [Google Scholar]
- 12.Nissen MH, Bregenholt S, Nording JA, Claesson MH. C1-esterase inhibitor blocks T lymphocyte proliferation and cytotoxic T lymphocyte generation in vitro. Int Immunol 1998;10:167–173 [DOI] [PubMed] [Google Scholar]
- 13.Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol 2010;126:918–925 [DOI] [PubMed] [Google Scholar]
- 14.Pittock SJ, Lennon VA, McKeon A, et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol 2013;12:554–562 [DOI] [PubMed] [Google Scholar]



