Abstract
Objective:
In bacterial meningitis, a decreased level of consciousness is predictive for unfavorable outcome, but the clinical features and outcome in patients presenting with a minimal score on the Glasgow Coma Scale are unknown.
Methods:
We assessed the incidence, clinical characteristics, and outcome of patients with bacterial meningitis presenting with a minimal score on the Glasgow Coma Scale from a nationwide cohort study of adults with community-acquired bacterial meningitis in the Netherlands from 2006 to 2012.
Results:
Thirty of 1,083 patients (3%) presented with a score of 3 on the Glasgow Coma Scale. In 22 of 30 patients (73%), the minimal Glasgow Coma Scale score could be explained by use of sedative medication or complications resulting from meningitis such as seizures, cerebral edema, and hydrocephalus. Systemic (86%) and neurologic (47%) complications occurred frequently, leading to a high proportion of patients with unfavorable outcome (77%). However, 12 of 30 patients (40%) survived and 7 patients (23%) had a good functional outcome, defined as a score of 5 on the Glasgow Outcome Scale. Patients presenting with a minimal Glasgow Coma Scale score on admission and bilaterally absent pupillary light responses, bilaterally absent corneal reflexes, or signs of septic shock on admission all died.
Conclusions:
Patients with community-acquired bacterial meningitis rarely present with a minimal score on the Glasgow Coma Scale, but this condition is associated with high rates of morbidity and mortality. However, 1 out of 5 of these severely ill patients will make a full recovery, stressing the continued need for aggressive supportive care in these patients.
Bacterial meningitis is a serious and life-threatening disease.1 Streptococcus pneumoniae and Neisseria meningitidis are the predominant causative pathogens of this disease in adults,2 causing 80%–85% of all cases, with high associated morbidity and mortality rates.3–5 Many patients with bacterial meningitis present with an abnormal conscious state, and 15%–20% of patients are comatose upon presentation.5 An abnormal conscious state, in most studies graded by the Glasgow Coma Scale, is a strong predictor for poor disease outcome in bacterial meningitis.5,6 The exact cause of an abnormal conscious state in bacterial meningitis remains unclear, but it is likely caused by a complex interaction between severe brain inflammation, raised intracranial pressure (ICP), and resulting complications such as hydrocephalus, cerebral (micro)infarctions, or epileptic seizures.7–9 The evaluation of patients with an abnormal conscious state in bacterial meningitis might further be complicated by the use of antiepileptic or sedative medication.
The clinical features and outcome of the most severely ill patients, those who present with a score on the Glasgow Coma Scale of 3, are unknown. The time frame for concluding that neurologic damage is irreversible in these patients is unclear. In this study we analyzed the incidence, clinical characteristics, complications, and outcome of patients with bacterial meningitis presenting with a minimal Glasgow Coma Scale score.
METHODS
In a prospective nationwide observational cohort study in the Netherlands, we included episodes of community-acquired bacterial meningitis confirmed by culture of CSF in adults. Methods of the MeninGene Study have been described in detail elsewhere.5,10 In summary, all patients were 16 years of age or older and were listed in the database of the Netherlands Reference Laboratory for Bacterial Meningitis from January 2006 to March 2012. This laboratory receives CSF isolates from approximately 90% of all patients with bacterial meningitis in the Netherlands. Patients with negative CSF cultures, hospital-acquired meningitis, a neurosurgical device, or who underwent a neurosurgical operation within 1 month before bacterial meningitis onset were excluded. Patients using immunosuppressive drugs and those with asplenia, diabetes mellitus, alcoholism, or infection with immunodeficiency virus were considered immunocompromised. Informed consent was obtained from all participating patients or, in case of decreased consciousness, from their legally authorized representatives. Clinical data, including specific queries about the Glasgow Coma Scale score on admission, had been prospectively collected by means of an online case record form. The Glasgow Coma Scale grades coma severity according to 3 categories of responsiveness: eye opening, motor responses, and verbal responses, with scores ranging from 3 to 15.11 Information about brainstem reflexes including pupillary light responses, respiratory drive, EEG data, intubation, and sedative medication at the time of presentation was not a standard part of the case record form. These data were all collected retrospectively by reanalyzing correspondence from ambulance and emergency departments.
At discharge, all patients underwent a neurologic examination performed by a neurologist, and outcome was graded according to the Glasgow Outcome Scale. This is a well-validated measurement scale with scores varying from 1 to 5.12 A favorable outcome was defined as a score of 5 and an unfavorable outcome as a score of 1–4. Two clinicians independently classified the cause of death. The interrater agreement (κ) for cause of death was 0.73. Differences were resolved by discussion.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the ethics committee of the Academic Medical Center, Amsterdam, the Netherlands.
RESULTS
From 2006 to 2012, 1,083 episodes of community-acquired bacterial meningitis were included in the cohort. Thirty of 1,083 patients (3%) presented with a Glasgow Coma Scale score of 3 on admission (figure 1). Fifteen patients (50%) were female and the median age was 65 years (interquartile range 49–76 years; table 1). Fifteen patients (50%) had one or more predisposing conditions for bacterial meningitis, consisting of otitis/sinusitis in 8, immunocompromised state in 6, and pneumonia in 2.
Figure 1. Distribution of scores on Glasgow Coma Scale for adults presenting with community-acquired bacterial meningitis.
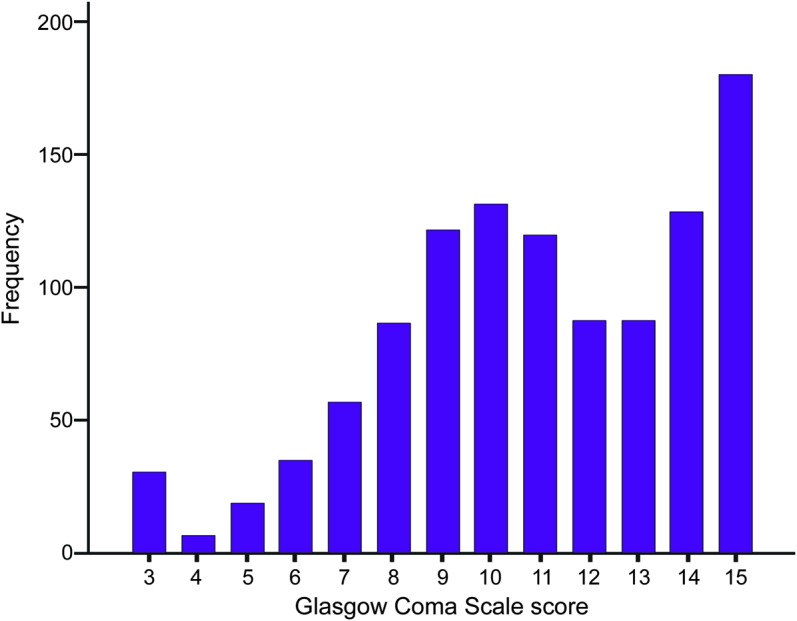
Table 1.
Clinical characteristics of 30 patients with bacterial meningitis presenting with a minimal coma scorea
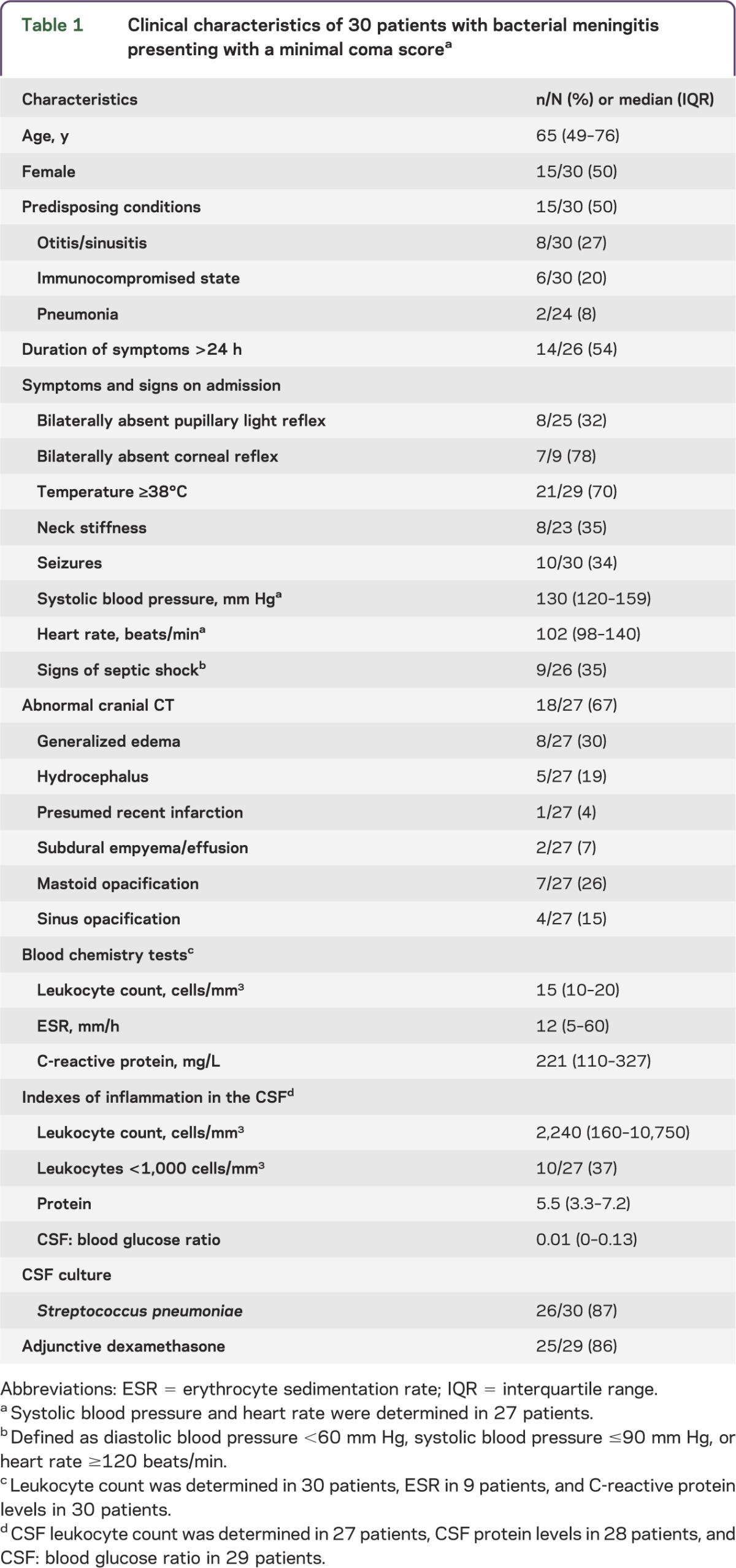
Data on pupillary light responses could be retrieved for 25 of 30 patients (83%). Pupillary light responses were bilaterally present in 15 patients (60%), unilaterally absent in 2 (8%), and bilaterally absent in 8 (32%; table 1). Anisocoria was noted in 6 of 20 patients (30%). Data on corneal reflexes could be retrieved for 9 patients, and corneal reflexes were bilaterally absent in 7 (including 4 patients with bilaterally absent pupillary light responses), unilaterally absent in 1, and bilaterally present in 1. One patient had an absent respiratory drive at time of presentation and died the next day. Of 30 patients, 13 (43%) were intubated prior to neurologic examination; 5 of these 13 patients (39%) received sedative drugs, 2 of whom also received muscle relaxant drugs. The remaining patients did not receive sedative mediation prior to initial neurologic examination. Eight of 23 patients (35%) with a minimal Glasgow Coma Scale score presented with neck stiffness, 10 of 30 patients (33%) presented with seizures, and 9 of 26 patients (34%) had signs of septic shock at time of presentation.
Seizures consisted of tonic-clonic seizures in all 10 patients; 4 patients received benzodiazepines to stop the seizures and 1 patient received sedative drugs and muscle relaxant drugs prior to intubation. One patient had prolonged seizures despite benzodiazepines; it is unknown how the prolonged seizures were treated in this patient.
Cranial CT on admission was performed in 27 of 30 patients (90%) and was abnormal in 67% (table 1; figure 2). In 22 of 30 patients (73%), the minimal Glasgow Coma Scale score could be explained either by complications resulting from the meningitis (seizures in 10, hydrocephalus in 5, and cerebral edema in 7) or by sedative medication (5 patients). In the other 8 patients, the minimal score on the Glasgow Coma Scale was explained by a direct effect of the meningitis (defined as a combination of severe brain inflammation and raised ICP).
Figure 2. Spectrum of abnormal CT scanning of patients with bacterial meningitis presenting with a minimal Glasgow Coma Scale score.
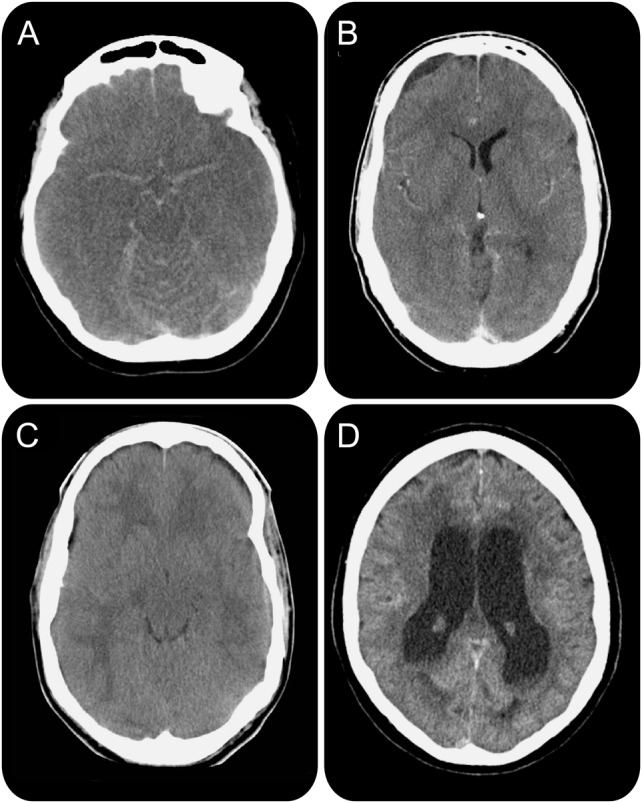
(A) Generalized cerebral edema with signs of pseudo-subarachnoid hemorrhage; (B) subdural empyema; (C) generalized cerebral edema indicating severe cerebral inflammation; (D) hydrocephalus.
Cranial imaging was repeated during admission in 16 patients (all patients underwent CT scan, 1 patient underwent CT and MRI scan) and was abnormal in 10 patients (63%). New abnormalities not present on admission were found in 2 patients and consisted of cerebral infarctions in both.
Twenty-nine of the 30 patients (97%) had at least one individual CSF finding predictive of bacterial meningitis (glucose level <34 mg/dL [1.9 mmol/L], ratio of CSF glucose to blood glucose <0.23, protein level >220 mg/dL, or leukocyte count >2,000/mm3).13 Ten of 27 patients (37%) had a CSF leukocyte count below 1,000 cells/mm3, which has previously been associated with poor outcome.5 CSF cultures grew S pneumoniae in 26 patients (87%) and Haemophilus influenzae, Salmonella enterica, Streptococcus pyogenes, and Staphylococcus aureus in 1 patient each. Initial antibiotic treatment was microbiologically adequate in all patients and 25 of 29 patients (86%) received adjunctive dexamethasone on admission.14 The clinical characteristics, results of cranial imaging, and CSF analysis were similar when considering only patients who did not receive sedative medication (table e-1 at Neurology.org/nn).
The majority of patients (87%; table 2) developed complications during the clinical course. Systemic complications consisted of respiratory failure (15 of 25 [60%]), pneumonia (6 of 26 [23%]), and hyponatremia (4 of 27 [15%]). Neurologic complications consisted of seizures (7 of 30 [23%]), hearing impairment (4 of 30 [13%]), cerebral infarction (3 of 30 [10%]), and subdural empyema (2 of 30 [7%]). EEG was performed in 5 patients during the clinical course. Four of these patients had an isoelectric EEG (1 to 3 days after admission) and treatment was withdrawn resulting in immediate death. In 1 patient, the EEG was performed on the eleventh day showing severe cortical dysfunction (with some residual cerebral activity). Care was withdrawn and the patient subsequently died of multiorgan failure. One patient with hydrocephalus was treated with external ventricular drainage on the day of admission but died 3 days later. Four patients with hydrocephalus were not treated with external ventricular drainage because of other poor prognostic factors (multiorgan failure, clinically brain dead) or because the hydrocephalus was mild and spontaneously resolved on follow-up imaging. None of the patients were treated with continuous ICP measuring and ICP-directed treatment.
Table 2.
Complications and outcome of 30 patients with bacterial meningitis presenting with a minimal coma score
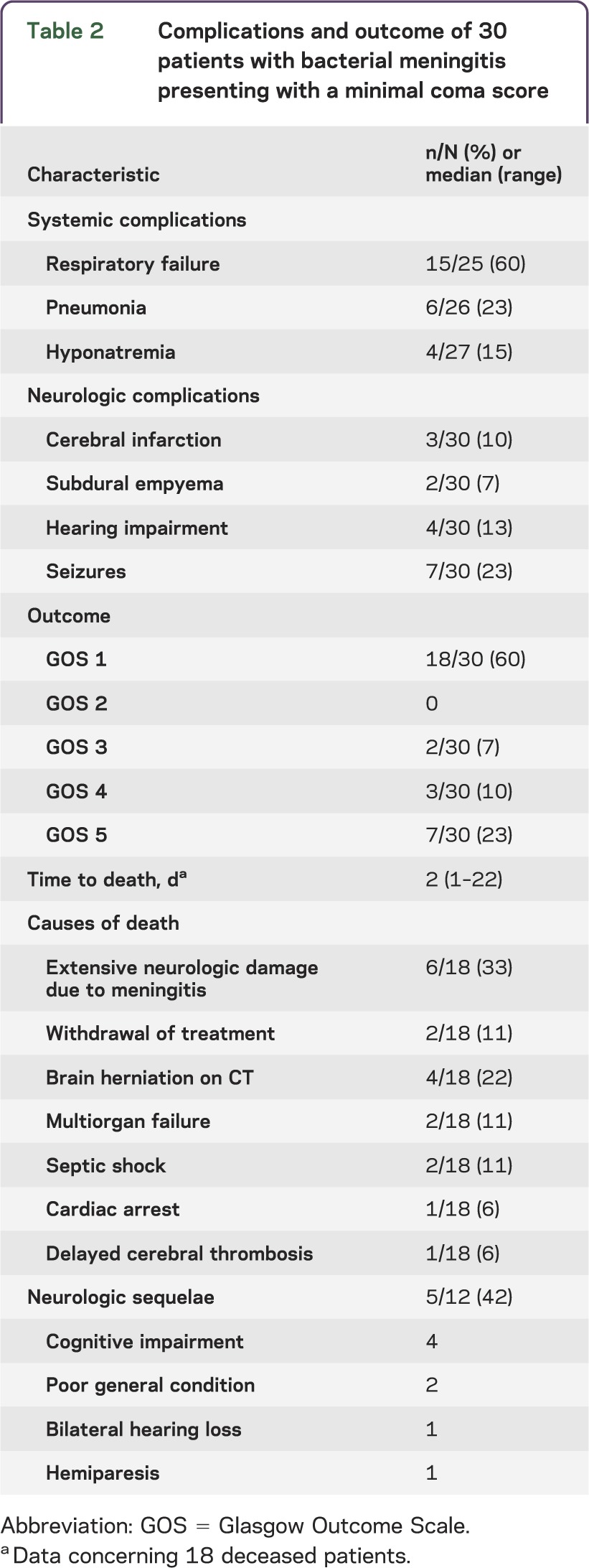
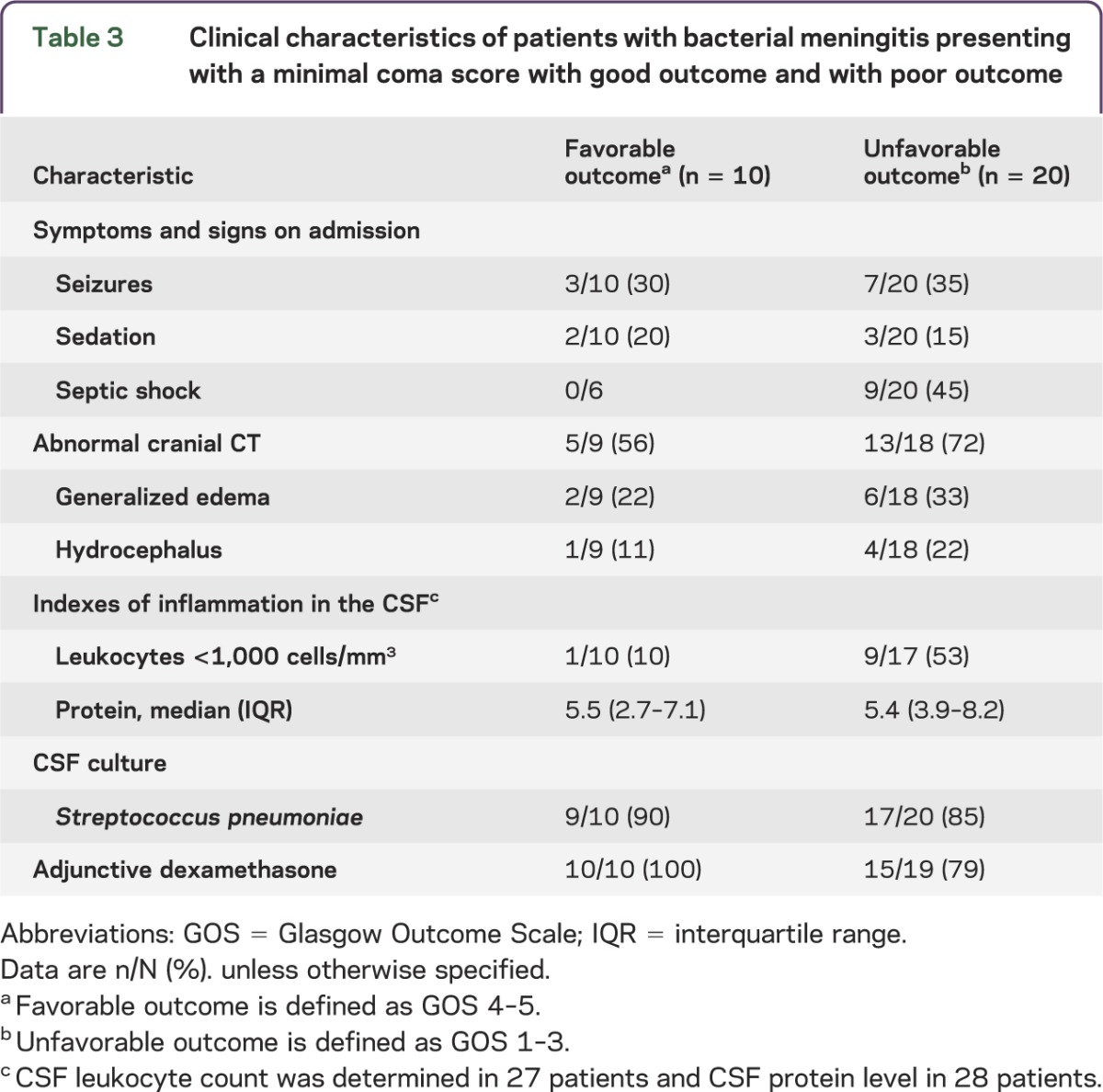
Twelve of the 30 patients (40%) survived and 7 patients (23%) had a good functional outcome, defined as a score of 5 on the Glasgow Outcome Scale. Neurologic sequelae were present on discharge in 5 of 12 survivors (42%) and consisted of cognitive impairment in 4, poor general condition in 2, and bilateral hearing loss and hemiparesis in 1 patient each; 3 surviving patients with sequelae were scored as moderately disabled on the Glasgow Outcome Scale and 2 as severely disabled requiring nursing home admission. All 11 patients presenting with either bilaterally absent pupillary light responses or bilaterally absent corneal reflexes died (table 3). Furthermore, all 9 patients with signs of septic shock on admission died; septic shock was defined as diastolic blood pressure <60 mm Hg, systolic blood pressure ≤90 mm Hg, or heart rate ≥120 beats per minute. The mortality rate and frequency of sequelae were similar when considering only patients who did not receive sedative medication on admission (table e-2).
Table 3.
Clinical characteristics of patients with bacterial meningitis presenting with a minimal coma score with good outcome and with poor outcome
The causes of death in the 18 deceased patients were as follows: extensive neurologic damage due to meningitis (6 patients), acute brain herniation shown on cranial imaging (4 patients), withdrawal of care because of poor neurologic prognosis (2 patients; care was withdrawn 2 and 3 days after admission), multiorgan failure or septic shock (2 patients each), and cardiac arrest or delayed cerebral thrombosis (1 patient each).15 Autopsy was performed in 3 patients showing severe brain damage in all patients. In 1 patient venous sinus thrombosis was identified that was not diagnosed prior to autopsy.
DISCUSSION
Our study shows that a small minority of patients with community-acquired bacterial meningitis presents with a minimal score on the Glasgow Coma Scale (3%). Although the majority of these patients had an unfavorable outcome (77%) and many patients died (60%), the number of patients who recovered completely was substantial. We could not identify clinical parameters predictive for favorable outcome, for example epileptic seizures or sedative drugs. However, patients presenting with a minimal Glasgow Coma Scale score on admission and bilaterally absent pupillary light responses, bilaterally absent corneal reflexes, or signs of septic shock on admission all died. Physicians confronted with a patient with bacterial meningitis presenting with a minimal Glasgow Coma Scale score should be aware that at least 1 out of 5 patients will make a full recovery.
Neuroimaging showed that 1 out of 4 patients with bacterial meningitis presenting with a minimal score on the Glasgow Coma Scale have cerebral abnormalities such as hydrocephalus or subdural empyema, which may require emergency neurosurgery. Hydrocephalus and empyema rarely complicate bacterial meningitis (3%–5% of patients) but have been associated with high rates of death and unfavorable outcome.16–18 Early identification and subsequent neurosurgical treatment of these complications may improve prognosis. Therefore, immediate cranial imaging should be a priority in patients with a minimal score on the Glasgow Coma Scale on admission to rule out treatable conditions. Because of the risk of increasing brain shift due to lumbar puncture, cranial imaging should precede lumbar puncture in all patients with a minimal Glasgow Coma Scale score to rule out space-occupying lesions causing brain shift.8 Antibiotic therapy and adjunctive dexamethasone therapy if indicated should be started before sending the patient to the imaging room. Although some authors have advocated the use of continuous ICP measuring and ICP lowering by use of CSF drainage and medication, the benefit of ICP management in these patients is still unclear.19,20
The abnormal conscious state could be explained by potentially reversible causes, such as seizures, sedative medication, or muscle relaxants, in a subgroup of patients (47%). However, these patients had similar outcomes compared to the other patients, suggesting that seizures and the need for intubation are markers of disease severity rather than indicators of a reversible cause of an abnormal conscious state. A minimal score on the Glasgow Coma Scale was preceded by epileptic seizures in 33% of patients and 5 patients received antiepileptic or sedative medication as treatment for seizures. Epilepsy has previously been described in 17% of patients with bacterial meningitis.21 If no clear explanation for a minimal coma score other than severe inflammation can be found, an EEG should be performed. This is also true for those patients with a waxing and waning level of consciousness. In 9 patients, signs of septic shock were present on admission and likely contributed to the minimal Glasgow Coma Scale score. Three of them also had seizures and one received sedative medication; in the other 5 patients, there was no explanation for the minimal coma score other than septic shock. Stabilization of hemodynamics is of the utmost importance early during sepsis and should have priority in patients with meningitis with concomitant sepsis. Despite aggressive management of the patients with sepsis included in our study, all patients with signs of septic shock on admission eventually died.
This study has several limitations. First, several clinical characteristics of patients with a minimal Glasgow Coma Scale score on admission were not routinely captured in the case record form (for example, brainstem reflexes, anisocoria, sedative medication), but these data were collected retrospectively. Furthermore, patients with a minimal score on the Glasgow Coma Scale may have been missed: patients with bacterial meningitis presenting with a minimal coma score may have severe brain edema, prohibiting a lumbar puncture. Another reason to withhold a lumbar puncture may be diffuse intravascular coagulation, which may occur due to concomitant sepsis. In patients with meningitis with predominant signs of septic shock, meningitis may be missed because the typical sign of meningitis, neck stiffness, is often absent in comatose patients. All these factors may have lead to an underestimation of the incidence of a minimal Glasgow Coma Scale score in patients with bacterial meningitis. Furthermore, this is an observational study, and all treatment decisions (for instance to place CSF catheters, operate for subdural empyema, or use ICP management) were at the discretion of the treating physician. Therefore, treatments between patients were different, which is likely to have influenced outcome. This implies our study cannot be used to determine treatment effects, but it does reflect clinical practice as it currently is in the Netherlands. Finally, only patients with positive CSF cultures were included. Negative CSF cultures are estimated to occur in 11%–20% of patients with bacterial meningitis.22–24 However, the clinical presentation of patients with culture-positive bacterial meningitis and patients with culture-negative bacterial meningitis was reported to be similar.
Patients with community-acquired bacterial meningitis rarely present with a minimal score on the Glasgow Coma Scale, but this condition is associated with high rates of morbidity and mortality. However, 1 out of 5 of these severely ill patients will make a full recovery, stressing the continued need for aggressive supportive care.
Supplementary Material
ACKNOWLEDGMENT
We are indebted to all Dutch physicians who participated in the study. We acknowledge research funding for the MeninGene study (Netherlands Organization for Health Research and Development, the Academic Medical Center, and the European Research Council).
GLOSSARY
- ICP
intracranial pressure
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Marjolein Lucas performed the data analysis and wrote the manuscript. Matthijs Brouwer performed the data analysis and wrote the manuscript. Arie van der Ende wrote the manuscript. Diederik van de Beek performed the data analysis, wrote the manuscript, is the principal investigator of the study, and provided funding.
STUDY FUNDING
This research has been supported by grants from the Netherlands Organization for Health Research and Development (Veni [916.76.023] and Vidi [016.116.358] to D.v.d.B.; Veni [916.13.078] to M.C.B.), the Academic Medical Center (AMC Fellowship 2008 to D.v.d.B.), and the European Research Council (ERC Starting Grant to D.v.d.B.).
DISCLOSURE
M.J. Lucas and A. van der Ende report no disclosures relevant to the manuscript. M.C. Brouwer has received research support from the Dutch Scientific Organization, The European Federation of Neurological Sciences, and the European Society of Clinical Microbiology and Infectious Diseases. D. van de Beek is an editor for BMC Infectious Diseases and Cochrane Acute Respiratory Infections Group and has received research support from Omeros, The Academic Medical Center, and the European Research Council. Go to Neurology.org/nn for full disclosures.
REFERENCES
- 1.van de Beek D, Brouwer MC, Thwaites GE, Tunkel AR. Advances in treatment of bacterial meningitis. Lancet 2012;380:1693–1702 [DOI] [PubMed] [Google Scholar]
- 2.McIntyre PB, O'Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet 2012;380:1703–1711 [DOI] [PubMed] [Google Scholar]
- 3.Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010;23:467–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 2003;126:1015–1025 [DOI] [PubMed] [Google Scholar]
- 5.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004;351:1849–1859 [DOI] [PubMed] [Google Scholar]
- 6.Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. A risk score for unfavorable outcome in adults with bacterial meningitis. Ann Neurol 2008;63:90–97 [DOI] [PubMed] [Google Scholar]
- 7.van de Beek D, Weisfelt M, de Gans J, Tunkel AR, Wijdicks EF. Drug Insight: adjunctive therapies in adults with bacterial meningitis. Nat Clin Pract Neurol 2006;2:504–516 [DOI] [PubMed] [Google Scholar]
- 8.van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med 2006;354:44–53 [DOI] [PubMed] [Google Scholar]
- 9.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 2011;24:557–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Beek D. Progress and challenges in bacterial meningitis. Lancet 2012;380:1623–1624 [DOI] [PubMed] [Google Scholar]
- 11.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81–84 [DOI] [PubMed] [Google Scholar]
- 12.Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet 1976;1:1031–1034 [DOI] [PubMed] [Google Scholar]
- 13.Spanos A, Harrell FE, Jr, Durack DT. Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. JAMA 1989;262:2700–2707 [PubMed] [Google Scholar]
- 14.van de Beek D, de Gans J, Spanjaard L, Vermeulen M, Dankert J. Antibiotic guidelines and antibiotic use in adult bacterial meningitis in The Netherlands. J Antimicrob Chemother 2002;49:661–666 [DOI] [PubMed] [Google Scholar]
- 15.Schut ES, Lucas MJ, Brouwer MC, Vergouwen MD, van der Ende A, van de Beek D. Cerebral infarction in adults with bacterial meningitis. Neurocrit Care 2012;16:421–427 [DOI] [PubMed] [Google Scholar]
- 16.Kasanmoentalib ES, Brouwer MC, van der Ende A, van de Beek D. Hydrocephalus in adults with community-acquired bacterial meningitis. Neurology 2010;75:918–923 [DOI] [PubMed] [Google Scholar]
- 17.Jim KK, Brouwer MC, van der Ende A, van de Beek D. Cerebral abscesses in patients with bacterial meningitis. J Infect 2012;64:236–238 [DOI] [PubMed] [Google Scholar]
- 18.Jim KK, Brouwer MC, van der Ende A, van de Beek D. Subdural empyema in bacterial meningitis. Neurology 2012;79:2133–2139 [DOI] [PubMed] [Google Scholar]
- 19.Lindvall P, Ahlm C, Ericsson M, Gothefors L, Naredi S, Koskinen LO. Reducing intracranial pressure may increase survival among patients with bacterial meningitis. Clin Infect Dis 2004;38:384–390 [DOI] [PubMed] [Google Scholar]
- 20.Abulhasan YB, Al-Jehani H, Valiquette MA, et al. Lumbar drainage for the treatment of severe bacterial meningitis. Neurocrit Care 2013;19:199–205 [DOI] [PubMed] [Google Scholar]
- 21.Zoons E, Weisfelt M, de Gans J, et al. Seizures in adults with bacterial meningitis. Neurology 2008;70:2109–2115 [DOI] [PubMed] [Google Scholar]
- 22.Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 1993;328:21–28 [DOI] [PubMed] [Google Scholar]
- 23.Sigurdardottir B, Bjornsson OM, Jonsdottir KE, Erlendsdottir H, Gudmundsson S. Acute bacterial meningitis in adults. A 20-year overview. Arch Intern Med 1997;157:425–430 [DOI] [PubMed] [Google Scholar]
- 24.Brouwer MC, Thwaites GE, Tunkel AR, van de Beek D. Dilemmas in the diagnosis of acute community-acquired bacterial meningitis. Lancet 2012;380:1684–1692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


